?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.Abstract
Introduction
In 2019, the prevalence of dialysis in Kuwait were 465 patient/million population, while the annual mortality rate among dialysis patients reached 12%. To improve resource allocation within the health care system, a cost-effectiveness model was conducted from a societal perspective to assess the cost-effectiveness of the use of dapagliflozin as an add-on-therapy against SoC (ramipril) among CKD patients with or without type-2 diabetes over their lifetime.
Methodology
A Markov process model was utilized to assess the cost-effectiveness of dapagliflozin + ramipril versus ramipril alone on a cohort of patients with an eGFR of 25 to 75 mL/min/1.73, with or without type-2 diabetes and a urinary ACR of 200 to 5,000 over their lifetime. The model included nine health states: (i) the six stages of CKD representing stages 1, 2, 3a, 3b, 4 and 5; (ii)ESRD, which represents RRT as dialysis or kidney transplant and (iii) death. Most of the clinical data were captured from the DAPA-CKD study. We assumed that the mortality risk of our study was similar to DAPA-CKD. The utility data were captured from different studies. Direct medical and indirect costs were captured from local data sources. Sensitivity analyses were conducted.
Results
The difference in QALY between dapagliflozin + ramipril versus ramipril was 0.2. The difference in cost between the two arms was KWD −4,120 (−USD25750). Dapagliflozin + ramipril generate better QALYs and lower costs than ramipril in CKD patients. Dapagliflozin improved the outcomes and generated cost savings in CKD patients.
Conclusion
Adoption of dapagliflozin + ramipril is considered to be a cost saving option in addition to the improvement in QALYs in CKD patients with or without type-2 diabetes due to its nephroprotective effect, regardless of the aetiology of CKD, which eventually leads to reduction of dialysis and the transplantation cost burden on the Kuwaiti health care system. This study was focussed only on DAPA-CKD cohort.
1. Introduction
Chronic kidney disease (CKD) has become a major public health issue. It is considered to be a global cause of morbidity and mortalityCitation1. In 2016, CKD was reviewed as the 16th leading cause of death and was forecasted to increase to the 5th top cause by 2040Citation2. The aetiology of CKD can vary from idiopathic to the presence of risk factors such as diabetes, hypertension and obesityCitation3. A systematic review showed that the prevalence of CKD among adults with diabetes, hypertension, and obesity was 31%, 27% and 14%, respectivelyCitation4. Over the past 30 years, CKD has been considered among the top ten contributors to global loss of health and increasing global burden of disease (GBD) among older adultsCitation5. Based on the global health estimated report of the World Health Organization (WHO), CKD was the 10th cause of global death in 2019Citation6. It represents a huge burden, especially in developing countries, due to the high prevalence of uncontrolled chronic risk factors, such as obesity, cardiovascular disease (CVD) and diabetesCitation7.
Based on epidemiological data, the estimated incidence of CKD in Kuwait was 366 patients per million population (PMP), while the incidence of end-stage renal disease (ESRD) was 78 PMPCitation3. The leading cause of ESRD in Kuwait was diabetes followed by glomerulonephritis (GN) Citation3. Kuwait, as a high-income countryCitation8, has a population with multiple risk factors for CKD, such as diabetes, hypertension and obesity, which increases the risk of CKD over timeCitation9. The number of patients receiving dialysis as a treatment for ESRD in 2015 and 2019 was 1720 and 2230, respectivelyCitation9,Citation10. In 2019, the prevalence and incidence of dialysis in Kuwait were 465 and 100 PMP, respectively, while the annual mortality rate among dialysis patients reached 12%Citation10.
CKD is classified into 5 stages based on the estimated glomerular filtration rate (eGFR), and stage 5 CKD represents patients with an eGFR <15 mL/min/1.73 m2Citation11. CKD contributed to approximately 35·8 million disability adjusted life years (DALYs) and to a global mortality rate among all ages of 41.5% in 2017Citation12. The burden of CKD is expressed over different levels, such as morbidity, increases the in health-care system resource utilization, and causes productivity loss and early mortalityCitation13. The significant burden of CKD is represented through complications in patients’ quality of life (QoL), in addition to the burden of dialysis or transplantation due to the development of ESRD as a sequence of improper management of CKDCitation14. The one-year overall survival (OS) rate among patients who are allocated to haemodialysis (HD) was 82.3%Citation15. As time goes on, the OS tends to decrease to reach a five-year survival rate of 35%, while the OS of patients with diabetes reached 25%Citation16. CKD is a major risk factor for the development of CVDCitation12. The greater the reduction in eGFR below 60 mL/min/1.73 m2, the greater the observed morbidity, mortality and CV eventsCitation17. The estimated CV mortality and DALY due to kidney dysfunction were 1.4 million and 25.3 million, respectivelyCitation12.
Dapagliflozin, a sodium–glucose cotransporter 2 (SGLT2) inhibitor, showed a kidney protective effect in patients with or without type 2 diabetes in the DAPA-CKD trialCitation18. DAPA-CKD, an international, phase-3, multicentre, double-blind, randomized controlled trial (RCT), evaluated the safety and efficacy of dapagliflozin as an add-on-therapy to standard of care (SoC) to prevent CKD progression or mortality due to renal or CV causes among patients with CKD, with or without type 2 diabetesCitation18. The available treatment options for CKD are considered to be limited. Experts recommend angiotensin-converting enzyme (ACE) inhibitors and angiotensin-receptor blockers (ARBs) as the gold standard to slow the progression of the diseaseCitation14.
