Abstract
Aims
Hepatocellular carcinoma (HCC) is a severe condition with poor prognosis that places a significant burden on patients, caregivers, and healthcare systems. Selective internal radiation therapy (SIRT) is a treatment available to patients with HCC which addresses some of the limitations of alternative treatment options. A cost-effectiveness analysis was undertaken into the use of SIRT using Y-90 resin microspheres for the treatment of unresectable intermediate- and late-stage HCC in Brazil.
Materials and methods
A partitioned-survival model was developed, including a tunnel state for patients downstaged to receive treatments with curative intent. Sorafenib was the selected comparator, a common systemic treatment in Brazil and for which comparative evidence exists. Clinical data were extracted from published sources of pivotal trials, and effectiveness was measured in quality-adjusted life-years (QALYs) and life-years (LYs). The analysis was conducted from the Brazilian private payer perspective and a lifetime horizon was implemented. Comprehensive sensitivity analyses were conducted.
Results
LYs and QALYs were higher for SIRT with Y-90 resin microspheres versus sorafenib (0.27 and 0.20 incremental LYs and QALYs, respectively) and costs were slightly higher for SIRT (R$15,864). The base case incremental cost-effectiveness ratio (ICER) was R$77,602 per QALY. The ICER was mostly influenced by parameters defining the sorafenib overall survival curve and SIRT had a 73% probability of being cost-effective at a willingness-to-pay threshold of R$135,761 per QALY (3-times the per-capita gross domestic product in Brazil). Overall, sensitivity analyses confirmed the robustness of the results indicating that SIRT with Y-90 resin microspheres is cost-effective compared with sorafenib.
Limitations
A rapidly evolving treatment landscape in Brazil and worldwide, and the lack of local data for some variables were the main limitations.
Conclusions
SIRT with Y-90 resin microspheres is a cost-effective option compared with sorafenib in Brazil.
Introduction
Hepatocellular carcinoma (HCC) is a severe condition with a poor prognosis, the consequences of which are further exacerbated by the significant physical and psychological burden patients experienceCitation1–7. In addition to the direct burden on patients, HCC poses a significant burden to caregivers, healthcare systems, and wider societyCitation8–11. International clinician associations have recognized that HCC constitutes both a current and a future major global health problemCitation12. In Brazil, liver cancer has the 11th highest cancer incidence, corresponding to 2.1% of new cases but 4.7% of the total cancer-related deaths, which highlights the burden and severity of this diseaseCitation13. To explain the high mortality rates of HCC, recently, dos Santos-Fernandes et al. reported that 76% of HCC patients in Brazil were diagnosed at an advanced stage, when treatments with a curative intent (TCIs) were no longer an optionCitation14. This underlines there is an unmet need, with limited interventions available, for HCC patients in Brazil.
International (e.g. European Association for the Study of the Liver [EASL], European Society for Medical Oncology [ESMO]) and local clinical practice guidelines in Brazil (e.g. Brazilian Society of Hepatology [SBH], Brazilian Society of Clinical Oncology [SBOC]) have been developed to help and guide healthcare professionals in the diagnosis and treatment of HCCCitation12,Citation15–17. Generally, these guidelines recommend allocating treatment according to disease stage defined by the Barcelona Clinic Liver Cancer (BCLC) systemCitation18,Citation19. Transarterial chemoembolization (TACE) has been uniformly adopted in clinical practice for the treatment of intermediate-stage (BCLC B) HCC, although its therapeutic efficacy remains a conflicting topicCitation20,Citation21. Also, TACE has been associated with safety concerns (e.g. pain, discomfort, liver decompensation, arteriopathy, or other SAEs) and health-related quality-of-life (HRQoL) reductions, aggravated by the need of multiple, sequential interventionsCitation6,Citation20,Citation22–25. The average number of TACE interventions per patient is estimated at three or four, with each session requiring a hospital stay of 3–6 daysCitation26–29. All this could in turn substantially increase the total cost of therapy of patients treated with TACE. For patients with advanced-stage (BCLC C) HCC, the treatment of choice according to guidelines is systemic therapy, with sorafenib being the most common treatment option in Brazil in the last decade. However, sorafenib has been shown to have an undesirable safety profile with a significant impact on patients’ HRQoL, and its downstaging efficacy to receive TCIs has been shown to be limited in randomized controlled trials (RCTs) as well as observational studiesCitation30–36. Lenvatinib, another systemic therapy, has been shown to have a similar efficacy and safety profile to sorafenibCitation37. The combination of atezolizumab and bevacizumab was associated with a significant improvement in overall survival (OS) and progression-free survival (PFS) versus sorafenib in IMbrave150, a phase 3 RCTCitation38; recent observational studies, however, indicate that the benefits for real-world patients may not be at the same level as those observed in IMbrave150Citation39,Citation40.
