Abstract
Background
The landscape of treatment strategies for relapsed/refractory multiple myeloma (RRMM) has dramatically changed due to the emergence of chimeric antigen receptor T (CAR-T) cell therapy. The aim of this study was to evaluate the cost-effectiveness of two CAR-T cell treatments for RRMM patients from the perspective of the Chinese healthcare system.
Methods
A Markov model was used to compare currently available salvage chemotherapy with Idecabtagene vicleucel (Ide-cel) and Ciltacabtagene autoleucel (Cilta-cel) for treatment of patients with RRMM. The model was developed based on data from three studies: CARTITUDE-1, KarMMa, and MAMMOTH. The healthcare cost and utility of RRMM patients were collected from a provincial clinical center in China.
Results
In the base case analysis, 3.4% and 36.6% of RRMM patients were expected to be long-term survivors after 5 years of Ide-cel and Cilta-cel treatment, respectively. Compared to salvage chemotherapy, Ide-cel and Cilta-cel were associated with incremental QALYs of 1.19 and 3.31, and incremental costs of US$140,693 and $119,806, leading to ICERs of $118,229 and $36,195 per QALY, respectively. At an ICER threshold of $37,653/QALY gained, the probability that Ide-cel and Cilta-cel are cost-effective were estimated to be 0% and 72%, respectively. With younger target people entering the model, and a partitioned survival model in scenario analysis, the ICERs of Cilta-cel and Ide-cel changed rather mildly and their cost-effectiveness results were the same as base analysis.
Conclusions
Based on the willingness-to-pay of 3-times China’s per capita GDP in 2021, Cilta-cel was considered to be a more cost-effective option compared to salvage chemotherapy for RRMM in China, while Ide-cel was not.
Introduction
The age-standardized incidence rate of multiple myeloma (MM) was about 2.1 per 100,000 persons worldwide in 2016Citation1. The advancement of new therapeutics for MM has resulted in improved survival rates and quality of life for patientsCitation2,Citation3. However, more than half of MM patients progress to relapse and/or refractory (RR) diseaseCitation4. Each successive salvage regimen is characterized by a decrease in response duration and a reduction in overall survival, which makes treating RRMM patients a challengeCitation5,Citation6.
Chimeric antigen receptor T-cell (CAR-T) therapies have achieved the most prominent responses in RRMM, with a high objective response rate (ORR)Citation7–9. Two anti-BCMA CAR-T therapies, Idecabtagene vicleucel (Ide-cel) and Ciltacabtagene autoleucel (Cilta-cel), were approved by the US Food and Drug Administration for patients with RRMM after four or more prior lines of therapy including an immunomodulatory agent, a proteasome inhibitor, and an anti-CD38 monoclonal antibodyCitation10,Citation11. In single-arm studies, the initial complete response (CR) rates for the two CAR-T therapies seem to be higher than those for salvage chemotherapy (33% and 82%, respectively, vs 8%)Citation12–14. Although CAR-T products have a good therapeutic effect on RRMM, their prices are also relatively high. In America, each wholesale acquisition of CAR-T therapy is generally approximately US$400,000; furthermore, when considering the related diagnosis and treatment costs (such as treatment costs, doctor remuneration, adverse reaction treatment costs, etc.), the overall health care cost of CAR-T treatment can reach $600,000–700,000Citation15,Citation16.
Therefore, it is increasingly important to determine whether the outcomes of CAR-T cell therapies justify the health care cost. Previous studies analyzed the cost-effective profile of CAR-T cell therapeutic products in different jurisdictions (e.g. USA, Canada, Switzerland, Singapore, Spain, Japan)Citation15–21. In China, two CAR-T therapeutic products for relapsed/refractory DLBCL were approved for commercial use, with prices of $187,597 and $200,000 respectively. Though no CAR-T products for RRMM are currently approved in China, the new CAR-T therapies for RRMM are in the process of applying for marketing and Ide-cel and Cilta-cel may also be approved for clinical use in the near future. This study aimed to explore the cost-effectiveness profile of Ide-cel and Cilta-cel for the treatment of RRMM compared to the salvage chemotherapy from the perspective of the Chinese health care system.