Within DAPA-CKD, the primary composite endpoint was the first manifestation of any of the following: a 50% decline in eGFR, onset of progression into CKD or mortality from a renal or a CV event, while the secondary outcome was the hierarchical order of a composite renal endpoint (either a 50% eGFR decline, ESKD or renal mortality), composite CV event (hospitalization due to HF or CV death) and time to death due to any cause. DAPA-CKD indicated that dapagliflozin, when compared to placebo, showed a significant reduction in the decline in eGFR of at least 50%, decrease in the progression of disease into ESRD and decrease in mortality due to renal or CV causes. Mortality from any cause among the dapagliflozin and placebo groups was 4.7% and 6.8% (hazard ratio (HR)=0.69), respectively. Other than mortality, approximately 12.7% and 14.4% of the dapagliflozin and placebo patients discontinued the administration of medication, respectively. The occurrence of the primary endpoint among the dapagliflozin and placebo groups was 9.2% and 14.5% (HR= 0.61), respectively. The HR for the composite renal end point was 0.56, while the HR for CV events was 0.71. The safety was similar between the 2 groups, considering that dapagliflozin was safe among nondiabetic patients, as no signs of diabetic ketoacidosis (DKA) or severe hypoglycaemia were observedCitation18.
2. Objective
We developed this economic model from the Kuwaiti societal perspective to provide evidence on the cost effectiveness of the use of dapagliflozin as an add-on-therapy to SoC (ramipril) against ramipril alone as a preventative strategy against CKD complications with or without the presence of type-2 diabetes over a life-time horizon to guide decision-makers to the best available therapy for this population. To the best of our knowledge, this is the first economic model built to evaluate the cost-effectiveness of dapagliflozin in Kuwaiti CKD patients.
3. Methodology
3.1. Model design
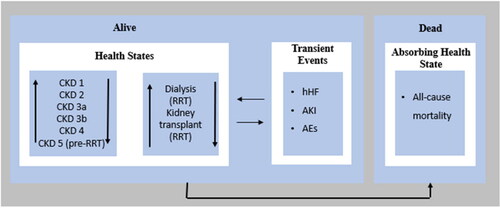
A Markov process model was established using Microsoft Excel® 365 to compare dapagliflozin 10 mg versus ramipril among patients with CKD with or without type 2 diabetes as a protection against both CV and renal outcomes. The model included nine health states (): (i) the six stages of CKD representing stages 1, 2, 3a, 3b, 4 and 5; (ii) ESRD that requires renal replacement therapy (RRT) as dialysis or kidney transplant (two health states) and (iii) death. The stages of CKD were categorized based on eGFR. The eGFRs of stages 1, 2, 3a, 3b, 4 and 5 were >90, 60–89, 45–59, 30–44, 15–29 and < 15 mL/min/1.73, respectively. The probability of transient events such as AEs, hospitalization due to heart failure (hHF) and acute kidney injury (AKI) were calculated at each stage for both treatment arms. This manuscript was reported in accordance with the Consolidated Health Economic Evaluation Reporting Standards (CHEERS) statementCitation19. Economic model validation including both clinical and programming validation was performed by using CHEERS checklistCitation19.
Figure 1. The model structure for CKD patients. Abbreviations. CKD, chronic kidney disease; RRT, renal replacement therapy; hHF, hospitalization due to heart failure; AKI: acute kidney injury; AEs, adverse events.
A comprehensive search of PubMed and MEDLINE was conducted for published English articles to retrieve the available published clinical data regarding the probabilities of the health states, the treatment lines options and doses, and the health states utilities. Randomized controlled trials (RCTs) and systematic reviews of RCTs were chosen because they provide the least biased evidence. Articles that addressed the management of patients with CKD were selected on the basis of terms related to the clinical conditions and the cost-effectiveness of dapagliflozin; these terms included the following: “cost-effectiveness,” “chronic kidney disease,” “dapagliflozin,” “end stage renal disease,” “Angiotensin converting enzyme inhibitor,” “ACEI,” “randomized controlled trial,” “randomized,” “controlled trial,” “meta-analysis,” and “systematic review.” Fifteen relevant articles and reports were identified by this electronic search and were reviewed, and four articles were excluded for irrelevance.
All inputs captured from the above literature review of all clinical trials were validated by an expert panel. This expert panel composed of three nephrologists affiliated to Dar Elshifa hospital and Al Amiri teaching hospital in Kuwait, and one payer from the Kuwaiti Ministry of Defence. We collected insights from experts through a focus group by using the quasi-Delphi panel approach. The experts’ insights included the current local clinical practice and treatment patterns of these patients within the Kuwait Healthcare settings. All model assumptions were validated by the expert panel to decrease the uncertainty within the model. Subsequently, the model simulated CKD patients with or without type 2 diabetes who were being allocated to ramipril.
The target population of our model resembled patients in DAPA-CKD, who were adults with an eGFR of 25 to 75 mL/min/1.73 m2, with or without type 2 diabetes and a urinary albumin-creatinine ratio (ACR) of 200 to 5,000 (albumin is assessed in mg, while creatinine is assessed in gm).
