 ?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.Abstract
Background
Factor VIII (FVIII) replacement and emicizumab are effective at preventing bleeds in patients with hemophilia A (HA). Though benefits of emicizumab among inhibitor patients with HA (PwHA) are well established, more real-world evidence among non-inhibitor patients is needed.
Methods
Using a United States healthcare claims database, we compared billed annualized bleed rates (ABRb) and the total cost of care (TCC) before and after switching from FVIII prophylaxis to emicizumab among non-inhibitor male PwHA. Bayesian inferences were used to assess the difference in ABRb and TCC per patient per year (PPPY) pre- versus post-prophylaxis switch.
Results
We included 101 non-inhibitor male PwHA aged between 3 and 63 years old who switched from FVIII prophylaxis to emicizumab prophylaxis in 2018 or 2019. The ABRb increased from 0.52 to 0.62 (p = 0.83) after switch. The posterior probability of the mean ABRb increasing after the switch was 75.54%. The TCC PPPY increased from $517,143 to $627,005 (p < 0.0001) after switch and the posterior probability of mean costs increasing after the switch was 99.80%.
Conclusions
Personalization of care through the identification of the most appropriate therapy for each patient can optimize clinical and economic outcomes. Future real-world evidence research could help establish the value of prophylactic options in targeted populations such as the non-inhibitor male PwHA.
Introduction
Hemophilia A (HA) is a rare recessive X-linked bleeding disorder caused by a deficiency of factor VIII (FVIII)Citation1,Citation2. The United States (US) Centers for Disease Control and Prevention (CDC) estimates that HA affects 1 in 5,000 male births, and approximately 33,000 males in the US are living with this diseaseCitation3.
Prophylactic care with FVIII involves long-term regular administration of FVIII concentrates by intravenous infusion to prevent bleeding and avoid long-term complications (such as arthropathy). Typical prophylaxis regimens for patients with moderate–severe HA involve prophylaxis with ∼2–3 weekly infusions of clotting factor concentrates such as plasma-derived or recombinant FVIII products (standard half-life [SHL] or extended half-life [EHL])Citation4,Citation5 with the goal of increasing FVIII levels to prevent spontaneous bleedingCitation4,Citation6 events and avoid subsequent sequelae such as arthropathyCitation7–9. FVIII products are used for prophylaxis, but also used as on-demand therapy for episodic careCitation3 and for perioperative management of bleeding episodes among patients with HA (PwHA) undergoing surgeriesCitation9.
Another option for severe PwHA who require prophylaxis is emicizumab (Hemlibra). Emicizumab is a recombinant, humanized bispecific monoclonal antibody that mimics FVIII by bringing together factors IXa and X to restore the blood clotting process. It was approved in the US in 2018 and is indicated for routine prophylaxis in PwHA with or without inhibitorsCitation6,Citation7,Citation10–12.
More data from the real-world are needed regarding the clinical and economic outcomes achieved among non-inhibitor PwHA comparing prophylaxis FVIII replacement and non-FVIII therapies. We aimed to estimate, among non-inhibitor PwHA, billed annualized bleed rates (ABRb) and the total cost of care (TCC) before and after switching prophylaxis from FVIII concentrates to emicizumab in a US real-world setting.
Methods
Data source
This retrospective pre–post cohort study queried the IBM MarketScan Research Commercial Claims and Encounters (CCAE) and Multi-State Medicaid databases. These are large US databases of healthcare claims from privately or publicly insured individuals capturing utilization and the associated costs of outpatient and inpatient medical services and pharmacy services, in addition to patient enrollment status and demographicsCitation13. The observation window spanned from January 2015 to September 2020. Since all patient data were de-identified, institutional review board approval was not required. International Classification of Diseases (ICD) codes, National Drug Codes (NDCs), and Healthcare Common Procedure Coding System (HCPCS) codes were used to identify diagnoses, therapies, and procedures.
