Abstract
Aims
The diagnostic history in the years leading up to the definitive diagnosis of patients with obstructive hypertrophic cardiomyopathy (HCM) has not been studied.
Methods
Patients with a delay in the definitive diagnosis of obstructive HCM from January 2009 to March 2019 were identified in the US IBM MarketScan Commercial and Medicare Supplemental Databases if they had an alternative diagnosis indicating a misdiagnosis during the 24 months before the definitive obstructive HCM diagnosis. Resource use and costs associated with the delay were estimated during the same period.
Results
Of 3,888 eligible patients with obstructive HCM, 59.5% had a delay in definitive diagnosis. Patients received a mean of 4.0 misdiagnoses before the definitive obstructive HCM diagnosis, most of which were other cardiovascular conditions. Consequently, 15.7% of patients may have received inappropriate treatment. Approximately 78.4% of patients visited a cardiologist (mean 4.7 visits) before the definitive obstructive HCM diagnosis. Additionally, 26.8% and 32.1% of patients had an inpatient and emergency room visit, respectively. Annualized healthcare costs associated with the delay were $4,379 per patient.
Limitations
The current study used administrative claims data for a commercially insured population. Therefore, the results may not be generalizable to other populations (e.g. those insured by Medicare or Medicaid and the uninsured). Like other database studies, the current study may have suffered from miscoding or undercoding, which may have caused misclassification of patients. Owing to insufficient data, the study could not evaluate all potential consequences of a delay in definitive diagnosis.
Conclusions
Most patients with obstructive HCM had a delay of ≤ 2 years before receiving the definitive diagnosis. The diagnostic journey involved multiple potential misdiagnoses, predominantly cardiovascular, as well as a substantial clinical and economic burden on patients and the healthcare system.
Introduction
Hypertrophic cardiomyopathy (HCM) is a myocardial disease characterized by left ventricular hypertrophy (LVH), hypercontractility, and impaired diastolic function in the absence of other identifiable diseases that could account for such remodeling and dysfunctionCitation1,Citation2. There is no reported distinct geographic, ethnic or sex pattern of distribution of the diseaseCitation3. However, HCM is most commonly caused by genetic mutationsCitation1,Citation2. Phenotypically, HCM is classified as either obstructive or nonobstructive. Obstructive HCM is defined clinically as a resting or provoked peak instantaneous left ventricular (LV) outflow gradient of ≥ 30 mm Hg and occurs in approximately 70% of patients with HCMCitation4,Citation5. Half of the patients with HCM are asymptomaticCitation4. The remaining half present with various symptoms, such as fatigue, chest pain, dyspnea, palpitations, and syncope, that are also common to other diseases, especially other cardiovascular diseasesCitation6. The current guideline-recommended pharmacologic treatment options for obstructive HCM focus on the treatment of symptoms rather than the underlying pathophysiological source of HCM. In cases where patients have persistent severe symptoms the treatment options also include septal reduction therapyCitation2. For symptomatic patients, the cumulative disease burden can be substantial, including heart failure (HF), atrial fibrillation, stroke, and sudden cardiac death (SCD)Citation2,Citation7,Citation8.
The diagnosis of HCM is established by the identification of LVH and assessment of LV outflow tract anatomy and physiologyCitation2. Based on the 2020 American Heart Association/American College of Cardiology joint clinical guidelines, a definitive HCM diagnosis requires cardiac imaging findings, based on echocardiography or cardiovascular magnetic resonance (CMR), showing maximal end-diastolic wall thickness of ≥ 15 mm anywhere in the LV or 13–14 mm in those with either a family history of HCM or a positive genetic testCitation2. Underdiagnosis and delay in the definitive diagnosis of HCM are common and may persist for yearsCitation9–11. This is due to the fact that in clinical practice, some patients are asymptomatic and others have nonspecific symptoms, which may pose a challenge when making HCM diagnoses. A study analyzing 711 patients diagnosed with HCM, found that in 54% of patients, HCM was only diagnosed after symptom onset or a cardiac eventCitation9. Furthermore, the prevalence of diagnosed HCM based on a large claims database in the US was reported as ≤ 1 in 3,000Citation11; in contrast, the prevalence in genetic and echocardiographic population studies based on clinical presentation, cardiac imaging, and genetic screening ranged from 1:200 to 1:500, respectively, highlighting that HCM is currently underdiagnosedCitation12,Citation13. In addition, the median age at diagnosis based on a large-scale registry was 46 yearsCitation8, whereas hypertrophy can be present much earlier in life, signifying a potentially long latency period during which symptoms may be subtle, missed, or attributed to other diseases.