Methods
Model structure
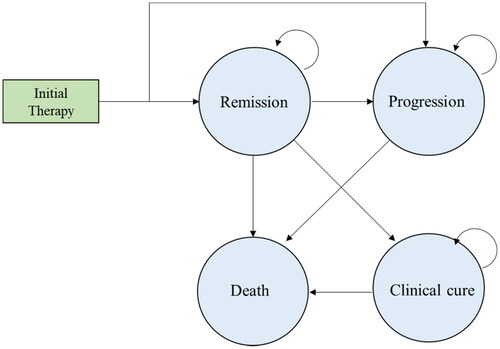
A Markov model was developed to assess the cost-effectiveness of Ide-cel and Cilta-cel for RRMM in the context of the Chinese healthcare systemCitation22 (). This study modeled a hypothetical cohort of Chinese adults (mean age, 60 years) with RRMM after four or more prior lines of therapy until patients died or were at age 79 years (mean life expectancy in China).
The Markov model included four health states: remission, progression, clinical cure, and death. After receiving CAR-T cell therapy or salvage chemotherapy, patients could transit to remission or progression. Patients in remission could stay in remission or transfer to progression or death. Patients in progression could stay in this state or transfer to death. More and more clinical evidences confirm that CAR-T therapies are a class of novel and potential cure therapies for patients with B-cell malignancesCitation15–21. Indeed, 52% of the RRMM patients in the CARTITUDE-1 have remained progress-free from the 30th month to follow-up cutoff (42nd month). Therefore, our model assumed that patients who stayed continuously in remission for more than 5 years were considered effectively cured and moved to clinical cure (long-term survival), and faced a very low probability of relapse and decreased lifespan compared with the general population. Patients who progressed after treatment may receive next line salvage chemotherapy but have particularly poor outcomes, which we model as poor survival, low quality-of-life, and high monthly costs. Additionally, we assumed that patients who were still in progression at the end of the fifth year had died by this time.
We used TreeAge Pro Healthcare (TreeAge Pro version 2021, Williamstown, MA) to create a Markov model and R (version 4.2.2) software to perform additional statistical analyses.
Comparator
Since CARTITUDE-1 and KarMMa are both single group trials and no patient-level data from these studies were published, it is difficult to conduct a matching-adjusted indirect comparison. Consistent with previous studies, we used patients from the MAMMOTH study as the reference for the standard care groupCitation14. The MAMMOTH study was a multicenter US-based retrospective analysis of 275 MM patients who were refractory to at least three lines of therapy (PI, IMiD, and anti-CD38 monoclonal antibody). Given that numerous salvage chemotherapies are used by clinicians at various lines of therapy, a therapeutic basket consisted of several salvage chemotherapies were used for comparison with each CAR-T therapy (Supplementary Table S5). The composition of the therapeutic basket was informed by real-world data from the Bone Marrow Transplantation Center, the First Affiliated Hospital of Zhejiang University School of Medicine, which provided inpatient personal information, diagnosis, treatment process, nursing records, and hospital billsCitation23.
Clinical data inputs
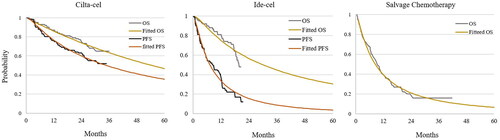
The Kaplan–Meier survival curves used to model overall survival (OS) and PFS were obtained from the CARTITUDE-1Citation12, KarMMaCitation13, and MAMMOTH studies using a method proposed by Na et al. to reconstruct individual patient dataCitation24. Then, parametric survival models under Weibull, log-logistic, log-normal, Gompertz, exponential, gamma, and generalized Gamma distributions were adopted to reconstruct the individual patient data. The best fitting distribution was chosen based on statistical information criteria, visual inspection of the curve and clinical plausibility (Supplementary Tables S1 and S2). For salvage chemotherapy, there were no published PFS curves in the MAMMOTH studies. Therefore, the PFS curve was derived from available OS data by a proportional relationship between PFS and OS reported in previous meta-analysesCitation25,Citation26. This best fitting parametric approach extrapolated the published survival curves to 5 years, after which patients who were alive and responding to treatment were considered to be clinically cured. In our study, patients in a clinical cure state were given a long-term probability of death derived from 2020 Chinese life tables adjusted with a standardized mortality ratio of 2.2Citation27. The final adjusted PFS and OS curves are presented in .