A recent study showed that HCC patients were willing to trade time alive to avoid certain treatment-related adverse events (AEs) and maintain their HRQoL, emphasizing how crucial safety and HRQoL associated with treatments is for HCC patientsCitation41. In addition, the study demonstrated that reducing hospital stay is important for HCC patients, a need that has not been fully addressed with current treatment optionsCitation41. The treatment with selective internal radiation therapy (SIRT) with Y-90 resin microspheres represents an alternative treatment option to help overcome some of the limitations of current treatments. This is a targeted treatment whereby radiation is directly delivered to the liver tumor site via selective hepatic arterial administrationCitation42,Citation43. The value of SIRT with Y-90 resin microspheres for the treatment of unresectable HCC, including intermediate- and advanced-stage patients, has been demonstrated in the SARAH and SIRveNIB RCTs, the only phase III RCTs comparing SIRT and sorafenibCitation30,Citation44. Even though the studies did not demonstrate a significant difference in OS between SIRT and sorafenib, the authors concluded that the favorable safety and HRQoL profile of SIRT may assist in choosing between the two treatmentsCitation30,Citation44. The results from SARAH and SIRveNIB have been further validated by robust observational studies in Europe and the United StatesCitation45–47, as well as other smaller studies demonstrating that survival outcomes with SIRT with Y-90 resin microspheres are maintained, or might even be improved, versus sorafenib in the real worldCitation48–54. Importantly, the benefits of SIRT with Y-90 resin microspheres, compared with sorafenib, are likely to be more substantial in the subgroup of patients with low tumor burden (≤25% of total liver volume) and good liver function (albumin-bilirubin [ALBI] grade 1), which may be used as a relevant patient selection criterionCitation55. The evidence base comparing SIRT with Y-90 resin microspheres and TACE or other systemic therapies, however, is scarce.
Recently, the National Regulatory Agency for Private Health Insurance (Agência Nacional de Saúde Suplementar [ANS]) in Brazil approved the inclusion of SIRT for the treatment of HCC in the list of procedures available in the private healthcare systemCitation56. More specifically, SIRT was approved for unresectable intermediate- (BCLC B) or advanced-stage (BCLC C) HCC for whom TACE is inappropriate, with or without portal vein thrombosis/involvement and without extrahepatic diseaseCitation56. Given the benefits that having access to SIRT can generate for HCC patients in Brazil, manifest by the ANS approval, we set out to present the cost-effectiveness analysis of SIRT with Y-90 resin microspheres for the treatment of HCC in Brazil that led to its inclusion in the list of available procedures.
Methods
The model was developed following the Brazilian Guidelines for Economic Evaluation and ISPOR Modeling Good Research PracticesCitation57,Citation58. The approaches adopted for modeling and local inputs were reviewed and validated by local experts in BrazilCitation59.
Target population, subgroups, and comparators
In the base case analysis, the cost-effectiveness of SIRT with Y-90 resin microspheres was estimated for patients eligible for systemic therapy, which encompassed the patient population approved by ANSCitation56. Pooled data from SARAH and SIRveNIB were used for this analysis, as per the National Institute for Health and Care Excellence (NICE) TA688 assessment group (AG) analysis and based on the results from a systematic literature review of clinical studiesCitation60. Sorafenib was the comparator of choice since it was the comparator in both RCTs and the most commonly used systemic therapy in Brazil at the time of model development and submission to ANSCitation30,Citation44.
Setting, perspective, and time horizon
The setting and perspective of the analysis was that of the private payer in Brazil, as recommended in the guidelinesCitation57. A lifetime time horizon (15 years) was selected for the base case analysis to capture all potentially relevant differences in costs and outcomes across treatment strategiesCitation57,Citation58,Citation61,Citation62. This was supported by the nature of the treatments assessed (e.g. the downstaging efficacy of SIRT with Y-90 resin microspheres is expected to be substantially better than that of sorafenib, leading to extended survival) and was in line with previous NICE and Scottish Medicines Consortium (SMC) appraisals in HCCCitation30,Citation48,Citation54,Citation63–66. Alternative time horizons (5, 10, and 20 years) were explored in sensitivity analyses.