To date, no studies have attempted to understand the diagnostic history of patients with obstructive HCM and the impact of a delay in definitive diagnosis. To fill this gap, the current study sought to provide real-world evidence of a delay in the definitive diagnosis of obstructive HCM, the more symptomatic subset of HCM. Specifically, this study aimed to estimate the proportion of patients with obstructive HCM who experienced a delay in definitive diagnosis, to increase understanding of the diagnostic history, and to assess the potential clinical and economic burden associated with a delay in definitive diagnosis. It was hypothesized that delays in the definitive diagnosis of obstructive HCM may result in additional annual healthcare costs.
Methods
Study design and data source
This retrospective, observational study used administrative healthcare claims data from the IBM MarketScan Commercial and Medicare Supplemental Databases (January 2009 to March 2019). These databases included de-identified medical and pharmacy claims of insured employees and their dependents covered by health benefits programs of US employers, as well as Medicare-eligible retirees with employer-provided Medicare supplemental plansCitation14. Available data include demographics, comorbidities, claims, clinical visits, and costs (assessed from the commercial payer’s perspective) for over 25 million patients annually. To account for inflation, all healthcare costs were adjusted to the 2020 USD value using the Medical Care Component of the Consumer Price Index.
Sample selection
Patients with obstructive HCM were identified as those meeting one of the following criteria: (1) at least one medical claim with an International Classification of Diseases, 9th/10th Revision, Clinical Modification (ICD-9/10-CM) diagnosis code for obstructive HCM (ICD-9-CM: 425.11; ICD-10-CM: I42.1) and another medical claim with a diagnosis code for HCM (ICD-9-CM: 425.1; ICD-10-CM: I42.1 or I42.2) that were ≥ 30 d apart; or (2) at least one medical claim with a diagnosis code for HCM and another claim on a different date for septal reduction therapy (SRT), including alcohol septal ablation and septal myectomy. The index date was the date of the first observed diagnosis for obstructive HCM or HCM. To be included in the study, patients had to be aged ≥ 18 years as of the index date and have ≥ 24 months of continuous eligibility prior to the index date (i.e. the observation period) and ≥ 12 months after the index date (i.e. the follow-up period) (Supplementary Figure S1). The baseline period was defined as the 12 months prior to the index date. Patients were excluded if they had a claim with a diagnosis code for Fabry disease or amyloidosis at any time. Patients with missing data were excluded from the analysis. Additionally, patients who had SRT prior to the index date were excluded because this indicated that the first HCM diagnosis observed in the database was not their initial HCM diagnosis.
An expert consensus process was used to identify a delay in the definitive diagnosis of obstructive HCM by selecting a list of potential conditions as common misdiagnoses among patients with obstructive HCM (Supplementary Table S1). A list of drugs used to treat these selected conditions not concurrently used in HCM was also compiled (Supplementary Table S2). Based on the selected condition and drug lists, eligible patients for this study were classified into the following groups (Supplementary Figure S2):
Group A. Patients who met one of the two criteria below were considered as having a delay in definitive diagnosis (misdiagnosis).
Criterion 1: (a) prescribed a drug listed in Supplementary Table S2 during the observation period and up to 6 months following the index date, and (b) had a diagnosis of the corresponding condition listed in Supplementary Table S1 during the observation period, and (c) prescribed the drug on or after the diagnosis of the corresponding condition, AND (d) did not have additional prescriptions for the same class of drugs ≥ 6 months following the index date.
Criterion 2: had a diagnosis for a condition listed in Supplementary Table S1 during the observation period but did not have any diagnoses for the same condition during the follow-up period.
To further evaluate the extent of delay in definitive diagnosis in Group A, the study defined two subgroups.
Subgroup A1 (diagnosis in progress: definitive obstructive HCM diagnosis within 12 months). Patients had to meet the following criteria: had a new diagnosis for an HCM-related condition (conditions marked with * in Supplementary Table S1) during the baseline period (i.e. within 12 months of their definitive HCM diagnosis) but did not have any diagnoses for these conditions before the baseline period (i.e. 12–24 months prior to definitive obstructive HCM diagnosis).
