1. Introduction
Hidradenitis suppurativa (HS), is a chronic inflammatory skin disease characterized by recurrent nodules, tunnels, and scarring in flexural skin locations that may lead to a severe reduction in quality of lifeCitation1. The prevalence of HS in the US is reported between 0.03–1% with onset at an average age of 22 years and a diagnostic delay between 7 and 10 yearsCitation2. For mild patients with HS, antibacterial treatments are recommended, and anti-inflammatory treatments are frequently used for moderate HS. Surgery is typically used to address recurrent lesions, symptomatic scars, and chronically inflamed tunnelsCitation3. Adalimumab is the only Food and Drug Administration-approved biologic therapy currently available in the US for patients with moderate-to-severe HS, with approximately half of the patients failing to achieve a meaningful clinical responseCitation3–5. With the expected introduction of novel treatment options such as bimekizumab and secukinumab which recently reported positive phase III results to address this heterogeneous disease, the importance of understanding patients’ preferences in treatment decision-making is criticalCitation6–8. Preference research is becoming increasingly important in regulatory- and reimbursement decision-making while accounting for preferences in clinical practice could improve shared-decision making and positively influence treatment outcomes, satisfaction, and adherence which in turn could reduce the high humanistic and socio-economic burden of HSCitation9–11. A discrete-choice experiment (DCE) was recently conducted with HS patients in Europe but the transferability of these preference findings to other geographies is uncertain due to potential differences in care pathwaysCitation12. At the time of this research, no DCE was yet conducted with HS patients in the US. Therefore, the aims of this study were to conduct a DCE with HS patients in the US that was similar to a recent DCE done with European patients to reveal the treatment preferences of US patients and to compare their characteristics and preferences with patients in EuropeCitation12.
2. Materials and methods
In this study, the same DCE questionnaire was used that elicited the treatment preferences of HS patients in EuropeCitation12. In the DCE questionnaire, participants were first asked about their demographics, socioeconomic characteristics and current health status using a pain visual-analogue scale (VAS), the EuroQoL 5-Dimension 5-Level Questionnaire (EQ-5D-5L) and the Hidradenitis Suppurativa Quality of Life (HiSQOL) before being asked to repetitively choose between one of two hypothetical treatmentsCitation13. The two hypothetical treatments differed in terms of [a] effectiveness on reducing the number of painful, inflammatory lesions, [b] reduction of pain, [c] duration of treatment benefit, [d] risk of mild side effects, [e] risk of serious infection and [f] mode of administration. Detailed information on the methodology of attribute and level selection was previously reportedCitation12. In short, a literature review and qualitative interviews with patients and clinicians were conducted to identify the most relevant attributes and levels for the DCECitation12,Citation14. The draft questionnaire was sequentially pilot tested by five preference researchers, three dermatologists, and two patients.
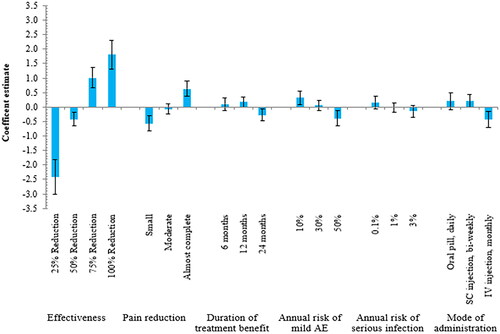
Figure 1. Random-parameters logit model estimates: coefficient estimate (N = 99). The vertical bars around each coefficient estimate (preference weight) represent the 95% confidence interval. Within each attribute, a higher coefficient estimate indicates a level being more preferred, and the sum of the coefficient estimates equals 0. Abbreviations. AE, adverse event; SC, subcutaneous; IV, intravenous
Adult patients with HS in the US were invited through patient advocacy and social media groups between August 2022 and December 2022 to complete the online questionnaire hosted in Qualtrics. Participants were only allowed to proceed in the survey if the location ‘United States’ was selected and if informed consent was provided online. After completing the socio-demographics questions, each participant was randomly assigned to one of three DCE blocks (designed in Ngene using an efficient experimental design to avoid ordering effects), each containing the identical 15 choice sets as previously usedCitation12. One choice set included a dominance test in which one hypothetical treatment had clearly better outcomes than the other, to assess the reliability of patients’ choices. Patients who failed the dominance test were excluded from the analyses. At the end of the questionnaire, participants were asked to rate the difficulty of completion on a 0–10 scale (0 = easy to 10 = difficult). Ethical approval for this study was obtained from the Medical Ethics Committee of the Academic Hospital Maastricht and Maastricht University.