For each treatment arm, costs were assigned to each health state. Utilities were also employed for each health state based on the patients’ wellbeing. As the model progressed for the assigned time horizon, cost and utility data were summed per treatment arm, thus allowing for the calculation of the total costs and effectiveness of each comparator at model completion. This cost-utility analysis output was the incremental cost-effectiveness ratio (ICER) of dapagliflozin as an add-on-therapy versus SoC among CKD patients with or without type 2 diabetes and an eGFR of 25 to 75 mL/min/1.73. ICER was calculated by computing the incremental monetary costs calculated in the Kuwaiti Dinar (KWD) and then dividing it by the incremental health outcomes calculated as quality-adjusted life-years (QALYs) according to the following equation:
In the meantime, there is no identified cost-effectiveness threshold for Kuwait. World health organization reported a cost-effectiveness threshold related to the country income (1–3 × gross domestic product per capita)Citation20. The cycle length was four weeks to ensure that multiple events do not occur within the same cycle, and a half-cycle correction was applied based on the International Society for Health Economics and Outcomes Research (ISPOR) modelling good research recommendationsCitation21 to adjust the distribution of costs and effects that occurred throughout the cycle. The life-time horizon (22 years) for both arms was considered long enough to enable capturing the long-term clinical and economic impact of CKD, which requires management until death. The cost and health-related outcomes were discounted at a rate of 3.5% in the base case (mean value) in accordance with the National Institute for Health and Care Excellence (NICE) appraisal for dapagliflozin for CKD patients as there is no, national HTA guideline for Kuwait and CHEERS statement did not mention the value of the discount rate that should be usedCitation19,Citation22.
3.2. Clinical inputs
Our model represented different health states of CKD categorized based on eGFR from stage 1 to 5 and ESRD. The starting age in our study was 61.8 years (the median age of DAPA CKD cohort). Our cohort, which simulates the patient characteristics of DAPA-CKD, was distributed at baseline as 10.5% in stage 2, 30.9% in stage 3a, 44.1% in stage 3b, and 14.5% in stage 4. Our expert panel confirmed that no patients were in stage 1, 5 or ESRD at the beginning, as CKD patients are rarely diagnosed at late or early stages. During the study, the patients who were laced into different stages of CKD either worsened and progressed to an advanced health state or regressed into a lower health state based on the transition probabilities extracted from the DAPA-CKD and NICE appraisal for dapagliflozin for CKD patientsCitation18,Citation22. Once the patient enters ESRD, there is no option to return to the pre-ESRD stages from 1 to 5. Eventually, all patients entered a death state.
The overall transition probabilities of dapagliflozin and ramipril between CKD stages 1 to 5 were mainly extracted from the DAPA-CKDCitation18. The DAPA-CKD is a phase-3, multicentre, randomized, placebo-controlled international clinical trial that included 4,304 patients with or without type 2 diabetes to compare the safety and nephroprotective efficacy of dapagliflozin as an add-on-therapy to SoC. The transition probabilities were divided into months 0–4 and month 5 onwards to simulate the change in eGFR mentioned in the DAPA-CKD, as dapagliflozin led to an initial decrease in eGFR followed by an increase in eGFR observed in the first four months.
The transition probabilities among ESRD between dialysis and kidney transplantation were mainly extracted from a review assessing the published economic models related to renal disease, as the events observed in the DAPA-CKD regarding this health state were not sufficient to extract those probabilities from themCitation23. The transition probabilities between the alive health state (CKD stages and ESRD) to absorbing health state of dapagliflozin and ramipril were also extracted from the DAPA-CKDCitation18. An extrapolation of the OS Kaplan–Meier (KM) curves was performed to evaluate lifetime extrapolation based on parametric models fitted to patient-level dataCitation18. A plausible fit for the OS curves was provided by the Weibull parametric function, as reported by NICECitation22. includes all transition probabilities of the model.
Table 1. The input transition probabilities of the model.
3.3. Outcomes
The health-related quality of life (HRQoL) for all health states and transient events, such as AEs, hHF and AKI, were included in the outcome measurements. The effect of dapagliflozin and SoC regarding hHF was extracted from a randomized trial assessing the effect of dapagliflozin compared to placebo as an add-on-therapy in 4,304 patients with CKD either having or not having a history of CVD and type 2 diabetes over a median follow-up duration of 2.4 yearsCitation24. The overall hHF for dapagliflozin and SoC were 1.7% and 3.3%, respectivelyCitation24. The effect of dapagliflozin and SoC on AKI was extracted from a prespecified randomized trial to assess the effect of dapagliflozin compared to placebo as an add-on-therapy to prevent an abrupt decline in renal function among 4,304 patients with CKD and albuminuria over a median follow-up of 2.4 yearsCitation25. AKI was defined as doubling of serum creatinine between two subsequent visits with a median of 100 days. The percentage of the patients who developed AKI among the dapagliflozin and placebo groups was 2.9% and 4.2%, respectively.