Design
The patients included in the analysis were non-inhibitor males on FVIII prophylaxis before initiating emicizumab. Patients were required to have at least 6 months of continuous medical and pharmacy insurance coverage (i.e. no lapse in coverage of greater than 45 days) before and after the first outpatient or pharmacy claim for emicizumab (defined as the index-date). Patients with an annualized FVIII utilization of ≥2,340 International Units (IU)/Kilogram (kg) (equivalent to an average of 45 IU/kg/week, regardless of number of infusions)Citation14 before switching to emicizumab met the inclusion criteria for continuous FVIII prophylaxis. As the weight of patients was unknown, FVIII utilization was calculated based on age-specific US national averagesCitation15,Citation16. Also, FVIII claims recorded within 7 days prior to a bleed related claim were excluded from the calculation of the annualized FVIII prophylaxis utilization, as the infusions were deemed likely associated with the bleeding eventCitation17. Patients who met the inclusion criteria were followed from the beginning of the study period or start of continuous enrollment (i.e. complete baseline period) until the end of the study period or end of continuous enrollment (i.e. complete follow-up period).
Patients were excluded if they had at least two diagnoses of another bleeding disorder other than HA (including von Willebrand disease, hemophilia B, acquired HA or acquired coagulation factor deficiency) recorded at least 30 days apart in the entire study period, or if they had a history of FVIII inhibitors in the complete baseline period. A history of FVIII inhibitors was defined as either a claim for a bypassing agent (Feiba-Activated prothrombin complex concentrate or recombinant activated factor VII/VIIa), a claim for rituximab, or immune tolerance induction (ITI) therapy. Patients were considered to have had ITI therapy if a claim for Bethesda/Nijmegen assays anytime in the complete baseline period was combined with high dose of FVIII (at least three times the median IU factors dispensed for patients of similar age for more than 5 consecutive 28-day intervals, allowing one interval gapCitation18) dispensed during the period preceding the switch to emicizumab.
It was hypothesized that the COVID-19 pandemic and associated societal changes have impacted the delivery of healthcare, specifically for PwHA. Therefore, we investigated the impact of the COVID-19 pandemic on the ABRb outcome by controlling for changes in prophylaxis options before and during the pandemic. Patients who were on emicizumab 6 months before and 6 months during the pandemic had noticeable decreases in ABRb (Algorithm A1 and Table A1 in Supplementary Appendix S1) which signaled a confounding impact of the COVID-19 pandemic on our outcome of interest. Therefore, we analyzed the outcomes of our cohort prior to 1 March 2020, when the COVID-19 pandemic policies were widely implemented (i.e. quarantine orders, shifts in healthcare delivery, cancelation of in-person school/sports). The selection criteria were applied in the truncated study period (1 January 2015 to 29 February 2020) to remove the potential impact of the pandemic on the outcomes. To get a similar amount of observation time when assessing the outcomes before and after the initiation of emicizumab, the length of the pre- and post-switch period was set equal to the duration of the shortest of the two periods. The study design schema is depicted in .
Figure 1. Study design schematic. COVID, coronavirus disease; FVIII, factor VIII. *Base case: Limited follow-up time to before 1 March 2020, to avoid COVID-19 confounding effect. **Sensitivity analysis: Included follow-up time during the COVID-19 pandemic.
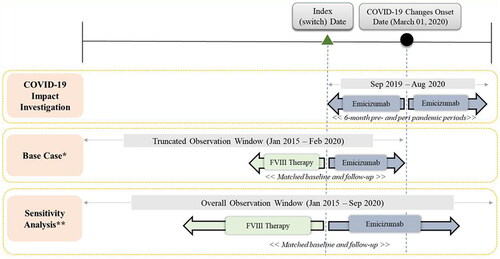
A sensitivity analysis was conducted in which the time period beyond 1 March 2020 was also included. The selection criteria for the sensitivity analysis were applied in the overall study period (1 January 2015 to 30 September 2020), including the time when the COVID-19 pandemic related changes were implemented. The details on the criteria and methodology of the investigation into the COVID-19 impact can be found in Algorithm A1 in Supplementary Appendix S1.
