Abstract
Aim
Evaluate the real-world costs over two years and costs by site of care for ocrelizumab (OCR), natalizumab (NTZ), and alemtuzumab (ATZ) in patients with multiple sclerosis (MS).
Methods
This retrospective study used HealthCore Integrated Research Database and included continuously enrolled adults with MS initiating OCR, NTZ, and ATZ between April 2017 and July 2019 (i.e. patient identification period). Annual total cost of care (pharmacy and medical costs) was evaluated for the first- and second-year of follow-up, further stratified by site of care. Costs were measured using health plan allowed amount and adjusted to 2019 US dollars. Sensitivity analyses were conducted in patients who completed yearly dosing schedule according to Food and Drug Administration approved prescribing information.
Results
Overall, 1,058, 166, and 46 patients were included in OCR, NTZ, and ATZ cohorts, respectively. Mean (standard deviation [SD]) total cost of care during first- and second-year follow-up were $125,597 ($72,274) and $109,618 ($75,085) for OCR, $117,033 ($57,102) and $106,626 ($54,872) for NTZ, and $179,809 ($97,530) and $108,636 ($77,973) for ATZ. Infusible drug cost was the main driver in all three cohorts accounting for >78% of the total costs. Annual total cost of care increased substantially after patients started/switched to infusible DMTs. Across site of care, hospital outpatient infusion was common (OCR 58%, NTZ 37%, ATZ 49%) and expensive followed by physician office infusion (OCR 28%, NTZ 40%, ATZ 16%); home infusion was the least common (<10%) and least expensive.
Limitations
The results were limited to commercially insured patients (specifically those with Anthem-affiliated health plans).
Conclusions
Real-world costs increased after patients started/switched to infusible DMTs. Drug cost is the main driver for the total costs, which varied substantially by site of care. Controlling drug cost markups and using home setting for infusion can reduce costs in the treatment of MS patients.
PLAIN LANGUAGE SUMMARY
Ocrelizumab (OCR), natalizumab (NTZ), and alemtuzumab (ATZ) are infusible drugs to treat patients with multiple sclerosis (MS). We did a study to understand the costs of these infusible MS drugs in real-world settings by analyzing a patients’ pharmacy and medical claims database. A total of 1,058 patients were included. We found that the annual total costs increased substantially after patients started to use these infusible MS drugs. Specifically, the average first- and second-year total costs for patients were $125,597 and $109,618 for OCR, $117,033 and $106,626 for NTZ, and $179,809 and $108,636 for ATZ, respectively. We also found that the cost of the drug itself is the main driver for the overall healthcare spending, accounting for >78% of the total costs. Additionally, we found that the cost varies depending on where patients receive these infusible MS drugs, and generally speaking, infusions received from hospital outpatient settings would be more expensive than received from home settings. In summary, this study showed that the real-world costs of these infusible MS drugs are very high. Shifting patients away from more costly hospital outpatient departments or using MS drugs that do not require infusion resources (e.g. oral/self-injectable) may help reduce the overall healthcare spending on MS.
Introduction
Multiple sclerosis (MS) is an immune-mediated, inflammatory, neurodegenerative disorder of the central nervous system (CNS) and is considered to be the second most expensive chronic disease after heart failureCitation1. The lifetime direct healthcare cost of MS is estimated to be $4.8 million (2020 US dollars)Citation1–3.
The introduction of disease-modifying therapies (DMTs) has revolutionized the prognosis for MS patients by reducing MS relapses, progression, and long-term disability. Currently, there are more than 20 DMTs approved by the Food and Drug Administration (FDA) in the United States (US) for the treatment of relapsing forms of MS with different mechanisms of action and routes of administration (self-injectable, oral, and intravenous infusion [IV])Citation4. The costs of DMTs, however, is a contributing factor for the increasing total healthcare expenditure for MSCitation5.