According to our expert panel, the most common AEs observed with dapagliflozin were genital infection (GI) and urinary tract infection (UTI). The probability of those AEs was mainly extracted from DECLARE–TIMI 58Citation26. DECLARE–TIMI 58 is a phase-3, multinational, randomized trial conducted on 17,160 patients with type 2 diabetes and atherosclerotic CVD over a follow-up median of 4.2 years to compare the safety and CV protective effect of dapagliflozin compared to placeboCitation26. The primary outcome of DECLARE–TIMI 58 was composite of major adverse cardiovascular events (MACEs), while the secondary endpoint was a renal composite (≥40% <60 mL/min/1.73 m2 decrease in eGFR, ESRD, or renal/CV death) and all-cause mortality. The percentages of the patients who developed GI and UTI with dapagliflozin were 0.9% and 1.5% and 0.1% and 1.6% with placebo, respectively.
The utility values applied in our model are health-state dependent, not a treatment-specific utility. The utility of CKD stages 1 to 3b was gathered at predialysis CKD stages through the assessment of HRQoL using the EuroQoL five-dimensional three-level questionnaire (EQ-5D-3 L). The assessment included five domains of health: depression, pain, self-care, usual activities and mobility. The utility score of stages 1 and 2 was 0.85, while the utility score of CKD 3a and 3b was 0.8Citation27. The utility values for CKD4, 5 and dialysis were extracted from a cross-sectional survey conducted in CKD by using EQ-5D-3 LCitation28. The utility for CKD4, CKD5 and dialysis was 0.566, 0.467 and 0.126, respectively. For renal transplant patients, the EQ-5D-5 L reported a utility score of 0.83 as an assessment of transplantation outcomes at six months after transplantationCitation29.
Disutility for unplanned hHF was extracted from a cost-effectiveness model among a hypothetical cohort with heart failure (HF) that assumed a disutility of −0.1Citation30, while disutility measurements of GI and UTI were extracted from EQ-5D-3 L for 20,705 diabetic patients with comorbidities of −0.038 and −0.025, respectivelyCitation31. The disutility of AKI was not considered in our model, as it was not estimated in DECLARE–TIMI 58. includes all the input parameters of the model.
Table 2. The input parameters of the model.
3.4. Treatments
The patients in CKD stages from 1 to 5 received either ramipril as SoC or dapagliflozin as an add-on-therapy to ramipril. During CKD 3a, 3b, 4, 5 and dialysis, the patients were allocated on concomitant medication to manage different complications of disease, and these medications included vitamin D supplements with calcium carbonate, sevelamer and darbepoetin alfa. Our cohort progressed or regressed among different CKD stages, but once patients entered ESRD, they could not regress to predialysis stages.
The required monitoring tests for the patients in different CKD stages were ACR, serum urea, serum creatinine, electrolyte assessment, and complete blood count (CBC) once every two months. For the patients who were allocated to receive dapagliflozin, a haemoglobin A1c (HbA1c) test was performed with the previously mentioned tests every two months.
At the dialysis stage, patients had HD for 4 h three times/week in the outpatient service, as the standardized form of dialysis in Kuwait. Before HD, the patients received conventional heparin as a loading dose of 1,000 iu and then 500 every hour. The patients were allocated to receive the previously mentioned concomitant medications, but neither ramipril nor dapagliflozin was administered at this stage. The standardized monitoring at the dialysis stage included electrolyte assessment, serum creatinine, serum urea, CBC, lipid profile and liver function test (LFT).
The transplantation phase is divided into pre/posttransplantation. In the pretransplantation phase, patients shall undergo some laboratory tests to assure the ability to undergo transplantation, such as echocardiogram (ECHO), myoview stress test, pulmonary function test (PFT), ultrasound (US) for abdomen and pelvis, upper GI endoscopy, micturating cystourethrogram (MCUG), virology scan, human leukocyte antigen (HLA) typing and blood typing test. The patients should also receive a single dose of cyclosporine and tacrolimus before transplantation. After transplantation, the patients should be allocated to receive steroids, tacrolimus with cyclosporine and mycophenolic acid (MPA) as immune suppressants. The patients should undergo serum urea, creatinine, CBC, 24-h urine protein tests and tacrolimus trough levels for monthly monitoring.
During administration of dapagliflozin or ramipril at CKD stages, transient events such as AEs (GI and UTI), hHF and AKI could occur. GI was managed by clotrimazole cream, while UTI was managed through the administration of phenazopyridine and ciprofloxacin. If patients went through an episode of AKI, they were hospitalized in a general ward for a duration of 4 days. The management of HF required hospitalization in the intensive care unit (ICU) for 7 days with the administration of the subsequent medications isosorbide dinitrate, furosemide and carvedilol. In addition, ECHO and X-rays should be obtained. shows the treatment line options and doses used in our model.
Table 3. Treatments lines options and doses used in our model.
3.5. Costs
Both direct medical and indirect costs were considered. The following direct medical costs were included: drug acquisition costs along different cycles, concomitant medications, monitoring tests and A/Es management costs. The resource use during routine follow-up was captured from our expert panel and was based on the Kuwaiti clinical practices within MoH settings. Only the possible treatment-related grade 3 and 4 AEs were considered. The AE costs were calculated based on the average resources used considering the length of hospital stay to treat a single episode and the unit costs. All unit costs of medications were obtained from the approved MoH tender list for the financial year 2022, while the unit costs of monitoring tests and examinations were captured from the MoH hospitals in Kuwait.