Analyses of the patient preference data were carried out using Nlogit software, version 5.0 and followed a similar approach as previously describedCitation12. Briefly, a random parameter logit (RPL) model was used to derive the mean coefficients and the distribution around them using standard deviations (SD). The conditional relative importance of the attributes was derived from the difference between the attribute level with the highest coefficient estimate and the one with the lowest. The coefficient indicated whether an attribute level led to an increase (positive) or a decrease (negative) of the participants’ utility. p values characterized the statistical difference between the coefficient of the attribute levels and the mean effect of the attribute; if the 95% confidence interval (CI) around the two levels did not overlap, the differences between were considered as statistically different. Non-overlapping SDs with zero indicate significant heterogeneity among patients’ preferences for a given attribute level. Subgroup analyses were not conducted due to sample size constraints, but a statistical comparison of the characteristics of patients with HS in the US and Europe was conducted using t-tests for continuous variables and chi-square tests for categorical variables in IMB SPPS Statistics 21.0. Descriptive statistics were used for the comparisons of conditional relative importance results between patients with HS in the US and Europe.
3. Results
A total of 100 patients with HS in the US completed the questionnaire, of whom 99 were included in the analysis as one patient (1%) did not pass the dominance test and was excluded from analyses as pre-specified. The demographics of patients included in the DCE are reported in . The mean age (SD) of participants was 41.7 (12.0) years and participants were predominantly female (90%) and of white/Caucasian ethnicity (69%). The HiSQOL median score (SD) of 37 (15.7) and pain median score (interquartile range) of 5 (2.5–7.0) indicated HS to have a profound effect on patients’ quality of life at the time of questionnaire completion. The difficulty to complete the questionnaire was stated on a 0–10 scale at 2.4 ± 2.4 (mean ± SD) by participants, which suggested a cognitively intuitive questionnaire.
Table 1. Demographic characteristics of participants.
The most important treatment attribute for patients in the US was effectiveness (conditional relative importance of 56.3%) followed by pain reduction (16.0%), the annual risk of mild AE (9.4%), mode of administration (8.3%), duration of treatment benefit (5.9%), and annual risk of serious infection (4.0%) as presented in and . In all treatment attributes, except the annual risk of serious infection, significant differences were observed between levels (as the 95% CI did not overlap), suggesting that effectiveness, pain reduction, duration of treatment benefit, the annual risk of mild AE, and mode of administration were important to patients. On average, patients in the US preferred treatment options offering higher effectiveness, greater pain reduction, a lower annual risk of mild AEs and serious infection which are either administered as a daily oral pill or bi-weekly subcutaneous injection as shown in the RPL model in . The directions of relationships were observed as expected with improved levels of each attribute resulting in higher coefficient values except for the duration of treatment benefit for which participants least preferred the 24 months duration ().
Figure 2. Comparison of the conditional relative importance of treatment attributes between US and European patients. Abbreviation. AE, adverse event. *Adapted from Willems et al.Citation12.
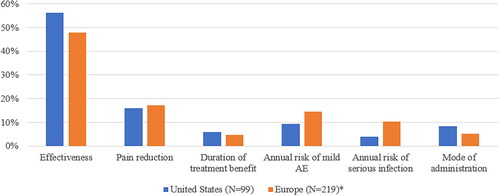
Table 2. Results from the random parameters logit model of the DCE with US patients.