Unlike self-injectable and oral MS DMTs, IV administered MS DMTs also have costs associated with administration of the drugCitation6. Administration of the drug requires healthcare professionals to prepare and administer the drug and any pre/post-medications and monitor/manage any adverse events. Administration of the IV DMT may occur in the hospital outpatient setting, freestanding infusion center, or home setting. These different sites of administration may also affect the real-world, actual costs of patients treated with IV DMTs.
The treatment costs for IV DMTs is usually calculated based on wholesale acquisition cost (WAC) in the cost-effectiveness analysis modelsCitation7–10, which may underestimate the actual amounts paid by US payers and patients, because this simplified approach neglects markups to the drug price. The price markups during acquisition of the IV DMTs (e.g. “buy and bill” through hospital facility or physician office) could substantially increase the real-world costs of IV DMTs. According to a study published in Milliman White Paper using IBM MarketScanFootnoteiv commercial claims data from 2017 to 2018, the observed average annual treatment costs of ocrelizumab (OCR) reimbursed by payers was $100,500, which is over 50% higher than its WAC price of $65,000Citation8. Additionally, another retrospective cohort study, conducted by Nicholas et al. using Optum Research Database from October 2016 to September 2018, found that the real-world first year cost of IV DMTs for commercially insured patients were much higher than published costs calculated based on WAC price, with mean annual costs estimated to be $87,787 for ocrelizumab (OCR), $143,086 for alemtuzumab (ATZ), and $99,422 for natalizumab (NTZ)Citation9. These previous studies evaluated only infusion costs using relatively older databases (2018 or earlier) with limited sample size and short follow-up (one year or less). Thus, it is important to continue to understand more recent costs including total costs and drug-related costs among patients treated with IV DMTs in different site of care settings, which may help inform the treatment decisions of health plans, healthcare systems, healthcare providers, and patients.
While the efficacy and safety of the IV DMT monoclonal antibodies have been demonstrated in randomized controlled trials, how these agents are administered in routine clinical practice and across settings to treat the known heterogeneous population of MS patients and the associated costs has not been described. The purpose of this study is to describe the real-world total cost of care for MS patients who initiated IV DMTs (OCR, NTZ, and ATZ) in the US. Additionally, the cost by site of care (e.g. hospital outpatient, physician office, and home) was also described to understand whether the cost of IV DMTs is different by site of care.
Methods
Study design and data source
This is a retrospective cohort study using HealthCore Integrated Research Database (HIRD)Footnotev with the study period spanning from 1 April 2016 to 31 July 2020 and the cost evaluation done from 2018 to 2020 (). This administrative claim database is a broad and geographically diverse repository of longitudinal medical and pharmacy claims data from health plan members across the US, representing over 70 million lives of commercially insured membersCitation11. All study data were de-identified and fully complied with US patient confidentiality requirements (HIPAA) of 1996 and therefore were exempted from Institutional Review Board (IRB) approval.
Patient selection criteria
Three study cohorts were studied comprising patients with at least one pharmacy or medical claim for OCR, NTZ, or ATZ. The first observed DMT of interest (OCR, NTZ, ATZ) in the corresponding cohort was regarded as the index drug and that date was regarded as the index date. Patients were required to be 18 years of age by the index date and have ≥1 medical claim with an MS diagnosis (International Classification of Diseases, Tenth Revision, Clinical Modification [ICD-10-CM] diagnosis code: G35) in any diagnosis position during the baseline period or index date. Additionally, patients were required to have continuous medical and pharmacy enrollment for ≥12 months before (baseline period) and ≥12 months (follow-up period) after the index date. Patients with prior use of their index DMT since 1 January 2006 or with multiple DMTs on index date were excluded.
The study period spanned from 1 April 2016 to 31 July 2020, and included a patient identification period (1 April 2017 to 31 July 2019), baseline period (12 months before the index date), and post-index period with ≥12 months but up to 24 months of follow-up ().
Study time periods
Index date: The date of first observed DMT of interest (OCR, NTZ, ATZ) within the patient identification period (i.e. between 1 April 2017 and 31 July 2019).
Baseline period: The 12 months period before the Index date.