Outcomes
Billed annualized bleed rates
ABRb was calculated based on bleeding episodes identified through claims for evaluation, treatment, or procedure pertaining to bleed treatment. A list of 535 ICD codes used to identify HA-related bleeding episodes in the primary diagnosis position was established by reviewing the diagnosis records found in PwHA and validated based on literature. A bleeding episode, referred to in this study as billed bleed, was defined as a bleeding event that resulted in an encounter with the healthcare system (evaluation, treatment, or procedure) where a medical insurance claim was submitted for services provided. The collection of bleeding-related claims could occur inpatient or outpatient and were grouped by location (body parts). Bleed-related claims at the same location within a 7-day window were grouped as one episode, and those at different locations were considered separate episodesCitation17.
Total cost of care
TCC included all hemophilia- and non-hemophilia-related healthcare costs paid by the payer, expressed as cost per patient per year (PPPY), and adjusted to 2021 US dollars using the medical care component of the Consumer Price Index (CPI). The TCC was categorized by costs on breakthrough bleeds, costs on prophylaxis, FVIII transitioning costs, concomitant FVIII use, and other costs (non-hemophilia related). The FVIII cost for breakthrough bleeds were those accrued during the window of ±7 days of a bleed-related claim. FVIII-related costs during the pre-switch period that were not associated with bleed-related claims were categorized as costs on prophylaxis. In the post-switch period, the costs of prophylaxis were those related to emicizumab. The cost for FVIII during the post-switch period were stratified between “FVIII transitioning”, the FVIII costs accrued during the first 4 weeks following the initiation of emicizumab, and “concomitant FVIII use”, the FVIII costs accrued more than 4 weeks after the initiation of emicizumab.
Statistical methods
Descriptive statistics were used to characterize patients. Gaussian and gamma Bayesian models (with 95% credible intervals [CrI]) were used to compare and generate inferences on the posterior probability of a mean change in ABRb and TCC, respectively, after switching from FVIII prophylaxis to emicizumab. The details on the Bayesian analysis methodologies can be found in Algorithm A2 in Supplementary Appendix S1.
Furthermore, pre- versus post-switch comparisons of ABRb and TCC were performed using Wilcoxon signed rank test as secondary endpoints. Correlation between the pre- and post-switch outcomes were captured using the following equation (Algorithm A3 in Supplementary Appendix S1)Citation19:
where r is the correlation coefficient, SDpre and SDpost are the standard deviations of the outcomes in the pre- and post-switch periods, respectively, and SDD is the standard deviation of the difference in outcomes in the post- and pre-switch periods.
Results
A total of 557 males were identified with a claim for emicizumab between 1 July 2015 and 31 March 2020. Of these, 395 were continuously enrolled for at least 6 months before and after the first claim for emicizumab, among whom 218 met the criteria for prophylactic FVIII therapy before emicizumab was initiated. There were 44 and 21 patients excluded due to evidence suggesting inhibitors and to diagnosis codes for either Hemophilia B, von Willebrand’s disease, acquired coagulation factor deficiency, or acquired Hemophilia prior to initiation of emicizumab, respectively. Another patient was excluded after clinical investigation for not being at least 3 years old.
Using the entire dataset covering the period from 1 January 2015 to 30 September 2020, a total of 152 patients met the criteria for inclusion. After truncating the data to the pre-COVID-19 period (i.e. before 1 March 2020), 101 patients who switched to emicizumab were included in the analysis. The mean (median) age was 18.6 (15) years and ranged from 3 to 63 years. The duration of the matched pre- and post-switch period was on average 0.87 years and ranged between 0.49 and 1.57 years. The sociodemographic and clinical characteristics of these patients are described in .