The characteristics of patients with HS in the US were significantly different from patients in Europe with regards to age (41.7 vs. 38.7 years; p = .024), ethnicity (p < .001), previous biologic treatment (47.5% vs. 29.7%; p = .002), previous wide excisional surgery (44.4% vs. 61.2%; p = .005) and HiSQOL (36.9 vs. 32.9 mean total score; p = .04). The observed differences in gender (90.9% vs 90.4% females), time since diagnosis (10.8 vs 10.9 years), disease severity (11.1% vs. 5.9% mild HS; 47.5% vs. 60.3% moderate HS; 41.4% vs. 33.8% severe HS), level of pain (4.92 vs. 4.74 median) and EQ-5D-5L (2.34 vs. 2.27 mean total score) were non-significant (p > .05)Citation12.
Considering the comparison of treatment preferences, patients in the US and Europe both stated effectiveness and pain reduction to be the two most important treatment attributes, with the conditional relative importance of 56.3% and 47.9%, and 16.0% and 17.3%, respectively as shown in . Patients in the US placed greater importance than patients in Europe on the mode of administration (8.3% vs. 5.3%) and less importance on the annual risk of mild AE (9.4% vs 14.4%) and serious infection (4.0% vs 10.3%)Citation12. Monthly IV injection was the least preferred mode of administration for patients in US and EuropeCitation12.
4. Discussion
This study revealed the treatment attributes patients with HS in the US valued most in therapy decision-making. Effectiveness, pain reduction, the annual risk of mild AE and mode of administration were most relevant to patients when deciding between two hypothetical treatment options. Effectiveness was the most important treatment attribute, which could be attributable to the high unmet needs reported by patients due to low treatment success and satisfaction with currently available therapies for HSCitation4,Citation5,Citation14. Higher levels of effectiveness aiming at a 75% and 100% reduction of abscess and nodule count, which represent more stringent effectiveness targets than the primary endpoint of most clinical trials in HS, were more important to patientsCitation15. Pain reduction being the second most important treatment attribute confirmed the findings of previous research that pain management is often not successful or overlooked in the management of HSCitation12,Citation14,Citation16. Patients preferred treatments with a duration of benefit of 6 and 12 months over 24 months, which may seem counter-intuitive but is in line with previous research reporting low willingness by patients to commit to treatment beyond one yearCitation17. Treatments offered as monthly IV injection were least preferred, likely attributable to the associated inconvenience for patients having to attend a clinic for IV injection compared to the comfort of treatment at home as previously concludedCitation18.
The statistical comparison of sample characteristics between patients in the US and Europe revealed the patients to be comparable in terms of gender, time since diagnosis, disease duration, current level pain, and EQ-5D-5L scoresCitation12. The statistically significant differences observed for age, ethnicity, biologic treatment experience, wide excisional surgery experience, and HiSQOL scores did not lead to strong variations in stated preferences between US and European patients as both groups considered effectiveness and pain reduction most important. The only considerable difference observed was US patients placing greater importance on the mode of administration than patients in EuropeCitation12.
These findings are further similar to another recently conducted DCE in Germany which also revealed therapeutic success to be the most important treatment attribute for patients with HS (N = 216), and safety attributes also to be the least important attributes in treatment decision-makingCitation19. The preferred mode of administration was oral tablets followed by subcutaneous injection, which is in line with the results of this studyCitation19.
This research adhered to high preference research standards but nevertheless has some limitations to be considered in the interpretation of the results. While most participants’ demographics were well-balanced and generally similar to recent preference research in other geographies, the ethnic variation of the sample may hinder the generalizability of findingsCitation12,Citation14,Citation19. The sample size further impaired subgroup analyses, but the sample characteristics and preference results were compared in detail with similar research in EuropeCitation12,Citation19. Despite having developed the questionnaire with patients and clinicians (of which 3 were located in the US), and selecting attributes and levels in accordance with best research practices, different attributes or levels could have led to varying preference results as recently revealed by Faverio et al.Citation19,Citation20. Recruitment through social media channels and patients advocacy groups hindered the estimation of participation rates and may have introduced bias as the biologic therapy use in the US is generally lower than the 47% observed with this study, which may indicate that more patients with prior treatment experiences and more severe disease were enrolledCitation2 Lastly, this study relied on patients’ self-diagnosis and self-rating of their disease severity rather than a clinician assessment.