Costs
This study considered the following direct medical costs: drug acquisition, routine follow-up costs, supporting care expenses, salvage chemotherapy costs, hospice costs, and treatment-related adverse event costs (). The cost of CAR T-cell treatment for RRMM was derived from a phase 1 open label single-institution clinical trial (ChiCTR1800017404)Citation28, which was initiated by the First Affiliated Hospital of Zhejiang University School of Medicine. The study was approved by the Institutional Review Board of the First Affiliated Hospital, School of Medicine, Zhejiang University, and was registered in the Chinese Clinical Trial Registry. A single-center retrospective observational study was conducted in the First Affiliated Hospital of Zhejiang University School of Medicine from September 2020 to May 2022 to identify the cost of salvage chemotherapy for RRMM. In our study, a proportion of patients mirrored the receipt rates of second treatment of CAR-T in the CARTITUDE-1 and KarMMa studies and only the base treatment cost associated with second CAR-T treatment was considered. This retrospective observational study was approved by the Institutional Review Board of the First Affiliated Hospital, School of Medicine, Zhejiang University.
Table 1. Key input parameters.
The cost of each inpatient and outpatient visit for CAR-T cell treatment and salvage chemotherapy can be divided into 10 component costs: drug acquisition, room charges, physicians, radiology examinations, oxygen supply, laboratory test, treatment, transfusion, nursing, and medical consumables. Considering that the national drug negotiation had a substantial impact on the price discount of chemotherapy drugs (e.g. daratumumab and bortezomib), the prices of all drugs in the study were adjusted by the latest negotiated prices derived from the Zhejiang Provincial Center for Drug & Medical Device ProcurementCitation29. Palliative therapy costs were estimated based on the data observed in a previous studyCitation30. Bootstrap with 1,000 resamples was used to generate 95% confidence intervals (CI) of cost collected.
Because Ide-cel and Cilta-cel have not yet been approved in China, this study assumed that the price of these two treatments was the average price of approved CAR-T products for relapsed/refractory DLBCL in China. Indeed, the prices of CAR-T products marketed in the United States are around $400,000, no matter what indications they are for. At present, the prices of two approved CAR-T products for relapsed or refractory DLBCL in China are $186,047 and $200,000, respectively. Therefore, we assumed that Cilta-cel and Ide-cel were the average of the prices of these two products, which is $193,023. Grade 3/4 AEs associated with Ide-cel and Cilta-cel, including cytokine release syndrome and neurotoxicity, were assumed to occur during the initial treatment and hospitalization period. All costs were presented in 2021 US dollars using an exchange rate of 6.45.
Utility
Although the KarMMa study determined health-related quality-of-life for patients treated according to their progression statusCitation31, health state utility values for PFS and PD in this published study were not reported. Therefore, we collected utility values for RRMM patients treated with CAR-T treatment according to their progression status alongside the clinical trial mentioned above (ChiCTR1800017404) using the general health EuroQol 5-dimension 5-level (EQ-5D-5L) instrumentCitation32. Written informed consent was obtained from patients for the data collecting who agreed to participate in the survey.
We assumed that the utility value of patients who received salvage chemotherapy in PFS and PD was the same as that of patients who received CAR-T therapy. Disutility for salvage chemotherapy was informed by the literature and experts’ opinionsCitation33. Because patients achieving long-term survival have the potential to be cured, it would be reasonable to use general population norm utility values rather than disease-specific estimates. Therefore, the utility of long-term survivors (clinical cure) was derived from a previous study that reported the Chinese urban population norm scores for the EQ-5DCitation34.
Main outcome
Outcomes of this study were life years, lifetime costs, quality adjusted life years (QALYs), and the incremental cost effectiveness ratio (incremental cost per QALY gained). According to the China Guidelines for Pharmacoeconomic Evaluations 2020, all costs and QALYs were discounted at 5% annually. The willingness-to-pay (WTP) threshold per QALY was set as three times the national per capita GDP in the incremental analysis based on the rule of thumb previously recommended by the World Health Organization. China’s per capita GDP in 2021 was $12,551 (from the website of the National Bureau of Statistics), so the WTP threshold of long-term cost-effectiveness analysis was set as $37,653.