Table 1. Demographic and clinical characteristics of non-inhibitor male patients with hemophilia A (base case cohort).
The mean (SD) ABRb increased from 0.52 (1.00) in the pre-switch period to 0.62 (1.37) in the post-switch period () (p = 0.83). The correlation between pre-switch and post-switch ABRb was 0.24 (Table A7 in Supplementary Appendix S1). The Bayesian model demonstrated a mean change in ABRb of +0.102 (95% CrI: −0.190 to 0.395) and a 75.54% posterior probability of increase in ABRb after the switch. The mean TCC PPPY increased from $517,143 to $627,005 (p <0.001) after patients switched from FVIII prophylaxis to emicizumab. The correlation between pre-switch and post-switch annualized TCC was 0.56 (Table A7 in Supplementary Appendix S1). The Bayesian model demonstrated a mean change in TCC of +$132,269 (95% CrI: $42,340 to $225,005) and a 99.80% probability of TCC increasing after the switch. The difference was mainly attributable to the increased cost of prophylaxis with emicizumab compared to FVIII replacement. The mean TCC PPPY by cost components, before and after the switch from FVIII prophylaxis to emicizumab, are described in . The estimated Bayesian probabilities distribution of range of difference in ABRb and TCC before and after the switch from FVIII prophylaxis to emicizumab are provided in Figure A1 and Figure A2 in Supplementary Appendix S1.
Figure 2. Total Cost of Care (TCC) Per Patient Per Year (PPPY) before and after switch from FVIII prophylaxis to emicizumab for the base case cohort with N = 101. FVIII, factor VIII; PPPY, per patient per year; TCC, total cost of care; USD, United States Dollars. *The total of all the cost components may not add up exactly to the overall costs as some of the claim lines were captured under both FVIII-specific and emicizumab-specific costs while rolling up costs at visit-level. **All costs are adjusted to 2021 USD. †Non-FVIII and non-emicizumab related costs. ‡Mean cost for first 4 weeks after switching. §PPPY cost accrued after 4 weeks of switching. ¶FVIII-specific cost related to breakthrough or traumatic bleeds. ¤Non-bleed related FVIII costs pre-switch and emicizumab related costs post-switch.
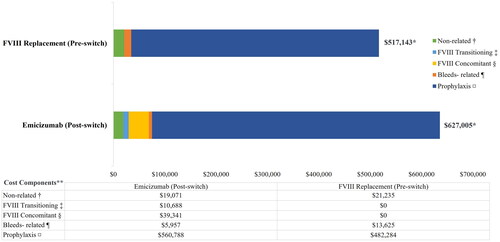
Table 2. Billed annualized bleed rate for the base case analysis.
The sensitivity analyses based on the 152 patients obtained with the post-pandemic period included yielded similar results (Figures A3, A4, and A5, Tables A5, A6, and A8 in Supplementary Appendix S1).
Stratification of outcomes by age-groups and insurance type
Pediatric patients (3–12 years) experienced a reduction in bleed rates, but adolescents (13–18 years) and adults (>18 years) experienced increased bleeds after the switch. The mean TCC PPPY increased for all ages after the switch, with a greater magnitude of increase among older age groups (Table A3 in Supplementary Appendix S1). The mean (SD) IU/kg/week utilizations of FVIII during the pre-switch period were 108.10 (66.85), 107.12 (49.22), 105.85 (50.35), and 88.04 (67.94) for the age-groups 3–6, 7–12, 13–18, and >18 years, respectively.
Of the 101 patients included in the analysis, 44 were commercially insured and 57 were covered by Medicaid. The sociodemographic and clinical characteristics of these patients by insurance type are described in Table A2 in Supplementary Appendix S1. The mean (SD) utilization of FVIII during the pre-switch period was 88.29 (47.07) and 109.24 (63.45) IU/kg/week for commercially insured and Medicaid patients, respectively. Commercially insured patients experienced a 0.28 increase in ABRb, whereas Medicaid patients had a 0.03 reduction in ABRb. TCC increased after the switch for both the groups (Table A4 in Supplementary Appendix S1).