Indirect cost represents loss of productivity over each cycle of death estimated from the Kuwaiti patient average wage/hour. The Kuwaiti patient average wage/hour was estimated using the most recently published gross domestic product (GDP) published by the World Bank in 2020Citation32. All unit costs were measured in the Kuwaiti Dinar (KWD) and were converted by the purchasing power parity factor for comparability of the results across countriesCitation33. includes all the costs used in our model.
Table 4. Unit costs used in our model.
3.6. Sensitivity analysis
Deterministic sensitivity analyses (DSA) and probabilistic sensitivity analysis (PSA) were performed to assure the robustness of the results. In DSA, various parameters were varied with 10–20% above or below their base case values, while in PSA, prespecified distributions were chosen (gamma distribution for the cost data and beta distribution for the utility and efficacy data). In DSA, the parameters tested were the utility data, clinical parameters and unit cost data for each treatment arm.
4. Results
4.1. Base-case results
Over a lifetime horizon, the difference in QALY between dapagliflozin versus ramipril was 0.2. The difference in cost between dapagliflozin and ramipril was KWD −4,120 (−USD 25,750). Dapagliflozin as an add-on therapy to ramipril generates better QALYs and lower costs than ramipril alone in CKD patients. Dapagliflozin improved the outcomes and generated cost savings in CKD patients. shows the total results for both treatment arms in our study.
Table 5. The model results of dapagliflozin.
A sub group analyses were conducted by diabetes mellitus status and the same conclusion reached. In both diabetic and non-diabetic patients, the difference in QALY between dapagliflozin as an add-on-therapy to ramipril versus ramipril alone was 0.17, while the difference in cost was KWD −4,123 (−USD 25,768).
Different time horizons (10 years and 25 years) were tested and no change in conclusion was occurred (savings in costs are − KWD 1,648, −KWD 4,434, while gain in QALYs is 0.11, 0.18, respectively).
4.2. Sensitivity analyses
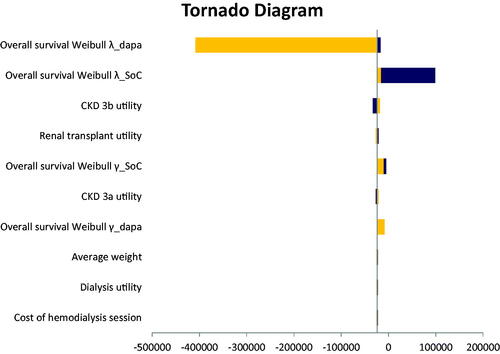
The DSA results are presented in a Tornado diagram (). The results showed that the most sensitive parameter when dapagliflozin was compared to ramipril in CKD patients was the overall survival distribution for dapagliflozin (the most impactful parameter).
Figure 2. Deterministic sensitivity analyses of dapagliflozin + ramipril versus ramipril. Orange colour indicates low value of the base case parameter and the dark blue colour indicates the high value of the base case parameter. Abbreviations. CKD, chronic kidney disease; SoC, standard of care; dapa, dapagliflozin.
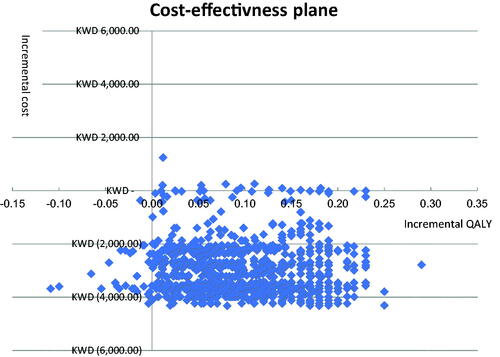
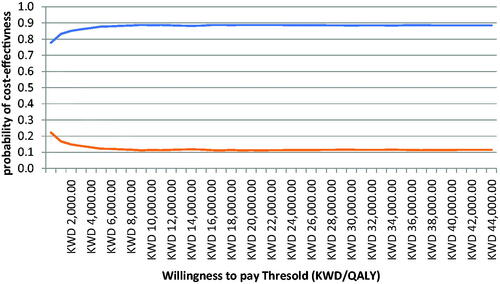
PSA using Monte Carlo simulation with 10,000 iterations explored the effects of joint uncertainty on the model results using prespecified distributions as mentioned above. A cost-effectiveness plane was used to graphically demonstrate the variation in incremental costs and QALYs for dapagliflozin compared to ramipril alone (). As shown, most difference pairs are found in the northeast and southeast quadrants of the cost effectiveness plane, which indicates that dapagliflozin use in CKD patients is more effective (positive incremental QALY scores) and that there is a large proportion in the southeast quadrant, suggesting that dapagliflozin is cost saving. A cost-effectiveness acceptability curve was generated to illustrate the probability that each regimen is cost-effective at varying levels of willingness to pay (per QALY gained; ).
5. Discussion
Implementation of new medication is needed to address the unmet need for CKD, as the current available treatment options, mainly ACE and ARBs, are considered to be of limited benefit in delaying disease progression into ESRD with no evidence of preventing CV-related morbidity and mortalityCitation14. Dapagliflozin is the main treatment strategy used as a preventative measure against renal complications among CKD patients with ACR and eGFR values between 200 and 5,000 mg/g and 25 and 75 mL/min/1.73 m2, respectivelyCitation34. Our study results reported that as an add-on-therapy, dapagliflozin leads to an increase in upfront cost, but eventually, it offsets the costs of delaying progression into dialysis or transplantation, as they represent a higher monthly cost compared to the initial stages of CKD in KuwaitCitation35.