Sensitivity analyses
We carried out both one-way sensitivity analysis and probabilistic sensitivity analysis to examine the robustness of the base case findings. In the one-way sensitivity analysis, parameters were adjusted independently within a reasonable range established by either published data or a 95% confidence interval (refer to Supplementary Table S6). If the confidence interval was not applicable, the values were modified by ± 20% of the corresponding base case value. To determine the price of CAR-T treatment required to achieve cost effectiveness at a threshold of $31,320 per QALY, the range of the CAR-T treatment price ranged from $20,000 to $300,000. The range of the discount rate was 0 to 8%.
To account for the combined uncertainties in input parameter values, we conducted probabilistic sensitivity analyses using second-order Monte Carlo simulations, with 10,000 iterations performed. Gamma distributions were applied to costs, while beta distributions were used for utilities and time-dependent transition probability (see Supplementary Table S7). To determine the likelihood of each strategy being cost-effective at a specific willingness-to-pay threshold, we counted the number of times the incremental cost-effectiveness ratio (ICER) fell below the specified threshold, out of 10,000 iterations.
Additionally, we ran two main scenario analyses: (1) a scenario in which the model starting age was changed from 60 to 55; and (2) a scenario in which partitioned survival model was used to accumulate the survival gained and costs. This model involved reconstructing IPD to calculate survival gain based on the first portion of the AUC during the follow-up period. After the cutoff of the clinical trial, the second portion of the AUC was calculated using a standard parametric survival model to determine survival gain.
Results
Base case analysis
The total discounted cost of Ide-cel was estimated at approximately $217,205, with a gain of 2.51 discounted life-years and 2.11 discounted QALYs (see ). Out of all patients, 3.4% were identified as long-term survivors. Cilta-cel had a total discounted cost of around $196,318, with a gain of 4.76 discounted life-years and 4.23 discounted QALYs. A total of 36.6% of patients were considered long-term survivors. Salvage chemotherapy was associated with a total discounted cost of approximately $76,512, with a gain of 1.19 discounted life-years and 0.92 discounted QALYs.
Table 2. Summary of base case and scenario analysis results.
None of the patients receiving salvage chemotherapy achieved long-term survival. The ICERs for Ide-cel and Cilta-cel compared to salvage chemotherapy were approximately $118,229 and $36,195 per QALY gained, respectively. Based on the WTP of 3-times China’s per capita GDP in 2021, Cilta-cel was considered to be a cost-effective option compared to salvage chemotherapy for RRMM patients, while Ide-cel was not.
Sensitivity analyses
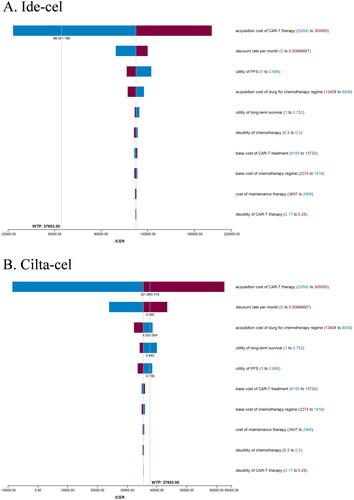
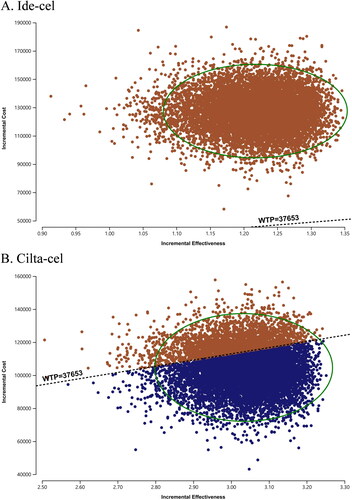
In one-way sensitivity analysis, the primary drivers of the model outcome were the prices of CAR-T therapy, discount rate, drug acquisition of usual care, utility for long-term survival, and PFS (). In the threshold analysis, the price of Ide-cel decreased to $88,451, which could make it cost-effective at a WTP threshold of $37,653/QALY. In probabilistic sensitivity analyses, Ide-cel and Cilta-cel were cost-effective at a WTP threshold of $37,653/QALY gained in 0% and 72% of simulations, respectively ().