Discussion
We assessed the rates of billed bleeding events and the costs of care to payers in non-inhibitors male PwHA initiating emicizumab after prophylaxis with FVIII. We used a geographically diverse US claims database that covers both pediatric and adult patients and applied operational algorithms and clinical practice definitions to identify prophylactic FVIII use, bleed events, and inhibitor statusCitation6,Citation14,Citation17,Citation18.
In the absence of a head-to-head trial comparing FVIII to emicizumab prophylaxis, this analysis provides important real-world insights about the cost and the effectiveness of FVIII and emicizumab prophylaxis in the non-inhibitors HA patient population. The application of the selection criteria identified 101 patients aged between 3 and 63 years old who initiated emicizumab in 2018 or 2019. Over a median of 11 months, the ABRb in the pre- and the post-switch period were similar (mean ABRb of 0.52 and 0.62, respectively). The posterior probability that the average ABRb was lower before switching to emicizumab than afterward was 75.54%. The TCC and the difference in TCC, were mainly attributable to the pharmacy cost of prophylaxis. During the pre-switch period, the mean FVIII prophylaxis cost was $482,284 as compared to $560,788 for emicizumab in the post-switch period. There were still costs on FVIII after switching to emicizumab for breakthrough bleeds, transitioning period, and concomitant use ($5,957, $10,688, $39,341, respectively). The FVIII concomitant use after the transition period (FVIII required during emicizumab loading dose-1st month) may represent FVIII dispenses for on-hand breakthrough bleeds, boost prophylaxis (physical activity), or maintaining chronic exposure to FVIII to minimize risk of inhibitor development. The 99.80% probability of an increase in TCC and an average increase of $109,862 after switch to emicizumab represents a substantial and certain impact on costs to payers to manage this population. When we stratified patients by insurance type, we found that the costs were lower in Medicaid population compared to the privately insured population. This might be because privately insured patients were older than Medicaid patients (mean age: 24.0 versus 14.5 years old), but also because public programs have more leverage over provident payment rates, which helps them to keep the cost down, such that private payers end up paying higher physician and hospital expensesCitation20. On the other hand, Medicaid had higher pharmacy costs, suggesting that these patients are likely to receive FVIII at pharmacies as opposed to treatment facilities.
Although several studies have examined health outcomes in patients treated with emicizumab in real-world settingsCitation11,Citation21–27, there is limited evidence regarding the role of switching to emicizumab on health outcomes and costs in non-inhibitor PwHA. Batt et al.Citation28 performed an analysis similar to ours using a large US-based commercial claims database and reported consistent findings, a non-significant impact on bleed prevention, and a substantial increase in cost. McCary et al.Citation29 and Samelson-Jones et al.Citation30 examined a cohort of 93 HA patients who initiated emicizumab using data from three hemophilia treatment centers in the US. The patient population differed from ours: 14% of patients were not on FVIII prophylaxis before the switch, the population was mostly pediatric, and both inhibitors and non-inhibitors patients were included. Unlike in our study, McCary et al.Citation29 found, among the non-inhibitor patients, that the annualized rate of treated bleed dropped from 1.6 to 0.4 (p = 0.0025) after switching to emicizumab. Moreover, the difference between the mean cost before and after initiating emicizumab ($204,987 versus $180,571 over 6-months) was not statistically significantCitation30. Differences in data sources and patient populations may explain divergent results. Another studyCitation31 assessing resource utilization and costs associated with emicizumab and FVIII concomitant use among adult male non-inhibitor PwHA reported higher total cost with emicizumab (annualized mean total cost: $814,592) as compared to that in our study ($627,005 PPPY) for the subgroup of patients who switched from FVIII prophylaxis to emicizumab. This difference may be explained by differences in patient selection criteria including age-groups under consideration, study time period, definition of non-inhibitor patients and FVIII prophylaxis, etc. The proportion of FVIII related costs (∼8%) while the patients were on emicizumabCitation31 was comparable to our study findings.