A cost-effectiveness analysis over a life-time horizon of dapagliflozin as an add-on-therapy to SoC compared to placebo among patients with CKD in the UK showed that dapagliflozin was considered to be a cost-effective option in addition to improvement of life expectancy (LE), slowing progression to ESRD and reducing the probability of CKD AEs, such as hHF and AKICitation36. Dapagliflozin generated a QALY of 8.722 compared to 7.88 in the placebo group. It increased the LE by 1.79 years and delayed CKD progression, with patients spending 1.72 additional years without ESRD. Our study results are different because the local clinical practice used in the management of each health state and its transient events (resource utilization), the unit costs and country specific mortality rate were different from the other countries/studies.
The effect of dapagliflozin on reducing CV/overall mortality was not limited to patients with previous CVD but was also seen in patients with no history of CV problemsCitation37, which indirectly led to productivity improvement, as CKD was the 9th mortality-cause in KuwaitCitation38. Furthermore, the protective effect of dapagliflozin against renal and CV endpoints shows consistency throughout the advanced stages of CKD, as it showed a beneficial effect among patients with a baseline characteristic stage 4 similar to those at stage 2/3Citation39.
As previously mentioned, the leading causes of CKD in Kuwait were mainly diabetes and GN. Dapagliflozin would be a beneficial treatment strategy for patients in Kuwait, as it controls the glucose level and consequently reduces the development of diabetic nephropathy (DN), as it is a type of CKDCitation40. A systematic review concluded that dapagliflozin, as an SGLT2 inhibitor, not only reduces the risk of ESRD managed by dialysis or transplantation among diabetic patients but also produces a nephroprotective effect against AKI and renal mortalityCitation41. In addition, dapagliflozin showed a significant reduction in the development of GN-related renal complications, such as a ≥ 50% reduction in eGFR, decreased the risk of developing ESRD and decreased renal/CV mortality. Approximately 8% of patients on dapagliflozin developed those endpoints compared to 11% in the placebo groupCitation42.
Dialysis is considered to be a huge burden on the government in Kuwait due to dialysis mortality (16%) and the cost of continuous RRT sessions (>US$2,000)Citation43. Dapagliflozin can help against this burden, as the overall mortality in the dapagliflozin group was 4.7% compared to 5.7% in the placebo groupCitation43. In addition, the all-cause mortality of dapagliflozin among patients with chronic dialysis compared to placebo was 17.6% vs. 22.2%, respectivelyCitation44.
There is an unmet need in CKD Kuwaiti patients, as those who are diagnosed with ESRD between 6 and 12 months have the worst QoL. Thus, it is very important to implement a preventive strategy such as dapagliflozin, which can slow the progression of CKDCitation18. This will help in the reduction of disease progression in the first place, will help with resource utilization of hHF and will help with the management of other comorbiditiesCitation45. There is additional value for dapagliflozin in nondiabetic CKD patients over a lifetime horizon, and dapagliflozin showed an improvement of QALY and life years (LYs), as it delays disease progression and decreases the number of patients developing ESRD from 17.4% to 11%Citation46.
Our study has several strengths that need to be mentioned. The major strength of our study is that all the cost parameters were obtained from MoH hospitals in Kuwait, representing the societal perspective. Furthermore, our study calculated the cost of transient events of hHF, AKI and AEs for each cycle for both patient cohorts. We also validated all the inputs of our model by surveying clinical experts with diversified profiles, thus reflecting real-life practice in Kuwait.
Nonetheless, some limitations have to be mentioned. Our study simulated the economic benefits of a cohort similar to the one used in DAPA-CKD, where the patients had a range of eGFR and a range of ACR of 25–74 mL/min/1.73 m2 and 200–5,000 mg/g, respectively. Therefore, it will not be possible to determine the economic benefits within a cohort with different baseline characteristics. In addition, the mortality risk of our study was extracted from DAPA-CKD with a median follow-up of 2.4 years with a constant mortality risk.
6. Conclusion
Adoption of dapagliflozin as an add-on-therapy to ramipril is considered to be a cost saving option in addition to the improvement in QALYs in CKD patients with or without type 2 diabetes due to its nephroprotective effect, regardless of the aetiology of CKD. This is because it slows the progression of disease into ESRD, which eventually leads to reduction of the dialysis and transplantation cost burden on the Kuwaiti health care system.
Transparency
Declaration of financial/other relationships
HTA Office was contracted by AstraZeneca for the creation of the cost-effectiveness model to Kuwaiti settings. MA is employee of AstraZeneca who are the manufacturers of dapagliflozin. GHE is employee of HTA Office. The funding body had no role in the design of the study and collection, analysis, and interpretation of data and in writing the manuscript. The rest of authors reported no conflict of interests.
Author contributions
GHE reviewed the literature, analysed and interpreted the data and drafted the manuscript. AS and MAS were major contributors in writing the manuscript. AS, MAS, HTS and MAJ edited the manuscript. All authors read and approved the final manuscript.