Follow-up period: ≥12 months to up to 24 months period after the Index date.
The dosage and formulation of these IV DMTs were based on FDA approved prescribing informationCitation12–14. OCR is administered intravenously twice a year in a hospital or an infusion center under medical supervision. The initial 600 mg (i.e. the loading dose) is administered as two separate infusions (i.e. 300 mg in 250 mL solution) over approximately 2.5 h two weeks apart. Subsequent doses are administered as a single infusion (i.e. 600 mg in 500 mL solution) for over 2 or 3.5 h every six monthsCitation12. NTZ is administered as IV infusion (i.e. 300 mg/15 mL in 100 mL solution) over 1 h every four weeksCitation13. ATZ is administered intravenously (i.e. 12 mg concentrate in 100 mL solution) over approximately 4 h as two annual treatment courses (i.e. the first course is administered once daily for five consecutive days and the second course is administered for three consecutive days 12 months later. Retreatment with up to two additional courses (12 mg/day for three consecutive days ≥12 months after the last course) was permitted in the event of disease relapse. Post-treatment, monthly monitoring for up to 48 months after the last dose is required to track potentially serious adverse eventsCitation14.
Sensitivity analyses were conducted in a subgroup of patients who completed FDA approved dosing schedules for the first and second year of follow-up. Patients on OCR were considered as “completed first year dosing schedule” if they received ≥3 infusions within the first year (includes two loading doses and one maintenance dose) and were considered as “completed first- and second-year dosing schedule” if they received ≥3 infusions within the first year AND ≥2 infusions within the second year. Patients on NTZ were considered as “completed first year dosing schedule” if they received ≥12 doses within the first year and were considered as “completed first- and second-year dosing schedule” if they received ≥12 doses within the first year AND ≥12 doses within the second year. Patients on ATZ were considered as “completed first year dosing schedule” if they received ≥5 doses within the first year and were considered as “completed first- and second year dosing schedule” if they received ≥5 doses within the first year AND ≥3 doses within the second year.
Study variables
Patient demographic and clinical characteristics
Patient demographic and clinical characteristics were captured at baseline, including age (on index date), gender, health plan type, geographic region (Northeast, Midwest, South, West), year of index date, length of follow-up period, proportion of patients with any MS relapse during 12-month pre-index period as defined by a previous published algorithmCitation15, comorbid conditions or symptoms, Quan-Charlson comorbidity index (QCI; an indicator of overall burden of illness)Citation16 during 12-month pre-index period and DMT use during 12-month pre-index period.
Total cost of care and drug cost
Total cost of care was measured at baseline (12 months before index date), and first and second year of follow-up. Total cost of care included all-cause direct pharmacy and medical (inpatient, emergency department, physician office, nursing facility, and other outpatient settings) costs based on the pharmacy and medical claims. Additionally, index drug cost defined as sum of costs for all index drug claims and index drug + infusion cost (i.e. IV DMT treatment cost) were also reported. Costs were measured using allowed amount, which refers to the maximum amount an insurance plan would pay for a covered health care service. Costs were adjusted to 2019 US dollars using the most recent Consumer Price Index (CPI) component (medical care price index) of the Bureau of Labor Statistics at the time of analysisCitation17.
Site of care
Within each of the three IV DMT cohorts, mutually exclusive study sub-cohorts were identified based on the site(s) of care observed on and after the index date, including hospital outpatient only, physician office only, home setting only, and multiple settings or sites (patients who received index treatment in multiple different sites during the follow-up).
Statistical analysis
All study variables were summarized using descriptive statistics. Categorical variables were reported as counts and percentages. Continuous variables were summarized as means and standard deviations (SDs). All statistical analyses were performed using SAS EG 9.4 (SAS Institute, Cary, NC) and/or R version 3.6.1 (R Core Team). All analyses were performed by HealthCore team.
Results
A total of 1,058 patients were included in the OCR cohort, 166 patients in the NTZ cohort, and 46 patients in the ATZ cohort, with at least 12 months of follow-up (). Results for patients who completed yearly dosing schedule are provided in Supplementary Materials as sensitivity analysis.