Subgroup A2 (incorrect diagnosis: definitive HCM diagnosis > 12 months). Patients had to have a diagnosis for an HCM-related condition during the observation period but prior to the baseline (i.e. 12–24 months prior to definitive obstructive HCM diagnosis).
Group B. Patients who did not have a delay in definitive diagnosis (no misdiagnosis/comorbidity). Specifically, patients (1) did not have a delay in obstructive HCM diagnosis, and (2) did not have any diagnoses for conditions listed in Supplementary Table S1 during the observation period (regardless of whether they had these conditions during follow-up).
Group C. Patients who did not have a delay in definitive diagnosis but who had a comorbidity. Specifically, patients (1) did not have a delay in obstructive HCM diagnosis, and (2) had a diagnosis for a condition listed in Supplementary Table S1 during both the observation and follow-up periods (i.e. possible comorbidities).
Study outcomes
Patient demographics and comorbidities in Groups A (Subgroups A1 and A2), B, and C were described. Specifically, age, sex, geographic region, index year, the Charlson Comorbidity Index (CCI)Citation15 and its components, and selected cardiac comorbidities were assessed during the baseline period. Physician specialty and treatment setting for the definitive obstructive HCM diagnosis on the index date were also described.
Diagnostic history and economic impact of a delay in definitive diagnosis were determined for patients in Group A. This included the proportion of patients with misdiagnosis and the number of misdiagnoses in cardiology and other specialties from the first observed misdiagnosis to the index date. The proportion of patients with a specialist visit and the number of visits in cardiology and other specialties were also described. Annualized healthcare resource utilization (HRU) and healthcare costs associated with misdiagnoses, including inpatient stays, outpatient visits, and emergency room (ER) visits, were assessed during the observation period. Specifically, HRU and costs were considered as associated with misdiagnoses if there was a diagnosis code for misdiagnosis in that visit. Because the misdiagnoses occurred at different times during the observation period, annualized HRU and healthcare costs were estimated.
Statistical methods
This was an observational, descriptive study, and no statistical hypothesis testing was conducted. Continuous variables were described by means, standard deviations (SDs), and medians. Categorical variables were presented as counts and proportions. Analyses were performed in SAS 9.4 (SAS Institute, Cary, NC, USA) and R 3.6.3.
Results
Obstructive HCM diagnosis and patient characteristics
Of 3,888 patients with obstructive HCM who were eligible for the study, 2,315 (59.5%) had a delay in definitive obstructive HCM diagnosis (i.e. misdiagnosis), as identified by alternative diagnoses or treatments during the 2 years before definitive diagnosis. These patients were classified as Group A (). Approximately 15.7% of patients in Group A also received treatment for a misdiagnosis during the observation period. Among patients in Group A, 1,006 (43.5%) were in Subgroup A1, which indicated an “HCM diagnosis in progress”, and 754 (32.6%) were in Subgroup A2, which denoted an “incorrect diagnosis” ().
Figure 1. Identification of patients with a delay in definitive obstructive HCM diagnosis. *HCM identified using ICD-9-CM (425.1) and ICD-10-CM (I42.1, I42.2) diagnosis codes. Obstructive HCM identified using ICD-9-CM (425.11) and ICD-10-CM (I42.1) diagnosis codes. †SRT procedures include alcohol septal ablation and septal myectomy. ICD-9-PCS (35.42, 37.34, 37.33), ICD-10-PCS (02BM3ZZ, 02TM3ZZ, 025M3ZZ, 02CM3ZZ, 02CM4ZZ, 02BM0ZZ, 025M0ZZ, 02TM0ZZ, 02CM0ZZ), and CPT/HCPCS (93583, 33416) codes were used to identify SRT. ‡Index date: first observed diagnosis for obstructive HCM. §Observation period: 24 months prior to index date. ¶Follow-up period: period from index date to death, end of continuous eligibility, or end of data availability, which ever happened first. ǁBaseline period: 12 months prior to index date. CPT: Current Procedural Terminology; HCM: hypertrophic cardiomyopathy; HCPCS: Healthcare Common Procedure Coding System; ICD-9-CM: International Classification of Diseases, 9th Revision, Clinical Modification; ICD-10-CM: International Classification of Diseases, 10th Revision, Clinical Modification; ICD-9-PCS: International Classification of Diseases, 9th Revision, Procedure Coding System; ICD-10-PCS: International Classification of Diseases, 10th Revision, Procedure Coding System; SRT: septal reduction therapy.