Reviewer disclosures
Peer reviewers on this manuscript have no relevant financial or other relationships to disclose.
Acknowledgements
None stated.
Data availability statement
All data generated or analysed during this study are included in this article and available upon request.
References
- Lv JC, Zhang LX. Prevalence and disease burden of chronic kidney disease. Adv Exp Med Biol. 2019;1165:3–15.
- Foreman KJ, Marquez N, Dolgert A, et al. Forecasting life expectancy, years of life lost, and all-cause and cause-specific mortality for 250 causes of death: reference and alternative scenarios for 2016-40 for 195 countries and territories. Lancet. 2018;392(10159):2052–2090.
- Farag YM, Kari JA, Singh AK. Chronic kidney disease in the Arab world: a call for action. Nephron Clin Pract. 2012;121(3-4):c120–c123.
- Shrestha N, Gautam S, Mishra SR, et al. Burden of chronic kidney disease in the general population and high-risk groups in South Asia: a systematic review and meta-analysis. PLoS One. 2021;16(10):e0258494.
- GBD 2019 Diseases and Injuries Collaborators. Global burden of 369 diseases and injuries in 204 countries and territories, 1990-2019: a systematic analysis for the global burden of disease study 2019. Lancet. 2020;396(10258):1204–1222.
- The Global Health Observatory of WHO. Global health estimates: life expectancy and leading causes of death and disability. 2019 [accessed 2022 May]. Available from: https://www.who.int/data/gho/data/themes/mortality-and-global-health-estimates
- Nugent RA, Fathima SF, Feigl AB, et al. The burden of chronic kidney disease on developing nations: a 21st century challenge in global health. Nephron Clin Pract. 2011;118(3):c269–c277.
- The World Bank. Data for Kuwait, high income. 2021 [accessed 2022 May]. Available from: https://data.worldbank.org/?locations=KW-XD
- AlSahow A, AlYousef A, AlHelal B, et al. Basic description of the dialysis population of Kuwait: the 2015 data. Saudi J Kidney Dis Transpl. 2016;27(6):1207–1210.
- AlSahow A, AlHelal B, Alyousef A, et al. Renal data from the Arab world dialysis in Kuwait: 2013-2019. Saudi J Kidney Dis Transpl. 2020;31(4):826–830.
- Levey AS, Eckardt KU, Tsukamoto Y, et al. Definition and classification of chronic kidney disease: a position statement from kidney disease: improving global outcomes (KDIGO). Kidney Int. 2005;67(6):2089–2100.
- GBD Chronic Kidney Disease Collaboration. Global, regional, and national burden of chronic kidney disease, 1990-2017: a systematic analysis for the global burden of disease study 2017. Lancet. 2020;395(10225):709–733.
- Elshahat S, Cockwell P, Maxwell AP, et al. The impact of chronic kidney disease on developed countries from a health economics perspective: a systematic scoping review. PLoS One. 2020;15(3):e0230512.
- National Institute for Health and Care Excellence (NICE). Dapagliflozin for treating chronic kidney disease. 2022 [accessed 2022 May]. Available from: https://www.nice.org.uk/guidance/ta775/resources/dapagliflozin-for-treating-chronic-kidney-disease-pdf-82611498049477
- Ferreira ES, Moreira TR, da Silva RG, et al. Survival and analysis of predictors of mortality in patients undergoing replacement renal therapy: a 20-year cohort. BMC Nephrol. 2020;21(1):502.
- Medscape, what are the mortality rates associated with chronic kidney disease (CKD). 2021 [accessed 2022 May]. Available from: https://www.medscape.com/answers/238798-105284/what-are-the-mortality-rates-associated-with-chronic-kidney-disease-ckd
- Go AS, Chertow GM, Fan D, et al. Chronic kidney disease and the risks of death, cardiovascular events, and hospitalization. N Engl J Med. 2004;351(13):1296–1305.
- Heerspink H, Stefánsson BV, Correa-Rotter R, et al. Dapagliflozin in patients with chronic kidney disease. N Engl J Med. 2020;383(15):1436–1446.
- Husereau D, Drummond M, Petrou S, et al. Consolidated health economic evaluation reporting standards (CHEERS)–explanation and elaboration: a report of the ISPOR health economic evaluation publication guidelines good reporting practices task force. Value Health. 2013;16(2):231–250.
- Marseille E, Larson B, Kazi DS, et al. Thresholds for the cost–effectiveness of interventions: alternative approaches. Bull World Health Org. 2015;93(2):118–124.
- Caro JJ, Briggs AH, Siebert U, et al. Modeling good research practices–overview: a report of the ISPOR-SMDM modeling good research practices task force-1. Med Decis Making. 2012;32(5):667–677.
- NICE, Single technology appraisal, Dapagliflozin for treating chronic kidney disease [ID3866]. 2021 [accessed 2022 May]. Available from: https://www.nice.org.uk/guidance/ta775/documents/committee-papers
- Sugrue DM, Ward T, Rai S, et al. Economic modelling of chronic kidney disease: a systematic literature review to inform conceptual model design. PharmacoEconomics. 2019;37(12):1451–1468.