Table 1. Patient selection.
Baseline patient demographic and clinical characteristics
Baseline patient demographic and clinical characteristics by each treatment cohort are presented in . Most patients across the treatment cohorts were female (63%–74%) and were between 35 and 54 years of age. Most of the patients were covered by a preferred provider organization (PPO) plan in all treatment cohorts with slight differences in the other plan types.
Table 2. Baseline demographic, clinical, and treatment characteristics.
The mean QCI score was highest in the OCR cohort, the five most common comorbidities in each of the cohorts are presented in . Majority of the patients had used at least one DMT during 12-month pre-index period, and the proportion was highest in the ATZ cohort. Mean follow-up time and the proportion of patients with at least two years of follow-up were comparable among the study cohorts.
Overall, among patients with at least one year of follow-up, 77.5% (n = 820) of the patients in OCR cohort, 65.1% (n = 108) of the patients in NTZ cohort, and 78.2% (n = 36) of the patients in ATZ cohort completed their first year FDA recommended dosing schedule. Among patients with at least two years of follow-up, 61.6% (n = 324) of the patients in OCR cohort, 49.4% (n = 38) of the patients in NTZ cohort, and 72.7% (n = 16) of the patients in ATZ cohort completed their first- AND second-year dosing schedule.
Total cost of care and treatment cost
For all three cohorts, the total cost of care (all-cause) for the patients increased substantially from baseline to the follow-up period (). Drug treatments costs, which include drug cost plus the cost of infusion (shown in yellow in ), accounts for most of the costs for all three treatment groups. The ATZ cohort showed the highest jump in treatment from baseline to year 1 with a return to lower costs for year 2, which is likely mainly due to the variation in dosing schedule for the first and second year. The cost of the drug itself what the main driver of total costs, accounting for more than 78% of the total cost of care for all three cohorts (). The infusion costs are a relatively minor component with the exception of NTZ, likely due to its more frequent infusion schedule. The detailed cost components by treatment at baseline, first year, and second year of follow-up are summarized in .
Figure 2. The mean total costs of care at baseline and first and second year of follow-up. The values above the bars are total costs and is inclusive of all-cause inpatient cost, emergency department cost, outpatient cost, skilled nursing facility cost and pharmacy cost. *Index drug refers to corresponding IV DMT drug. Abbreviations. ATZ, alemtuzumab; DMT, disease-modifying therapy; IV, intravenous; NTZ, natalizumab; OCR, ocrelizumab.
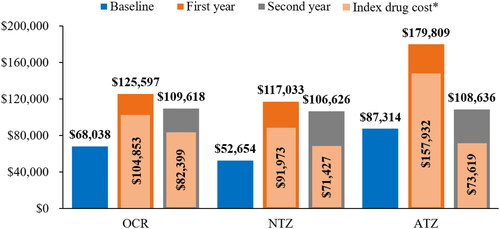
Figure 3. Cost components during the first year of follow-up. Other costs include cost due to inpatient visit, outpatient visit, emergency department visit, physician office visit, laboratory test, skilled nursing facility and pharmacy. *Index drug refers to corresponding IV DMT drug. Abbreviations. ATZ, alemtuzumab; DMT, disease-modifying therapy; IV, intravenous; NTZ, natalizumab; OCR, ocrelizumab.
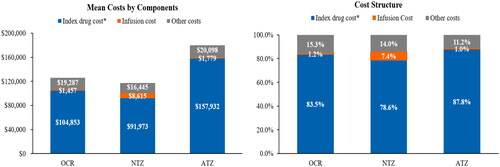
Table 3. Total cost of care and treatment-related costs for OCR, NTZ, and ATZ for all included patients.
To assess if treatment discontinuation introduced bias on the cost estimates, the total cost of care and treatment-related costs were also estimated for those who completed yearly treatment dosing schedule (Supplementary Appendix Figures 1 and 2). Overall, the costs for all patients were slightly less than the costs for those who completed yearly dosing schedule.