Our study has several limitations, including the fact that administrative claim databases do not contain clinical and laboratory information such as whether factor concentrates are used for prophylaxis or on-demand treatment, nor does it provide the severity of HA, the inhibitor status of patients, or their weights. We used pre-defined algorithms and existing guidelines to identify patients most likely on FVIII prophylaxis and to distinguish between FVIII prophylaxis and on-demand treatment costs. We had to rely on age-specific national averages to estimate weight-based dosing targets, however. To accurately identify bleed events, the administrative billing codes were carefully reviewed by clinical experts and validated based on the literature. Nonetheless, risks of biases inherent to studies conducted through the health administrative claims database remain, as there are variations and inconsistencies in coding and billing as health services are not always claimed. Our study is limited to billed bleed events that are claimed and those not resulting in healthcare resource utilization were not captured. This, along with the lack of clinical details, may have affected the ABRb estimation in our study. Importantly, the definition of billed bleed events was applied to both observation periods (pre-switch and post-switch) and, while it may underestimate “all bleeds”, the risk of differential bias is low. Furthermore, we had no data about medications administered during inpatient admissions. This, however, affects TCC but not the assessment of prophylaxis drug use. As the inhibitor status was unknown, we determined it based on the reported usage of bypassing agents or ITI therapy. However, low-titer inhibitor patients could simply have been treated with higher concentration of FVIII product; because of that, some might have been erroneously identified as non-inhibitor patients. Moreover, the circumstances and indications for FVIII usage after switching to emicizumab were not evaluated due to the lack of clinical details in the data. In addition, differences between the pre- and the post-switch period that affect the comparisons may exist, such as changes in the age or internal states. Also, younger patients may have more bleeds as they are more likely to exercise or get involved in sports but, given the pre–post nature of our analysis (i.e. the selected patients serve as their own control) and relatively shorter study period, the patients will most likely remain in the same age-group in both the time periods, which will help minimize any potential bias arising due to age (or any other patient characteristics). Furthermore, while the IBM MarketScan Research Databases covers diverse regions in the US and a broad range of ages, the results may not be generalizable to the entire US population of non-inhibitor PwHA.
Conclusions
To conclude, we found no evidence of an improvement in ABRb after switching from prophylaxis with FVIII replacement to emicizumab. However, the costs of care were considerably higher after initiating emicizumab.
Personalization of care through the identification of the most appropriate patients for each therapy can optimize clinical and economic outcomes. As more real-world data become available, future research could help establish the value of alternative prophylactic drugs in the targeted population, such as the non-inhibitor male PwHA.
Transparency
Author contributions
ME, BGS, JC, and MB conceptualized and designed the study. NA, SC, and SB contributed to the data analysis and interpretation of results. All authors contributed to revising the paper critically for intellectual content. All authors approved the final version to be published and agree to be accountable for all aspects of the work.
Reviewer disclosures
Peer reviewers on this manuscript have no relevant financial or other relationships to disclose.
Previous presentations
Poster presented at Thrombosis & Hemostasis Summit of North America (THSNA). 25–27 August 2022, Chicago, IL – Dr Miguel Escobar, Neha Agrawal, Sagnik Chatterjee, Swastik Bhattacharya, Jorge Caicedo, Michael Bullano, and Bob G. Schultz. Impact of Switching Prophylaxis Treatment from Factor VIII to Emicizumab in Hemophilia A Patients without Inhibitors.