These findings emphasize the importance to understand and account for patients’ preferences in research-, clinical-, regulatory- and reimbursement decisions. Future treatments for HS should allow patients to experience more stringent levels of effectiveness than primarily investigated in clinical trials, lead to greater pain reduction, minimize the risk of adverse events when possible, and preferably be offered as an oral pill or subcutaneous injection. However, given the observed heterogeneity in patients’ preferences, a variety of treatments should become available to allow individualization of HS therapy to patients’ unique preferencesCitation12.
5. Conclusions
This research presented the results of the first patient preference study with HS patients in the US using a DCE. Faced with high unmet needs and low success rates of limited treatment options available, patients considered effectiveness and pain reduction to be the most important when selecting a treatment. The preferences of patients with HS in the US were revealed to be generally similar to those of patients in Europe. Future HS treatments can be better tailored to the individual needs of patients when accounting for the revealed preferences in decision-making.
Transparency
Author contributions
All authors contributed to the study conception and design. Material preparation, data collection, and analysis were performed by DW, ELH, CB and MH. The manuscript was written by DW and all authors provided feedback throughout the development of the manuscript and read and approved the final manuscript. All authors sufficiently contributed to this research according to ICMJE criteria to qualify as listed author.
Reviewer disclosures
Peer reviewers on this manuscript have no relevant financial or other relationships to disclose.
Ethics approval/compliance with Ethical standards
All procedures performed in studies involving human participants were in accordance with the ethical standards of Maastricht University and with the 1964 Helsinki Declaration and its later amendments or comparable ethical standards. Ethics approval was granted by the Faculty of Health, Medicine and Life Sciences of Maastricht University on January 3rd 2022 (ref: ‘FHML-REC/2021/115’)
Consent form
Informed consent was obtained from all individual participants included in the study
Acknowledgements
The authors would like to thank all patients for completing the questionnaire. The authors would also like to thank the patient organizations (HS Connect, HS Warriors, HS Heroes, and Hope for HS) that supported this research with the invitation of participants.
Declaration of funding
No funding was received for the conduct of this study.
Declaration of funding/other relationships
DW is a registered PhD student at Maastricht University and an employee of UCB Pharma, UCB Pharma had no role in the design, conduct, and analysis of the study, or in the writing/reviewing of this manuscript. CS is a speaker for Abbvie and Novartis, a consultant for Abbvie, Novartis, UCB, Incyte, InflaRx, and Alumis, has received education grants from Abbvie, and has been an investigator with fees paid to his institution for Abbvie, Novartis, Incyte, InflaRx, Chemocentryx, and UCB. CS has an unpaid position on the board of the Hidradenitis Suppurativa Foundation and is a member of the European Hidradenitis Suppurativa Foundation. HZ received consultancy fees from Abbvie, InflaRX, Novartis and Insmed. JRI received a stipend as Editor-in-Chief of the British Journal of Dermatology and an authorship honorarium from UpToDate. He is a consultant for Boehringer Ingelheim, ChemoCentryx, Citryll, Novartis and UCB Pharma and has served on advisory boards for Insmed, Kymera Therapeutics and Viela Bio. He is the co-copyright holder of HiSQOL, Investigator Global Assessment and Patient Global Assessment instruments for HS. His department receives income from the copyright of the Dermatology Life Quality Instrument (DLQI) and related instruments. ELH is a registered PhD student at Maastricht University and an employee of EY-Parthenon, EY-Parthenon had no role in the design, conduct, and analysis of the study, or in the writing/reviewing of this manuscript. CB: none. SE: none. MH: none
Data availability statement
Maastricht University is committed to share the data that support the findings of this study with qualified external researchers. The requests are to be made to the corresponding author and will be appraised based on their scientific merit.
References
- Montero-Vilchez T, Diaz-Calvillo P, Rodriguez-Pozo JA, et al. The burden of hidradenitis suppurativa signs and symptoms in quality of life: systematic review and meta-analysis. Int J Environ Res Public Health. 2021;18(13):6709.
- Garg A, Naik HB, Alavi A, et al. Real-World findings on the characteristics and treatment exposures of patients with hidradenitis suppurativa from US claims data. Dermatol Ther. 2022;13(2):581–594.