Cost by site of care
The hospital outpatient setting was the most common and most expensive site of infusion among patients on OCR and ATZ. For NTZ cohort, physician office was the most common site of infusion, followed by hospital outpatient setting. Home setting was the least common and least expensive site of infusion for all drugs (). The increase in cost by site of care was almost entirely accounted by an increase in index drug cost. As an example, for OCR in the first year, total costs increased $41,347 in going from home to hospital outpatient infusions with mean drug costs accounting for $40,285 of these costs, an increase of 53.7% in the cost of OCR. Similarly, the drug cost of OCR in year 2 between these two sites increased 58.2%. The infusions costs for OCR in year 1 increased from $634 for home infusions to $1,397 for hospital outpatient infusions, which was an increase of $763 although this did represent an increase of 120.3%.
Table 4. Cost by site of care for OCR, NTZ, and ATZ.
Discussion
To our knowledge, this is the first study that evaluated the real-world cost for MS patients with infusible DMTs and cost by site of infusion over two years of follow-up. This study found that the total costs of care for MS patients increased substantially after they initiated infusible DMTs. Drug cost, including price markups during the supply chain to patients, is the main driver for the overall healthcare expenditure for MS patients using IV DMTs. Additionally, this study found that the real-world costs vary by site of care, with hospital outpatient setting being the most common and expensive site of care for patients treated with OCR.
This study shows that the real-world IV DMT drug costs are substantially higher than costs calculated based on WACCitation7,Citation9,Citation10. Specifically, the real-word IV DMT drug costs during first year of follow-up were $104,853 for OCR, $91,973 for NTZ, and $157,932 for ATZ, whereas the annual WAC price in 2019 were $65,000 for OCR, $76,756 for NTZ, and $114,519 for ATZ (for the first year)Citation18. The difference between IV DMT cost in real-world settings and WAC represents the markups during acquisition of the drug, ignoring which may underestimate the actual budget impact to the US payers. The highest price markup is observed in patients treated with OCR, with real-world drug costs over 60% higher than the WAC price during first year of follow-up ($104,853 vs. $65,000). When restricted the analyses to patients who completed FDA approved yearly dosing schedules, the difference between real-world drug costs and WAC price is even higher (Supplementary Appendix 3).
The high costs of infusible DMTs in real-world have been reported in previous studies as well. A real-world study using IBM MarketScan commercial claims data (2017–2018) reported that the annual treatment costs of OCR was $100,500 which was similar to the treatment cost for OCR observed in our studyCitation8. Another retrospective cohort study using Optum Research Database (2016–2018) reported that the annual treatment costs were $80,582, $121,053, and $93,807 for OCR, ATZ, and NTZ, respectively. That study has a smaller sample size (OCR n = 162, NTZ n = 56, ATZ n = 18) including both commercially and Medicare insured patients with only one-year of follow-up, and just reported drug costsCitation9. Compared to the prior Optum study, the current study used a different and more recent claims database, focused on commercially insured patients, had a larger sample size (total patients N = 1,269 vs. N = 236), and reported both treatment costs and total costs of care.
Additionally, the current study assessed costs by site of care including hospital outpatient, physician office, and home setting. Hospital outpatient setting was found to be common and relatively more expensive for patients treated with IV DMTs, whereas home setting is the less common and less expensive. This is not surprising because the costs at hospital settings is usually higher for services that are not commonly seen in home settings such as operation and administration-related services (e.g. handling and transfer, examinations)Citation18. Depending on the manner of acquiring the drug, hospital outpatient facilities may include additional markups to the drug price (e.g. profits, tax) and bill to the patients’ medical insurance, along with administration costs (“buy and bill”)Citation8. Some payers have already started to attempt to shift patients from hospital settings to non-hospital setting such as home infusion, when it’s safeCitation19. However, based on the current study, hospital outpatient is still a common site of care for IV MS DMTs. Shifting patients away from more costly hospital outpatient departments, negotiating site-neutral pricing for specialty medicationsCitation20, or using DMTs that do not requiring infusion-related resources (e.g. oral/self-injectable DMTs) may help reduce the healthcare expenditure. Future studies can also explore the trend in site of care after pandemic and its impact on real-world costs.