Supplemental Material
Download MS Word (543.2 KB)Acknowledgements
The authors thank Qi Fan for reviewing and revising the manuscript. QF is an employee and stockholder of Takeda Pharmaceuticals U.S.A., Inc., Lexington, MA, USA. The authors thank Martin Senecal for medical writing and editorial assistance. MS is an employee of Complete HEOR Solutions, North Wales, PA and has received financial compensation for writing the manuscript. Funding for medical writing was provided by Takeda Pharmaceuticals U.S.A., Inc., Lexington, MA.
Declaration of funding
Funding was provided by Takeda Pharmaceuticals U.S.A., Inc., Lexington, MA
Declaration of financial/other interests
ME is a consultant for Takeda, Bayer, NovoNordisk, Genentech, Biomarin, CSL Behring, Sanofi, Pfizer, Kedrion, Hemobiologics/LFB, UniQure, and National Hemophilia Foundation. BGS, JC, and MB are employees of Takeda Pharmaceuticals U.S.A., Inc., Lexington, MA, USA, and current holders of individual stock/stock options. NA, SC, and SB are employees of Complete HEOR Solutions, which has received financial compensation for conducting the study analysis. The authors have no other relevant financial relationships or otherwise to disclose.
Data availability statement
Data to support the findings of this study was derived from IBM MarketScan research databases, https://www.ibm.com/in-en/products/marketscan-research-databases/databases, but restrictions apply to the availability of these data, which were used under license for the current study and so are not publicly available.
References
- Rind D, Walton S, Agboola F, et al. Valoctocogene roxaparvovec and emicizumab for hemophilia A: effectiveness and value; evidence report. Inst Clin Econ Rev. 2020.
- Lyons J, Desai V, Xu Y, et al. Development and validation of an algorithm for identifying patients with hemophilia a in an administrative claims database. Value Health. 2018;21(9):1098–1103.
- Data & Statistics | Hemophilia | NCBDDD |. Centers for Disease Control and Prevention, September 2020. [cited March 2021]. Available from: https://www.cdc.gov/ncbddd/hemophilia/data.html
- Simpson ML, Valentino LA. Management of joint bleeding in hemophilia. Expert Rev Hematol. 2012;5(4):459–468.
- Chhabra A, Spurden D, Fogarty PF, et al. Real-world outcomes associated with standard half-life and extended half-life factor replacement products for treatment of haemophilia a and B. Blood Coagul Fibrinolysis. 2020;31(3):186–192.
- Srivastava A, Santagostino E, Dougall A, et al. WFH guidelines for the management of hemophilia, 3rd edition. Haemophilia. 2020;26(S6):1–158.
- Hemophilia A. Genetic and Rare Diseases Information Center (GARD) – an NCATS Program. [cited 2021 March]. Available from: https://rarediseases.info.nih.gov/diseases/6591/hemophilia-a#ref_5891
- Treatment of Hemophilia. Centers for Disease control and Prevention. [cited 2021 March]. Available from: https://www.cdc.gov/ncbddd/hemophilia/treatment.html
- Aledort L, Mannucci PM, Schramm W, et al. Factor VIII replacement is still the standard of care in haemophilia A. Blood Transfusion. 2019;17(6):479.
- Powell K, Williams A, Sheen J, et al. Uptake of Emicizumab-kxwh (Hemlibra) in the MO HealthNet population and impact on medical and pharmacy cost and utilization. Poster presented at: the AMCP eLearning Days, 2020 April 20–24.
- Ebbert PT, Xavier F, Seaman CD, et al. Emicizumab prophylaxis in patients with haemophilia a with and without inhibitors. Haemophilia. 2020;26(1):41–46.
- Hemlibra (emicizumab). Roche. 2022. Available from: https://www.roche.com/media/releases/med-cor-2017-11-16
- IBM. IBM MarketScan research databases. [cited 2022 June 6]. Available from: https://www.ibm.com/in-en/products/marketscan-research-databases/databases
- Srivastava A, Brewer AK, Mauser‐Bunschoten EP, et al. Guidelines for the management of hemophilia. Haemophilia. 2013;19(1):e1–e47.