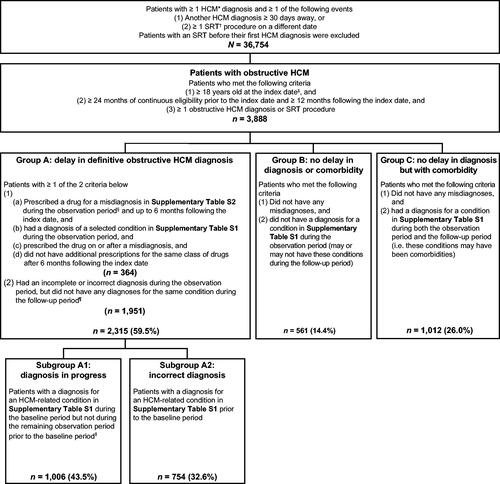
Among those who did not have a delay in definitive diagnosis, 561 (14.4%) did not have a comorbidity (Group B) and 1,012 (26.0%) had at least one comorbidity during the observation and follow-up periods (Group C) ().
Overall, the study population had a mean (SD) age of 59.9 (14.0) years and 45.5% were women (). Patients in Groups A, B, and C had similar demographic characteristics compared to the overall study population. The mean (SD) CCI for the overall study population was 0.9 (1.4). The most common comorbidities included hypertension (64.9%), dyspnea (31.6%), and coronary artery disease (27.6%). Patients in Group A generally had higher rates of other comorbidities than those in Groups B and C ( and Supplementary Table S3).
Table 1. Characteristics of patients receiving a diagnosis of obstructive HCM.
Some differences in patient characteristics between Subgroups A1 and A2 were noted. Patients in Subgroup A2 were, on average, older than those in Subgroup A1; Subgroup A2 had more women and shorter follow-up times (). However, comorbidities in both subgroups were similar ( and Supplementary Table S4).
Table 2. Characteristics of subgroups of patients with possible delayed obstructive HCM diagnosis.
The majority of patients (57.7%) in the overall study population received the definitive obstructive HCM diagnosis from cardiologists. This proportion was relatively consistent across different groups, except for a slightly lower proportion in Group B (54.2%) (). About 17–18% of patients in all groups received a diagnosis from generalists. Most patients (83.9%) received the diagnosis in an outpatient setting (). This proportion was lower in Group B, with 75.8% receiving the diagnosis in an outpatient setting and 23.5% in an acute setting.
Table 3. Obstructive-HCM-diagnosing physician and setting.
Diagnostic history of obstructive HCM among patients with a delay in definitive diagnosis
Among patients in Group A, the mean (SD) number of misdiagnoses was 4.0 (5.6) during the 2-year observation period prior to the index date. About 90.2% of patients in Group A had at least one misdiagnosis for a cardiovascular condition, with a mean (SD) of 3.2 (3.5) misdiagnoses for the selected conditions during the observation period. For other noncardiovascular specialties, 28.8% of patients had at least one misdiagnosis, with a mean (SD) of 3.8 (7.3) misdiagnoses during the observation period.
Most patients in Group A (78.4%) visited a cardiologist, with a mean (SD) of 4.7 (5.4) visits from the first observed misdiagnosis to the index date. Over half of the patients (56.2%) had at least one cardiologist visit related to a misdiagnosis, with a mean (SD) of 2.1 (2.1) visits per patient (). Only 7.6% of patients had a documented visit related to a misdiagnosis of a noncardiovascular condition, with a mean (SD) of 3.0 (6.6) visits.
Table 4. Diagnostic history in patients with a delay in obstructive HCM diagnosis during observation period.
There was a higher number of misdiagnoses during the observation period in Subgroup A2 than in Subgroup A1 (mean, 3.5 vs. 2.6) (). Subgroup A2 also had a higher proportion (83.0% vs. 79.0%) and number (mean, 6.5 vs. 3.5) of cardiologist visits of any type than Subgroup A1. However, the proportion and number of cardiologist visits related to a misdiagnosis of a cardiovascular condition were comparable between the two groups ().