The results of this study should be interpreted within the context of its limitations. First, for all the cost analyses, health plan allowed amount was used. While this might not be the actual paid costs, the allowed amount is often equal to the final adjudicated paid amount (inclusive of patient out of pocket costs and coordination of benefit, if any) in most cases in the HIRD as it is the maximum amount a plan would pay for a covered health care service. Second, the study was limited to commercially insured health plan members, and thus, results may not be generalizable to government-sponsored health insurance members or those uninsured or underinsured who may not have access to the healthcare resources of interest. Third, the ICD-10-CM code used for MS does not distinguish between different MS subtypes (i.e. relapsing-remitting MS, secondary progressive MS, or primary-progressive MS) so any differences in the treatment due to the MS subtype could not be evaluated. Fourth, restricting the study follow-up to up to 24 months is a limitation for ATZ which is infused in year 1 and year 2, and can achieves disease control over four years. However, CARE-MS I and II trials showed that through year 6, 63% of ATZ-only patients received neither additional ATZ nor other DMT, and 44% patients across both studies needed retreatment to sustain disease controlCitation21. Fifth, only descriptive statistics were used in this study since the main objective was to understand real-world costs and no formal comparison was intended. It is possible that confounders could have impacted the overall costs. For example, the patients in OCR cohort had older age and more comorbidities, which could inevitably lead to higher overall healthcare costs. However, this study also measured drug cost, which is not directly impacted by these baseline patient characteristics. Sixth, the HIRD data is a repository of claims data from Anthem affiliated health plans. Rebates, pricing, and other formulary considerations that may be caused by health plan policies may make these results less generalizable to other commercially insured patients. Moreover, the proportion of payment made by patients was not available to the study and information on rebate is not available as it was a confidential information. Finally, as with any research using administrative data, medical care coding errors or omission may have occurred.
This study contributed to the understanding of real-world costs of IV DMTs as well as costs by site of care. However, there are a few additional things that are valuable to investigate but were not included in the current study, for example, whether the change in costs of care from baseline to post initiation of IV DMTs differ for DMT naïve vs. DMT experienced patients, whether there is a difference in real-world costs between each DMTs after adjusting for baseline confounding factors, whether there is a change in site of care before and after the pandemic period. Future studies may explore these topics to obtain a more comprehensive understanding on the real-world utilization and costs of DMTs.
In conclusion, the study found that the overall healthcare costs for MS patients increased a lot after they initiated infusible DMTs including OCR, NTZ, and ATZ. The cost of IV DMTs, including price markups, was the main driver of the total healthcare expenditure of patients with MS. Additionally, this study found that total costs of care differ by site of care, with hospital outpatient setting still being common and more expensive as compared to home settings for patients treated with IV DMTs. This study adds to our understanding on the real-world costs of IV DMTs, which can help providers and patients in treatment selection and US payers to guide formulary decisions.
Transparency
Declaration of financial/other relationships
CD, QS, and EMM are employees of Novartis Pharmaceuticals Corporation. HT and NG are employees of HealthCore, which is a consultancy whose activities on research projects are funded by various life sciences companies and health plans. KR was an employee of HealthCore at the time of study conduct. KVN serves as a consultant to Bristol Myers Squibb, Genentech, Novartis, TG Therapeutics, and PhRMA Foundation. She serves on the Speakers Bureau of Sanofi-Genzyme and Alexion, and she has received research grants from Genentech, PhRMA Foundation, Bristol Myers Squibb and Novartis. KVN is also paid by the American Academy of Neurology for her speaking and course director efforts. EA has received compensation for activities such as advisory boards, lectures and consultancy with the following companies and organizations: Actelion/Janssen, Alexion, Bayer, Biogen, Celgene/BMS, EMD Serono/Merck, Genentech/Roche, Genzyme, Novartis, Sanofi, and TG Therapeutics and research support from: Biogen, Genentech/Roche, Novartis, TG Therapeutics, Patient-Centered Outcomes Research Initiative, National Multiple Sclerosis Society, Bristol Meyers Squib, Genentech and Novartis and has received grants from Novartis, Genentech, and Biogen. The reviewers on this manuscript have disclosed the following:
One acts a consultant for multiple MS pharma, including Novartis.