- McMurray J, Wheeler DC, Stefánsson BV, et al. Effects of dapagliflozin in patients with kidney disease, with and without heart failure. JACC Heart Fail. 2021;9(11):807–820.
- Heerspink H, Cherney D, Postmus D, et al. A pre-specified analysis of the dapagliflozin and prevention of adverse outcomes in chronic kidney disease (DAPA-CKD) randomized controlled trial on the incidence of abrupt declines in kidney function. Kidney Int. 2022;101(1):174–184.
- Wiviott SD, Raz I, Bonaca MP, et al. Dapagliflozin and cardiovascular outcomes in type 2 diabetes. N Engl J Med. 2019;380(4):347–357.
- Jesky MD, Dutton M, Dasgupta I, et al. Health-related quality of life impacts mortality but not progression to end-stage renal disease in pre-dialysis chronic kidney disease: a prospective observational study. PLoS One. 2016;11(11):e0165675.
- Kularatna S, Senanayake S, Gunawardena N, et al. Comparison of the EQ-5D 3L and the SF-6D (SF-36) contemporaneous utility scores in patients with chronic kidney disease in Sri Lanka: a cross-sectional survey. BMJ Open. 2019;9(2):e024854.
- Li B, Cairns JA, Draper H, et al. Estimating health-state utility values in kidney transplant recipients and waiting-list patients using the EQ-5D-5L. Value Health. 2017;20(7):976–984.
- Bertoldi EG, Rohde LE, Zimerman LI, et al. Cost-effectiveness of cardiac resynchronization therapy in patients with heart failure: the perspective of a middle-income country’s public health system. Int J Cardiol. 2013;163(3):309–315.
- Sullivan PW, Ghushchyan VH. EQ-5D scores for diabetes-related comorbidities. Value Health. 2016;19(8):1002–1008.
- World Bank. GDP per capita (current LCU) – Kuwait. 2020 [accessed 2022 Aug]. Available from: https://data.worldbank.org/indicator/NY.GDP.PCAP.CN?locations=KW
- World Bank Organization. PPP conversion factor, GDP (LCU per international $). 2021 [accessed 2022 May] Available from: https://data.worldbank.org/indicator/PA.NUS.PPP
- HAS. Committee meeting of dapagliflozin FORXIGA 10 mg film-coated tablets, Microsoft Word - FORXIGA_271021_SUMMARY_CT19370 (has-sante.fr). 2020 [accessed 2022 August]. Available from: https://www.has-sante.fr/upload/docs/application/pdf/2021-03/forxiga_18112020_summary_ct18815.pdf
- Vareesangthip K, Deerochanawong C, Thongsuk D, et al. Cost-utility analysis of dapagliflozin as an add-on to standard of care for patients with chronic kidney disease in Thailand. Adv Ther. 2022;39(3):1279–1292.
- McEwan P, Darlington O, Wheeler D, et al. POS-335 cost-effectiveness of dapagliflozin as a treatment for chronic kidney disease: a health-economic analysis of DAPA-CKD. Clin J Am Soc Nephrol. 2022;17(12):1730–1741.
- McMurray JJV, Wheeler DC, Stefánsson BV, et al. Effect of dapagliflozin on clinical outcomes in patients with chronic kidney disease, with and without cardiovascular disease. Circulation. 2021;143(5):438–448.
- Kuwait, Life expectancy, Kidney disease in Kuwait. 2020 [accessed 2022 Aug]. Available from: worldlifeexpectancy.com
- Chertow GM, Vart P, Jongs N, et al. Effects of dapagliflozin in stage 4 chronic kidney disease. J Am Soc Nephrol. 2021;32(9):2352–2361.
- Huang Y, Lu W, Lu H. The clinical efficacy and safety of dapagliflozin in patients with diabetic nephropathy. Diabetol Metab Syndr. 2022;14(1):47.
- Neuen BL, Young T, Heerspink HJL, et al. SGLT2 inhibitors for the prevention of kidney failure in patients with type 2 diabetes: a systematic review and meta-analysis [published correction appears in Lancet Diabetes Endocrinol. 2019;7(12):e23]. Lancet Diabetes Endocrinol. 2019;7(11):845–854.
- Wheeler DC, Jongs N, Stefansson BV, et al. Safety and efficacy of dapagliflozin in patients with focal segmental glomerulosclerosis: a prespecified analysis of the dapagliflozin and prevention of adverse outcomes in chronic kidney disease (DAPA-CKD) trial. Nephrol Dial Transplant. 2022;37(9):1647–1656.
- AlSahow A, AlYousef A. Global dialysis perspective: Kuwait. Kidney360. 2021;2(6):1015–1020.
- Heerspink HJL, Sjöström CD, Jongs N, et al. Effects of dapagliflozin on mortality in patients with chronic kidney disease: a pre-specified analysis from the DAPA-CKD randomized controlled trial. Eur Heart J. 2021;42(13):1216–1227.
- Albatineh AN, Ibrahimou B. Factors associated with quality-of-life among Kuwaiti patients on maintenance hemodialysis. Psychol Health Med. 2019;24(8):1005–1014.
- Tisdale RL, Cusick MM, Aluri KZ, et al. Cost-Effectiveness of dapagliflozin for non-diabetic chronic kidney disease. J Gen Intern Med. 2022;37(13):3380–3387.