- Alikhan A, Sayed C, Alavi A, et al. North American clinical management guidelines for hidradenitis suppurativa: a publication from the United States and Canadian hidradenitis suppurativa foundations: part II: topical, intralesional, and systemic medical management. J Am Acad Dermatol. 2019;81:91–101.
- Ingram JR, Bettoli V, Espy JI, et al. Unmet clinical needs and burden of disease in hidradenitis suppurativa: real-world experience from EU5 and US. J Eur Acad Dermatol Venereol. 2022;36(9):1597–1605.
- Kjaersgaard Andersen R, Loft IC, Hansen T, et al. Incidence and remission rates of self-reported hidradenitis suppurativa – A prospective cohort study conducted inandom blood donors. J Eur Acad Dermatol Venereol. 2022;36:717–725.
- Kimball AB, Zouboulis CC, Sayed C, et al. Bimekizumab in patients with moderate-to-severe hidradenitis suppurativa: 48-week efficacy and safety from BE HEARD I & II, two phase 3, randomized, double-blind, placebo controlled, multicenter studies. Presented at Late-breaking Research Session 1 of the Annual Academy of Dermatology Annual Meeting. March 17-21, 2023; New Orleans, LA.
- Kimball AB, Jemec GBE, Alavi A, et al. Secukinumab in moderate-to-severe hidradenitis suppurativa (SUNSHINE and SUNRISE): week 16 and week 52 results of two identical, multicentre, 15 andomized, placebo-controlled, double-blind phase 3 trials. Lancet. 2023;401(10378):747–761.
- Martora F, Megna M, Battista T, et al. Adalimumab, ustekinumab, and secukinumab in the management of hidradenitis suppurativa: a review of the real-life experience. Clin Cosmet Investig Dermatol. 2023;16:135–148.
- Gaspar K, Hunor Gergely L, Jenei B, et al. Resource utilization, work productivity and costs in patients with hidradenitis suppurativa: a cost-of-illness study. Expert Rev Pharmacoecon Outcomes Res. 2021;22:399–408.
- Ho MP, Gonzalez JM, Lerner HP, et al. Incorporating patient-preference evidence into regulatory decision making. Surg Endosc. 2015;29:2984–2993.
- Whichello C, Levitan B, Juhaeri J, et al. Appraising patient preference methods for decision-making in the medical product lifecycle: an empirical comparison. BMC Med Inform Decis Mak. 2020;20:114.
- Willems D, Hinzpeter EL, Van der Zee HH, et al. Patient preferences in the management of hidradenitis suppurativa: results of a multinational discrete choice experiment in Europe. Patient. 2023;16(2):153–164.
- Kirby JS, Thorlacius L, Villumsen B, et al. The hidradenitis suppurativa quality of life (HiSQOL) score: development and validation of a measure for clinical trials. Br J Dermatol. 2020;183:340–348.
- Willems D, Hiligsmann M, van der Zee HH, et al. Identifying unmet care needs and important treatment attributes in the management of hidradenitis suppurativa: a qualitative interview study. Patient. 2022;15:207–218.
- Zouboulis CC, Gulliver W, Ingram J, et al. Endpoints of clinical trials for hidradenitis suppurativa: proceedings of a round-table session. Exp Dermatol. 2020;29(Suppl 1):67–72.
- Thorlacius L, Ingram JR, Villumsen B, et al. A core domain set for hidradenitis suppurativa trial outcomes: an international Delphi process. Br J Dermatol. 2018;179:642–650.
- Ring HC, Maul JT, Yao Y, et al. Drug survival of biologics in patients with hidradenitis suppurativa. JAMA Dermatol. 2022;158(2):184–188.
- Overton PM, Shalet N, Somers F, et al. Patient preferences for subcutaneous versus intravenous administration of treatment for chronic immune system disorders: a systematic review. Patient Prefer Adherence. 2021;15:811–834.
- Faverio K, Peitsch WK, Gorig T, et al. Patient preferences in hidradenitis suppurativa (APProach-HS): a discrete choice experiment. J Dtsch Dermatol Ges. 2022;20:1441–1452.
- Bridges JF, Hauber AB, Marshall D, et al. Conjoint analysis applications in health–a checklist: a report of the ISPOR good research practices for conjoint analysis task force. Value Health. 2011;14:403–413.