HRU and healthcare costs associated with misdiagnosis
During the observation period, 26.8% of patients in Group A had an inpatient admission for a misdiagnosis, with a mean (SD) annualized number of inpatient admissions of 0.2 (0.5) per patient. The mean (SD) annualized number of inpatient days was 1.1 (3.6). Almost all patients (99.3%) had an outpatient visit associated with a misdiagnosis, with a mean (SD) annualized number of outpatient visits of 12.3 (16.5). Close to one-third of patients had an ER visit for a misdiagnosis, with a mean (SD) annualized number of ER visits of 0.3 (0.9) (). Overall, mean (SD) annualized total healthcare costs associated with misdiagnosis were $4,379.0 ($20,515.3) (median [IQR], $409.1 [$81.9, $1,456.7]) per patient. Most of these costs were attributable to medical costs (mean [SD] annualized, $4,352.1 [$20,497.1]; median [IQR], $395.5 [$80.1, $1,402.9]), specifically inpatient costs (mean [SD] annualized, $3,189.1 [$19,909.6]; median [IQR], $0.0 [$0.0, $0.0]). Among patients in Group A, 470 (20.3%) patients had undergone diagnostic imaging and tests for a misdiagnosis in the outpatient setting, for a mean cost of $1,134 per patient.
Table 5. HRU associated with misdiagnoses in patients with a delay in obstructive HCM diagnosis.
Subgroup A2 had higher HRU associated with misdiagnoses in all categories. Notably, the proportion of patients with an inpatient admission was over 13 percentage points higher in Subgroup A2 than in Subgroup A1 (). The rate of ER visits was also higher in Subgroup A2 than in Subgroup A1 (42.4% vs. 26.2%). The mean (SD) annualized number of outpatient visits was 19.7 (21.0) in Subgroup A2, which was more than double the number in Subgroup A1 (mean [SD], 7.3 [12.5]).
Discussion
HCM is an underrecognized cardiovascular disease that can lead to serious complicationsCitation1,Citation2,Citation8. To our knowledge, this is the first study to evaluate the diagnostic history of patients with obstructive HCM in the 2 years prior to a definitive diagnosis. This study provides an opportunity to better understand the extent of delay in definitive diagnosis (misdiagnosis) in patients with obstructive HCM and its associated real-world clinical and economic impact.
Delay in obstructive HCM diagnosis affected approximately 60% of patients who ultimately received a diagnosis of the disease. Another quarter of patients had comorbidities associated with obstructive HCM before the definitive obstructive HCM diagnosis (Group C). Only 14% of patients did not have a misdiagnosis or comorbidity associated with obstructive HCM (Group C). Furthermore, approximately 16% of patients with a delay in obstructive HCM diagnosis (Group A) received treatments for an incomplete or incorrect diagnosis, which might have been ineffective or potentially detrimental because they were terminated after the definitive obstructive HCM diagnosis was made.
There were demographic differences between Subgroups A1 (diagnosis in progress) and A2 (incorrect diagnosis). Specifically, older patients and female patients were more likely to be in Subgroup A2, suggesting they may experience longer delays in obstructive HCM diagnosisCitation16.
The reasons for the delay in obstructive HCM diagnosis are multifaceted. The heterogeneous clinical presentation of HCM coupled with diverse symptomology can make diagnosis challenging, which can label HCM as a more common disorder with similar features. Indeed, many conditions with similar symptoms, especially cardiovascular, may have complicated the diagnostic journey for patients with obstructive HCM and delayed confirmation of the diagnosis. Typically, the diagnosis of HCM occurs only after symptoms present or at the time an emergent event occursCitation9,Citation17.
The diagnosis of obstructive HCM requires a detailed assessment of clinical presentation, family history, and imaging evidenceCitation2. Echocardiography is the primary diagnostic tool for HCMCitation2. However, as LV outflow tract gradients are dynamic, the diagnosis can be missed on resting echocardiography in approximately half of patients with obstructive physiologyCitation18. A study of 333 patients with HCM in the US found that LVH would have been underestimated by echocardiography in 12% of patients who underwent CMRCitation19. This finding emphasizes the importance of CMR in diagnosing obstructive HCM; however, CMR is not routinely conducted in patients presenting with cardiovascular diseases. It is therefore important to prescribe postexercise echocardiography and CMR in a timely manner during the process of diagnosing obstructive HCM, and to have HCM-specific protocols of measuring wall thickness in multiple locations, including the basal septum.