When the median onset age of MM was set as 55 instead of 60, the ICER for Ide-cel and Cilta-cel compared to salvage chemotherapy was approximately $113,735 and $32,247/QALY, the latter of which was considered cost-effective (). When a partitioned survival model was used, the ICER for Ide-cel and Cilta-cel compared to salvage chemotherapy was approximately $117,169/QALY and $36,484/QALY.
Discussion
Given that CAR-T therapy is a class of novel and effective therapies for patients with B-cell malignancies, understanding the value of CAR-T therapy is crucial to informing funding decisions made by public health insurance plans. Currently, there are no approved CAR-T cell therapies for RRMM in China and this study explores the cost-effective analysis of Ide-cel and Cilta-cel for RRMM with hypothetical marketed prices. Our analysis found that, compared to salvage chemotherapy, Ide-cel and Cilta-cel improve effectiveness by 1.19 and 3.31 QALYs and increase the overall cost by $140,693 and $119,805, leading to an ICER of $118,229 and $36,195/QALY. Then Cilta-cel could be considered cost-effective when assuming the price of these two CAR-T therapies was $193,023.
In a study conducted by the Institute for Clinical and Economic Review from the perspective of the American healthcare systemCitation35, ICERs of approximately $319,000 and $253,000 per QALY were gained for Ide-cel and Cilta-cel versus the comparator, respectively. This study demonstrated that Ide-cel and Cilta-cel were not cost-effective at $50,000 per QALY, $100,000 per QALY, or $150,000 per QALY gained. In our study, Cilta-cel was possibly cost-effective. The main reason for this was that this study adopted the latest follow-up evidence to extract the survival curve of Cilta-cel, which reduced the uncertainty to a certain extent.
Price reductions could allow Ide-cel to be cost-effective if the price of Ide-cel decreased by 54%. At present, some core raw materials (e.g. viral vectors) in the process of CAR-T manufacturing in China are imported from abroad, resulting in high production costs of CAR-T therapies. When these core raw materials can be produced independently in China, the production cost of CAR-T cell treatment will be greatly reduced, as will the price. Greater improvement in clinical efficacy may be another approach to increasing the cost-effectiveness of CAR-T. Although Ide-cel and Cilta-cel achieved a high complete response (CR) rate in heavily pretreated populations, the disease progressed or relapsed soon after achieving responses in a certain proportion of patients, and some patients had no responses to anti-BCMA CAR T cells at allCitation12,Citation13. In the base analysis, Ide-cel and Cilta-cel resulted in 3.4% and 36.6% of patients being long-term survivors, which is a substantial gap between their ICERs. Whether from the perspective of drug development or from the perspective of economic evaluation, it is necessary to develop a more effective CAR T-cell regimen for RRMM, especially to achieve a substantial proportion of long-term survivors.
The starting age of patients with RRMM in our study was 60, which meant that the maximum time horizon for which QALYs were accumulated was approximately 20 years. Previous studies confirmed that CAR-T products were more cost-effective in treating young people (<22 years old) than in treating elderly people (>60 years old), because the QALY gained in the young cohort was substantial in long-term simulationsCitation15,Citation16,Citation36. Therefore, we conducted a scenario analysis to assume that the median age of patients with RRMM entering the model was set as 55, which was rational given that the prevalence of RRMM was getting younger. This scenario analysis demonstrated that the incremental QALY gained from two CAR-T therapy increased rather mildly and the cost-effectiveness results of two CAR-T therapies did not change. This implied the change caused by patients who were 5 years younger entering the model was slight in our study. We also utilized the partitioned survival model, a classical method, to estimate the QALYs and costs. Finally, we observed a slight increase in the incremental QALYs and costs obtained from the partitioned survival model compared to those from the Markov model. Furthermore, the ICERs were found to be similar for both models. This was also confirmed in previous studies, which showed that the results based on both models were very similarCitation37. Actually, when the method (for example, the IPDfromKM proposed by Na Liu et al.Citation23) can reconstruct individual patient data accurately and the model structure is not complicated, the partitioned survival model is more appropriateCitation38. However, it should be noted that the Markov model is more flexible than the partitioned survival model, especially when considering more heath stages and sequential therapies. In this study, we used the Markov model to assume that patients achieve clinical cure after staying in remission for 5 years.