- Fryar CD, Kruszon-Moran D, Gu Q, et al. Mean body weight, height, waist circumference, and body mass index among adults: United States, 1999–2000 through 2015–2016. Natl Health Stat Report. 2018;122:1–16.
- Ogden CL, Kuczmarski RJ, Flegal KM, et al. Centers for disease control and prevention 2000 growth charts for the United States: improvements to the 1977 national center for health statistics version. Pediatrics. 2002;109(1):45–60.
- Shrestha A, Eldar‐Lissai A, Hou N, et al. Real-world resource use and costs of haemophilia A-related bleeding. Haemophilia. 2017;23(4):e267–e275.
- Su J, Zhou J, Buckley B, et al. The immune tolerance induction factor utilizations and costs for the management of male hemophilia-A patients who developed inhibitors. Blood. 2016;128(22):4758–4758.
- Morris SB, DeShon RP. Combining effect size estimates in meta-analysis with repeated measures and independent-groups designs. Psychol Methods. 2002;7(1):105–125.
- Shelby Livingston. Medicare, Medicaid contain costs better than private insurers, study says. 11 February 2019. [cited 2019 May 1]. Available from: https://www.modernhealthcare.com/article/20190211/NEWS/190219996/medicare-medicaid-contain-costs-better-than-private-insurers-study-says.
- Levy-Mendelovich S, Brutman-Barazani T, Budnik I, et al. Real-world data on bleeding patterns of hemophilia a patients treated with emicizumab. J Clin Med. 2021;10(19):4303.
- Barg AA, Avishai E, Budnik I, et al. Emicizumab prophylaxis among infants and toddlers with severe hemophilia a and inhibitors—a single‐center cohort. Pediatr Blood Cancer. 2019;66(11):e27886.
- Barg AA, Livnat T, Budnik I, et al. Emicizumab treatment and monitoring in a paediatric cohort: real‐world data. Br J Haematol. 2020;191(2):282–290.
- Barg AA, Budnik I, Avishai E, et al. Emicizumab prophylaxis: prospective longitudinal real‐world follow‐up and monitoring. Haemophilia. 2021;27(3):383–391.
- Misgav M, Brutman‐Barazani T, Budnik I, et al. Emicizumab prophylaxis in haemophilia patients older than 50 years with cardiovascular risk factors: real‐world data. Haemophilia. 2021;27(2):253–260.
- Lewandowska M, Randall N, Bakeer N, et al. Management of people with haemophilia a undergoing surgery while receiving emicizumab prophylaxis: real‐world experience from a large comprehensive treatment Centre in the US. Haemophilia. 2021;27(1):90–99.
- Caplan EO, Patel AM, DeClue RW, et al. Real-world treatment, clinical outcomes and healthcare resource utilization among persons with hemophilia a by age. J Comp Eff Res. 2021;10(15):1121–1131.
- Batt K, Schultz BG, Caicedo J, et al. A real-world study of pre-post annualized bleed rates and all cause costs among non-inhibitor patients with hemophilia a switching from FVIII prophylaxis to emicizumab. Blood. 2021;138(Supplement 1):3028–3028.
- McCary I, Guelcher C, Kuhn J, et al. Real-world use of emicizumab in patients with hemophilia with and without inhibitors. Blood. 2019;134(Supplement_1):1137–1137.
- Samelson‐Jones BJ, Guelcher C, Kuhn J, et al. Real‐world cost estimates of initiating emicizumab in US patients with haemophilia A. Haemophilia. 2021;27(4):591–598.
- Cafuir L, Estrin A, Chen E, et al. Early real-world experience with emicizumab and concomitant factor VIII replacement products in adult males with hemophilia a without inhibitors. J Med Econ. 2022;25(1):984–992.