Another has received personal compensation for participation in scientific advisory boards, steering committees, and/or for speaking engagements from Alexion pharmaceuticals, Banner Life Sciences, Biogen, Biologix, Bristol Myers Squibb, Celgene, EMD Serono, Genentech, GW Pharma, Jazz Pharma, Horizon therapeutics, Novartis (local and global), Sandoz Pharmaceuticals, Sanofi/Genzyme, TG therapeutics, and Viela Bio. Consultant fee for serving as a scientific reviewer for Exploration-Hypothesis Development Award (EHDA) peer review panel of the 2020 Multiple Sclerosis Research Program (MSRP) for the Department of Defense Congressionally Directed Medical Research Programs (CDMRP). Honoraria from Medscape, WebMD, and MJH Life Sciences. AZO serves as a site PI for studies funded (directly paid to MCW) by National MS Society/PCORI; Atara biotherapeutics, Biogen, Celgene, CorEvitas, LLC, Bristol Myers Squibb, EMD Serono, Genentech, GW pharma, Immunic, Sanofi/Genzyme. TScan therapeutics, and Novartis. AZO received research funds from Central for immunology, Research Affairs committee, Neuroscience research center, Clinical & Translational Science Institute (CTSI) and the National Institute of Health.
Another reviewer’s team completed research on the impact of MS on Caregivers for EMD Serono in 2021. Some of that research is under review for publications and I have provided EMD Serono with comments about this manuscript. These comments were not compensated.
Within the past three years, my research has used the IQVIA (formerly IMS Health), Mericata (formerly IBM Watson/Marketscan), and Workpartners databases. I have limited experience with the HIRD data.
The other peer reviewers on this manuscript have no other relevant financial relationships or otherwise to disclose.
The Editors in Chief helped with adjudicating the final decision on this paper.
Previous presentations
This data has been presented as poster at 2021 Consortium of Multiple Sclerosis Annual Meeting in Orlando, Florida, USA (October 25–28).
Supplemental Material
Download MS Word (119 KB)Acknowledgements
Vijayalakshmi Vasanthaprasad (Novartis Healthcare Pvt Ltd, Hyderabad, India) provided medical writing support for this manuscript.
Additional information
Funding
Notes
i Lemtrada is a registered trademark of Genzyme Corporation, Cambridge, MA, USA.
ii Ocrevus is a registered trademark of Genentech, Inc, San Francisco, CA, USA.
iii Tysabri is a registered trademark of Biogen Inc., Cambridge, MA, USA.
iv IBM MarketScan is a registered trademark of IBM Corp, New York, NY, USA.
v HIRD is a registered trademark of HealthCore Inc., Wilmington, NC, USA.
References
- Hartung DM. Health economics of disease-modifying therapy for multiple sclerosis in the United States. Ther Adv Neurol Disord. 2021;14:1756286420987031.
- Adelman G, Rane SG, Villa KF. The cost burden of multiple sclerosis in the United States: a systematic review of the literature. J Med Econ. 2013;16(5):639–647.
- Owens GM, Olvey EL, Skrepnek GH, et al. Perspectives for managed care organizations on the burden of multiple sclerosis and the cost-benefits of disease-modifying therapies. J Manag Care Pharm. 2013;19(1 Suppl A):S41–S53.
- National Multiple Sclerosis Society. Medications. [accessed 2022 Nov 8]. Available from: https://www.nationalmssociety.org/Treating-MS/Medications
- Kim Y, Krause TM, Blum P, et al. Disease modifying therapies continue to drive up health care cost among individuals with multiple sclerosis. Mult Scler Relat Disord. 2019;30:69–75.