The cost-effectiveness threshold of a QALY in this study was set as three times the national per capita GDP based on the rule of thumb previously recommended by the World Health Organization. It has been argued that the commonly used metric of 3-times the gross domestic product (GDP) per capita CETs of a QALY was not necessarily empirically supported in all countriesCitation39,Citation40. Recently, a study conducted an estimation of the cost-effectiveness threshold of a QALY in Chinese settings based on the value of statistical life, and the result of this study was 1.16 (range: 1.45–2.90) times the GDP per capita in ChinaCitation41. Another study using a marginal productivity approach estimated that the cost-effectiveness threshold of a DALY in Chinese settings was 63% (range: 47–88%) of GDP per capitaCitation42. The cost‑effectiveness threshold of a QALY or DALY was much lower than 3-times the national per capita GDP, which demonstrated that the use of the two CAR-T therapies could not be cost-effective in base case analysis or scenario analysis when adapting these WTPs.
Because this study was based on evidence from a single-arm clinical trial, the selection of the appropriate comparator for cost-effective analysis could be challenging. Patient characteristics treated with a comparator should be consistent with those treated with CAR-T therapies in cost-effectiveness analysis. In this study, a multicenter US-based retrospective analysis and real-world data from the Bone Marrow Transplantation Center, the First Affiliated Hospital of Zhejiang University School of Medicine were adopted to construct the comparator cohort. The short follow-up period of clinical trials of CAR-T therapy makes it necessary to extrapolate the trial survival curves over a lifetime horizon to estimate the lifetime incremental benefits associated with CAR-T therapies. In this study, a standard parametric model and partitioned parametric model were used to approximate the aggregated data with fewer assumptions. When data from randomized controlled clinical trials with longer follow-up periods become available in the future, the cost-effectiveness of CAR-T can be more accurately measured.
There were several limitations to this study. The PFS and OS data from clinical trials abroad were used to inform the transition probability between health states of CAR-T therapy and comparator cohorts. However, the sample of these clinical trials were not entirely composed of Asian patients, and differences between ethnic groups and uncertainty in the results may exist. Additionally, the utility value and cost were derived from a single-center database, which will lead to biases in the model results.
Conclusion
In the context of the Chinese healthcare system, Cilta-cel was considered to be a more cost-effective option for patients with RRMM compared to salvage chemotherapy at a WTP threshold of $37,653 per QALY, while Ide-cel was not. With younger target people entering the model and the partitioned survival model used to capture long-term survival benefits, the ICERs of Cilta-cel and Ide-cel changed rather mildly and their cost-effective results did not change.
Transparency
Author contributions
WW and SD developed the economic model, performed the analyses, collected and reviewed the data. YZ, XS, and YY interpreted the results and drafted the manuscript. SD, MZ, and YZ reviewed the data, interpreted the results, and revised the manuscript. HD supervised the whole study.
Reviewer disclosures
A reviewer on this manuscript has disclosed that they are an investigator on the Janssen ciltacel studies and have received an honoraria from Janssen and BMS. The other peer reviewers on this manuscript have no other relevant financial relationships or otherwise to disclose.
Supplemental Material
Download MS Word (556.4 KB)Acknowledgements
None reported.
Declaration of funding
This study is funded by the China Scholarship Council [CSC, 2022062320370] and Zhejiang Pharmaceutical Association Economics and Health Technology Evaluation Special Scientific Research Funding Project [2022-SKY-A07054-0007].
Declaration of financial/other interests
No potential conflict of interest was reported by the author.
References
- Cowan AJ, Allen C, Barac A, et al. Global burden of multiple myeloma: a systematic analysis for the global burden of disease study 2016. JAMA Oncol. 2018;4(9):1221–1227.
- Michels TC, Petersen KE. Multiple myeloma: diagnosis and treatment. Am Fam Physician. 2017;95(6):373–383.
- Martin T, Huff CA. Multiple myeloma: current advances and future directions. Clin Lymphoma Myeloma Leuk. 2019;19(5):255–263.
- Bazarbachi AH, Al Hamed R, Malard F, et al. Relapsed refractory multiple myeloma: a comprehensive overview. Leukemia. 2019;33(10):2343–2357.
- Yang Y, Li Y, Gu H, et al. Emerging agents and regimens for multiple myeloma. J Hematol Oncol. 2020;13(1):150.