Low awareness of HCM in the clinical community may be a major contributor to the delay in obstructive HCM diagnosis, even among cardiologists. Indeed, in this study, among patients who were seen by cardiologists, it took a mean of 4.7 visits and 3.2 cardiovascular condition misdiagnoses before the obstructive HCM diagnosis was confirmed. It is unlikely that the extent of LVH progressed to the HCM level during this short period, indicating that HCM-level LVH was present during the entire diagnostic process. Although a minority of patients received their definitive diagnosis from generalists, most patients received theirs from cardiologists. These findings suggest that even among cardiologists, obstructive HCM is underrecognized. Therefore, it is crucial to include HCM initially in the differential diagnosis alongside these other possible diagnoses that share similar clinical presentation and cardiac features to avoid a delay in obstructive HCM diagnosis.
Delay in obstructive HCM diagnosis also incurs economic impacts on both patients and the healthcare system. The HRU associated with the misdiagnoses is substantial. Nearly one-quarter of patients in the current study had an inpatient admission, close to one-third had an ER visit, and almost all patients had an outpatient visit. These patients had a mean annualized cost of over $4,000, mainly due to inpatient admissions. HRU was higher in patients with an incorrect diagnosis than in those with a diagnosis in progress. Rates of inpatient admissions, ER visits, and outpatient visits related to incorrect diagnoses were 36%, 42%, and 100%, respectively, in patients with an incorrect diagnosis. Notably, HRU observed in the present study was higher than that of most patients with atherosclerotic cardiovascular disease and was comparable to that observed in patients with coronary artery disease with diabetes and those with acute coronary syndrome in a similar claims database analysisCitation20. The difference in HRU between Subgroups A1 and A2 suggests that the longer the delay in HCM diagnosis, the more healthcare resources are devoted to potential incomplete or incorrect diagnoses, particularly among women. While this HRU may not be entirely avoided with timely diagnosis, if patients received a diagnosis earlier, an opportunity would exist to optimize HRU and focus on the correct treatment of obstructive HCM.
Understanding the complex process for making an obstructive HCM diagnosis highlights the need for disease awareness that might enable timely diagnosis and treatment. Therefore, education of patients and healthcare professionals is crucialCitation10. HCM education may be incorporated into continuing medical education programs for healthcare professionals providing primary care, cardiology, and psychology. The goal of such programs would be to ensure that HCM is considered as a possible diagnosis when patients present with relevant but nonspecific symptoms. Generalists and other specialists should be encouraged to refer patients to cardiologists or centers of excellence in HCM in a timely manner if they are not equipped with the knowledge and skills to diagnose HCM. Expanding the role of these centers could provide state-of-the-art care with consistent outcomes for patients with HCMCitation21. Additionally, it is important for healthcare professionals and patients to recognize the genetic basis of HCM. Genetic screening may help identify HCM in the subclinical stage among individuals with a family historyCitation2. Algorithms for electrocardiograms and echocardiography values to trigger HCM consideration may also prove beneficial. Ideally, early diagnosis and initiation of treatment may lead to a reduced burden from cardiovascular complications, including HF, stroke, and SCD through early initiation of treatments.
Limitations
This study has several limitations. First, the current study used administrative claims data. Additional narrative details from other sources such as medical charts were not available. Like other database studies, the current study may have suffered from miscoding or undercoding, which may have caused misclassification of patients. Furthermore, when using treatments to identify a delay in definitive diagnosis, the study excluded treatments used off-label for obstructive HCM, which likely underestimated the extent of the delay. When classifying patients into Subgroups A1 and A2, the study applied a somewhat arbitrary timeline (i.e. 12 months prior to the definitive obstructive HCM diagnosis). Despite these limitations, the two subgroups contribute to our understanding of the characteristics and potential economic burden associated with different degrees of delay in obstructive HCM diagnosis.
Second, to achieve a sufficient sample size, this study only required a 2-year observation period before the definitive obstructive HCM diagnosis. Therefore, the selected patients may not reflect all patients with obstructive HCM but rather may represent those who had a more expedited diagnostic process. Future studies with longer observation periods may be needed to better understand the average time taken to confirm an obstructive HCM diagnosis.