- Slen BM. Infused therapies: cost savings benefits through home infusion [online]. Pharmacy Times; [accessed 2022 Aug 8]. Available from: https://www.pharmacytimes.com/view/infused-therapies-cost-savings-benefits-through-home-infusion
- Institute for Clinical and Economic Review [Internet]. Disease-modifying therapies for relapsing-remitting and primary progressive multiple sclerosis: effectiveness and value. Final evidence report; 2017 [cited 2022 Apr 14]. Boston (MA): ICER. Available from https://icer.org/wp-content/uploads/2020/10/CTAF_MS_Evidence_Report_012617.pdf
- Dieguez G, Engel T, Jacobson N. Site of service and cost dispersion of infused drugs [online]; [accessed 2022 Jul 1]. Available from: https://www.milliman.com/en/insight/site-of-service-and-cost-dispersion-of-infused-drugs
- Nicholas J, Halpern R, Ziehn M, et al. Real-world cost of treatment for multiple sclerosis patients initiating and receiving infused disease-modifying therapies per recommended label in the United States. J Med Econ. 2020;23(8):885–893.
- Yang H, Duchesneau E, Foster R, et al. Cost-effectiveness analysis of ocrelizumab versus subcutaneous interferon beta-1a for the treatment of relapsing multiple sclerosis. J Med Econ. 2017;20(10):1056–1065.
- Esposito DB, Banerjee G, Yin R, et al. Development and validation of an algorithm to identify endometrial adenocarcinoma in US administrative claims data. J Cancer Epidemiol. 2019;2019:1938952.
- Ocrevus®. (Ocrelizumab) prescribing information; [accessed 2022 Aug 3]. Available from: https://www.gene.com/download/pdf/ocrevus_prescribing.pdf
- Tysabri®. (Natalizumab) prescribing information; [accessed 2022 Aug 3]. Available from: https://www.tysabrihcp.com/content/dam/commercial/tysabri/hcp/en_us/pdf/tysabri_prescribing_information.pdf
- Lemtrada®. (Alemtuzumab) prescribing information; [accessed 2022 Aug 3]. Available from: https://products.sanofi.us/Lemtrada/Lemtrada.html
- Chastek BJ, Oleen-Burkey M, Lopez-Bresnahan MV. Medical chart validation of an algorithm for identifying multiple sclerosis relapse in healthcare claims. J Med Econ. 2010;13(4):618–625.
- Quan H, Li B, Couris CM, et al. Updating and validating the Charlson Comorbidity Index and score for risk adjustment in hospital discharge abstracts using data from 6 countries. Am J Epidemiol. 2011;173(6):676–682.
- US Department of Labor, Bureau of Labor Statistics. Consumer Price Index. [accessed 2022 Aug 8]. Available from: https://www.bls.gov/cpi/tables/supplemental-files/home.htm
- First data bank. Wholesale acquisition cost package pricing history. [accessed 2022 Jul 1]. Available from: https://www.fdbhealth.com/solutions/medknowledge-drug-database/medknowledge-drug-pricing
- Trebes N, Keenan C, Conway L. Payers are shifting where patients receive infusions. Here’s what that means for 4 key industry players [online]; 2020 [accessed 2022 Jul 1]. Available from: https://www.advisory.com/blog/2020/10/infusion-site-of-care
- Fronstin P. Location, location, location: spending differences for physician-administered outpatient medications by site of treatment. EBRI. 2021;536:1–13. [accessed 2022 Aug 3]. Available from: https://www.ebri.org/docs/default-source/ebri-issue-brief/ebri_ib_536_locationx3-19aug21.pdf?sfvrsn=cf6c3b2f_4
- Coles AJ, Arnold DL, Bass AD, et al. Efficacy and safety of alemtuzumab over 6 years: final results of the 4-year CARE-MS extension trial. Ther Adv Neurol Disord. 2021;14:1756286420982134.