- Joseph NS, Tai YT, Anderson KC, et al. Novel approaches to treating relapsed and refractory multiple myeloma with a focus on recent approvals of Belantamab Mafodotin and selinexor. Clin Pharmacol. 2021;13:169–180.
- Larson RC, Maus MV. Recent advances and discoveries in the mechanisms and functions of CAR-T cells. Nat Rev Cancer. 2021;21(3):145–161.
- MacKay M, Afshinnekoo E, Rub J, et al. The therapeutic landscape for cells engineered with chimeric antigen receptors. Nat Biotechnol. 2020;38(2):233–244.
- June CH, O’Connor RS, Kawalekar OU, et al. CAR-T cell immunotherapy for human cancer. Science. 2018;359(6382):1361–1365.
- Celgene Corporation, a Bristol-Myers Squibb Company: ABECMA® (idecabtagene vicleucel) prescribing information. https://packageinserts.bms.co-m/pi/pi_abecma.pdf.
- Janssen Biotech, Inc and Legend Biotech: CARVYKTI® (ciltacabtagene autoleucel) prescribing information. https://www.janssenlabels.com/package-insert/product-monograph/prescribing-information/CARVYKTI-pi.pdf.
- Munshi NC, Anderson LD, Jr, Shah N, et al. Idecabtagene vicleucel in relapsed and refractory multiple myeloma. N Engl J Med. 2021;384(8):705–716.
- Martin T, Usmani SZ, Berdeja JG, et al. Ciltacabtagene autoleucel, an anti-B-cell maturation antigen chimeric antigen receptor T-cell therapy, for relapsed/refractory multiple myeloma: CARTITUDE-1 2-year follow-up. J Clin Oncol. 2023;41(6):1265–1274.
- Gandhi UH, Cornell RF, Lakshman A, et al. Outcomes of patients with multiple myeloma refractory to CD38-targeted monoclonal antibody therapy. Leukemia. 2019;33(9):2266–2275.
- Lin JK, Muffly LS, Spinner MA, et al. Cost effectiveness of chimeric antigen receptor T-cell therapy in multiply relapsed or refractory adult large B-Cell lymphoma. J Clin Oncol. 2019;37(24):2105–2119.
- Whittington MD, McQueen RB, Ollendorf DA, et al. Long-term survival and value of chimeric antigen receptor T-Cell therapy for pediatric patients with relapsed or refractory leukemia. JAMA Pediatr. 2018;172(12):1161–1168.
- Furzer J, Gupta S, Nathan PC, et al. Cost-effectiveness of tisagenlecleucel vs standard care in high-risk relapsed pediatric acute lymphoblastic leukemia in Canada. JAMA Oncol. 2020;6(3):393–401.
- Moradi-Lakeh M, Yaghoubi M, Seitz P, et al. Cost-Effectiveness of tisagenlecleucel in paediatric acute lymphoblastic leukaemia (pALL) and adult diffuse large B-Cell lymphoma (DLBCL) in Switzerland. Adv Ther. 2021;38(6):3427–3443.
- Cher BP, Gan KY, Aziz MIA, et al. Cost utility analysis of tisagenlecleucel vs salvage chemotherapy in the treatment of relapsed/refractory diffuse large B-cell lymphoma from Singapore’s healthcare system perspective. J Med Econ. 2020;23(11):1321–1329.
- Ribera Santasusana JM, de Andrés Saldaña A, García-Muñoz N, et al. Cost-Effectiveness analysis of tisagenlecleucel in the treatment of relapsed or refractory B-Cell acute lymphoblastic leukaemia in children and young adults in Spain. Clinicoecon Outcomes Res. 2020;12:253–264.
- Wakase S, Teshima T, Zhang J, et al. Cost-Effectiveness analysis of tisagenlecleucel for the treatment of pediatric and young adult patients with relapsed or refractory B cell acute lymphoblastic leukemia in Japan. Transplant Cell Ther. 2021;27(3):241.e1–241.e11.
- Diaby V, Adunlin G, Montero AJ. Survival modeling for the estimation of transition probabilities in model-based economic evaluations in the absence of individual patient data: a tutorial. Pharmacoeconomics. 2014;32(2):101–108.
- Liu N, Zhou Y, Lee JJ. IPDfromKM: reconstruct individual patient data from published Kaplan-Meier survival curves. BMC Med Res Methodol. 2021;21(1):111.