Third, owing to insufficient data, the study could not evaluate all potential consequences of a delay in definitive diagnosis. Studies with longer follow-up are indicated to compare the clinical and economic outcomes between patients with and without a delay in definitive diagnosis.
Fourth, for this analysis, the geographic location, socioeconomic status, race, and ethnicity were not assessed in the population. Therefore, the results are not adjusted for the presence of potential bias or barriers which may have resulted in delays in the presentation of symptoms, diagnosis, or referral.
Lastly, this study is based on claims data for a commercially insured population. Therefore, the results may not be generalizable to other populations (e.g. those insured by Medicare or Medicaid and the uninsured), which may have distinct sociodemographic profiles.
Conclusions
This study showed that the majority of patients with obstructive HCM had a delay in diagnosis (i.e. misdiagnosis or incorrect diagnosis in the 2 years prior to a definitive diagnosis) in a real-world US sample primarily evaluated by cardiologists. These patients’ diagnostic journey involved multiple misdiagnoses, predominantly cardiovascular, as well as multiple specialist visits and treatments for misdiagnoses, prior to the definitive diagnosis of obstructive HCM. In addition to the increased clinical burden associated with misdiagnosis, these delays in diagnosis were also associated with economic costs, with mean (SD) annualized total healthcare costs of $4,379.0 ($20,515.3) per patient. Improving HCM education among patients and healthcare professionals, including protocolized imaging algorithms, may enable timely diagnosis of obstructive HCM, which may, in turn, help mitigate the substantial clinical and economic burden associated with a delay in diagnosis.
Transparency
Reviewer disclosures
Peer reviewers on this manuscript have no relevant financial or other relationships to disclose.
Author contributions
All authors were involved in the conception and design of the study or in the analysis and interpretation of the data. All authors were involved in the drafting of the manuscript or revising it critically for intellectual content. All authors provided final approval of the version to be published and agreed to be accountable for all aspects of the work.
Previous presentations
The results reported here have not been presented previously.
Supplemental Material
Download MS Word (196.7 KB)Acknowledgements
Editorial assistance was provided by Anne Kangethe, an employee of Oxford PharmaGenesis, Inc. Support for this assistance was funded by MyoKardia, Inc., a wholly-owned subsidiary of Bristol Myers Squibb.
Disclosure statement of funding
Funding for this study was provided by MyoKardia, Inc., a wholly owned subsidiary of Bristol Myers Squibb.
Disclosure statement of financial/other interests
JTF was an employee of MyoKardia, Inc., a wholly owned subsidiary of Bristol Myers Squibb, at the time the study was conducted and holds stock/options. MBS was an employee of MyoKardia, Inc., a wholly owned subsidiary of Bristol Myers Squibb, at the time the study was conducted. WG is an employee of Analysis Group, Inc., which has received consulting fees from MyoKardia, Inc., a wholly owned subsidiary of Bristol Myers Squibb. JX was an employee of Analysis Group, Inc., at the time of manuscript development. SSN is a consultant and serves on advisory boards for Bristol Myers Squibb and Cytokinetics, and is on the Executive Committee of the VALOR trial for Bristol Myers Squibb. NRD works under contract with the Centers for Medicare & Medicaid Services to develop and maintain performance measures used for public reporting and pay-for-performance programs. He reports research grants and/or consulting for Amgen, AstraZeneca, Boehringer Ingelheim, Cytokinetics, MyoKardia, Inc., a wholly owned subsidiary of Bristol Myers Squibb/Bristol Myers Squibb, Novartis, scPharmaceuticals, and Vifor Pharma. ATO reports consulting for Cytokinetics, MyoKardia/Bristol Myers Squibb, Pfizer, and Renovacor.
Data availability statement
BMS policy on data sharing may be found at https://www.bms.com/researchers-and-partners/independent-research/data-sharing-request-process.html.
References
- Elliott PM, Anastasakis A, Borger MA, et al. 2014 ESC guidelines on diagnosis and management of hypertrophic cardiomyopathy: the Task Force for the Diagnosis and Management of Hypertrophic Cardiomyopathy of the European Society of Cardiology (ESC). Eur Heart J. 2014;35(39):2733–2779.
- Ommen SR, Mital S, Burke MA, et al. 2020 AHA/ACC guideline for the diagnosis and treatment of patients with hypertrophic cardiomyopathy: a report of the American College of Cardiology/American Heart Association joint committee on clinical practice guidelines. J Am Coll Cardiol. 2020;76(25):e159–e240.
- Marian AJ, Braunwald E. Hypertrophic cardiomyopathy: genetics, pathogenesis, clinical manifestations, diagnosis, and therapy. Circ Res. 2017;121(7):749–770.
- Jain SS, Li SS, Xie J, et al. Clinical and economic burden of obstructive hypertrophic cardiomyopathy in the United States. J Med Econ. 2021;24(1):1115–1123.
- Ammirati E, Contri R, Coppini R, et al. Pharmacological treatment of hypertrophic cardiomyopathy: current practice and novel perspectives. Eur J Heart Fail. 2016;18(9):1106–1118.
- Finocchiaro G, Magavern E, Sinagra G, et al. Impact of demographic features, lifestyle, and comorbidities on the clinical expression of hypertrophic cardiomyopathy. J Am Heart Assoc. 2017;6(12):e007161.
- Guttmann OP, Rahman MS, O'Mahony C, et al. Atrial fibrillation and thromboembolism in patients with hypertrophic cardiomyopathy: systematic review. Heart. 2014;100(6):465–472.
- Ho CY, Day SM, Ashley EA, et al. Genotype and lifetime burden of disease in hypertrophic cardiomyopathy: insights from the Sarcomeric Human Cardiomyopathy Registry (SHaRe). Circulation. 2018;138(14):1387–1398.
- Adabag AS, Kuskowski MA, Maron BJ. Determinants for clinical diagnosis of hypertrophic cardiomyopathy. Am J Cardiol. 2006;98(11):1507–1511.
- Magnusson P, Palm A, Branden E, et al. Misclassification of hypertrophic cardiomyopathy: validation of diagnostic codes. Clin Epidemiol. 2017;9:403–410.
- Maron MS, Hellawell JL, Lucove JC, et al. Occurrence of clinically diagnosed hypertrophic cardiomyopathy in the United States. Am J Cardiol. 2016;117(10):1651–1654.
- Maron BJ, Gardin JM, Flack JM, et al. Prevalence of hypertrophic cardiomyopathy in a general population of young adults: echocardiographic analysis of 4111 subjects in the CARDIA study. Circulation. 1995;92(4):785–789.
- Semsarian C, Ingles J, Maron MS, et al. New perspectives on the prevalence of hypertrophic cardiomyopathy. J Am Coll Cardiol. 2015;65(12):1249–1254.
- IBM Watson Health. IBM MarketScan Research Databases for Health Services Researchers [White Paper]. 2019 [cited 2021 April 20]. Available from: https://www.ibm.com/products/marketscan-research-databases/databases
- Quan H, Li B, Couris CM, et al. Updating and validating the Charlson comorbidity index and score for risk adjustment in hospital discharge abstracts using data from 6 countries. Am J Epidemiol. 2011;173(6):676–682.
- Lakdawala NK, Olivotto I, Day SM, et al. Associations between female sex, sarcomere variants, and clinical outcomes in hypertrophic cardiomyopathy. Circ Genom Precis Med. 2021;14(1):e003062.
- Lemasters CS, Grosel JM. Hypertrophic cardiomyopathy: an often missed diagnosis in athletes. JAAPA. 2010;23(6):42–46.
- Maron MS, Olivotto I, Zenovich AG, et al. Hypertrophic cardiomyopathy is predominantly a disease of left ventricular outflow tract obstruction. Circulation. 2006;114(21):2232–2239.
- Maron MS, Maron BJ, Harrigan C, et al. Hypertrophic cardiomyopathy phenotype revisited after 50 years with cardiovascular magnetic resonance. J Am Coll Cardiol. 2009;54(3):220–228.
- Zhao Z, Zhu Y, Fang Y, et al. Healthcare resource utilization and costs in working-age patients with high-risk atherosclerotic cardiovascular disease: findings from a multi-employer claims database. J Med Econ. 2015;18(9):655–665.
- Williams BR, Salberg L. Building a hypertrophic cardiomyopathy center of excellence. In: SS Naidu, editor. Hypertrophic cardiomyopathy. Switzerland: Springer Cham; 2019. p. 419–427.