- Guyot P, Ades A, Ouwens MJ, et al. Enhanced secondary analysis of survival data: reconstructing the data from published Kaplan-Meier survival curves. BMC Med Res Methodol. 2012;12(1):9.
- Raje NS, Siegel DS, Jagannath S, et al. 3234 Idecabtagene vicleucel (ide-cel, bb2121) in relapsed and refractory multiple myeloma: analyses of high-risk subgroups in the KarMMa Study. ASH; December 7, 2020, 2020.
- Wang B, Liu J, Zhao W-H, et al. 2304 Chimeric Antigen Receptor T cell therapy in the relapsed or refractory multiple myeloma with extramedullary disease–a single institution observation in China. ASH; December 6, 2020, 2020.
- Binder M, Nandakumar B, Rajkumar SV, et al. Mortality trends in multiple myeloma after the introduction of novel therapies in the United States. Leukemia. 2022;36(3):801–808.
- Clinical trial: Clinical and basic research on BCMA-CAR T cells in the treatment of multiple myeloma, ChiCTR1800017404. http://www.chictr.org.cn/showproj.aspx?proj=28864.
- Zhejiang Provincial Center for Drug&Medical Device Procurement, https://www.zjyxcg.cn/?pageNow=88.
- Li Z, Pan Z, Zhang L, et al. End-of-life cost and its determinants for cancer patients in urban China: a population-based retrospective study. BMJ Open. 2019;9(3):e026309.
- Delforge M, Shah N, Rodriguez-Otero P, et al. 3465 Health state utility valuation in patients with triple-class-exposed relapsed and refractory multiple myeloma treated with the BCMA-Directed CAR T cell therapy, idecabtagene vicleucel (ide-cel, bb2121): results from the KarMMa trial. Blood. 2020;136(Supplement 1):14–15.
- Liu GG, Wu H, Li M, et al. Chinese time trade-off values for EQ-5D health states. Value Health. 2014;17(5):597–604.
- Li X, Liu J, Chen M, et al. Health-related quality of life of patients with multiple myeloma: a real-world study in China. Cancer Med. 2020;9(21):7896–7913.
- Yang Z, Busschbach J, Liu G, et al. EQ-5D-5L norms for the urban Chinese population in China. Health Qual Life Outcomes. 2018;16(1):210.
- Beinfeld M, Lee S, McQueen B, et al. Anti B-cell maturation antigen CAR T-cell and antibody drug conjugate therapy for heavily pretreated relapsed and refractory multiple myeloma. J Manag Care Spec Pharm. 2021;27(9):1315–1320.
- Sarkar RR, Gloude NJ, Schiff D, et al. Cost-effectiveness of chimeric antigen receptor T-cell therapy in pediatric relapsed/refractory B-Cell acute lymphoblastic leukemia. J Natl Cancer Inst. 2019;111(7):719–726.
- Rui M, Shi F, Shang Y, et al. Economic evaluation of cisplatin plus gemcitabine versus paclitaxel plus gemcitabine for the treatment of First-Line advanced metastatic Triple-Negative breast cancer in China: using markov model and partitioned survival model. Adv Ther. 2020;37(9):3761–3774.
- Rui M, Wang Y, Fei Z, et al. Will the markov model and partitioned survival model lead to different results? A review of recent economic evidence of cancer treatments. Expert Rev Pharmacoecon Outcomes Res. 2021;21(3):373–380.
- Robinson LA, Hammitt JK, Chang AY, et al. Understanding and improving the one- and three-times GDP per capita cost-effectiveness thresholds. Health Policy Plan. 2017;32(1):141–145.
- Marseille E, Larson B, Kazi DS, et al. Thresholds for the cost-effectiveness of interventions: alternative approaches. Bull World Health Organ. 2015;93(2):118–124.
- Cai D, Shi S, Jiang S, et al. Estimation of the cost-effective threshold of a quality-adjusted life year in China based on the value of statistical life. Eur J Health Econ. 2022;23(4):607–615.
- Ochalek J, Wang H, Gu Y, et al. Informing a cost-effectiveness threshold for health technology assessment in China: a marginal productivity approach. Pharmacoeconomics. 2020;38(12):1319–1331.