Model structure
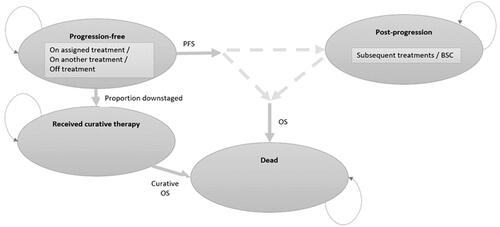
The economic model was developed using a partitioned survival approach with three main health states: “progression-free”, “progressed”, or “dead”. This approach is widely reported in the literature and has also been used and approved by health technology assessment (HTA) agencies worldwide for the assessment of health technologies in HCCCitation61. The mean age of patients entering the model was 66 yearsCitation30. The OS of patients downstaged to receive TCIs (i.e. liver transplant, liver resection, and ablation) were modeled separately from those not downstaged, as TCIs offer significantly extended survival. For this reason, a further tunnel state capturing the costs and outcomes of patients receiving TCIs was incorporated, with the following characteristics: downstaged patients remained progression-free until they received the TCI; only after receiving a TCI did these patients move to the “received curative therapy” health state, which was associated with a longer OS than those patients without TCI; for patients that did not receive a TCI, survival was modeled via an OS curve that did not include patients that had received a TCI. This approach ensured that overestimation of survival was avoided. The appropriateness of the model structure capturing the probability of downstaging was validated by clinicians in Brazil (two hepatologists and one interventional radiologist, plus co-authors of this manuscript) and was aligned with the literatureCitation67. The model had a cycle length of 1 month and half-cycle correction was implemented. The model structure diagram is displayed in and further details are available elsewhereCitation67,Citation68.
Figure 1. Model structure. Abbreviations. BSC, best supportive care; OS, overall survival; PFS, progression-free survival.
The effectiveness of health interventions was measured in quality-adjusted life-years (QALYs) and life-years (LYs). The key output of the model was the incremental cost-utility ratio (commonly referred to as incremental cost-effectiveness ratio [ICER]), calculated as the incremental cost divided by the incremental QALYs of interventions being compared.
Inputs
Survival curves
Following the guidelines developed by the NICE Decision Support Unit (DSU), parametric models were fitted to patient-level data reconstructed from OS and PFS Kaplan-Meier curves of pooled SARAH and SIRveNIB data published by the NICE TA688 AGCitation60,Citation69,Citation70. The curves generated by the NICE TA688 AG captured per protocol populations from both studies for the SIRT arm and the intention-to-treat (ITT) populations for the sorafenib arm. This way, the model accounted for the proportion of patients who fail the work-up procedure and subsequently do not receive treatment with SIRT with Y-90 resin microspheres. Independent parametric models were fitted to each treatment arm, as this involves fewer assumptions than jointly fitting curves and potentially relying on the proportional hazards assumption. Parametric curves were chosen based on goodness-of-fit statistics (Akaike information criterion [AIC] and Bayesian information criterion [BIC]), visually inspecting predicted and observed Kaplan-Meier curves and clinical plausibility.
For PFS, the generalized gamma was selected in the base case, as the AIC and BIC were superior to other functions in both treatment arms (Supplementary Table S1), with all parametric curves resulting in similar long-term estimates (Supplementary Figure S1). For OS, the AIC and BIC of the generalized gamma were superior to other functions in the case of SIRT with Y-90 resin microspheres (Supplementary Table S2); for sorafenib, the AIC and BIC values of the generalized gamma, log-logistic, and log-normal functions were very close (Supplementary Table S2) and yielded very similar long-term estimates, providing little justification to discriminate between models (Supplementary Figure S1). According to the NICE guidelines, fitting different types of parametric models to different treatment arms would require a strong justification, as different models allow very differently shaped distributionsCitation69. Because the generalized gamma distribution was by far the best-fitting distribution for SIRT, the generalized gamma model was selected in the base case analysis to keep consistency across treatment arms. Additionally, this was supported by the NICE TA688 AG statement saying that the generalized gamma model had the best fit for the pooled OS data and, importantly, it contains the exponential, Weibull, gamma, and lognormal distributions as special casesCitation60. The curve selection for sorafenib was tested in sensitivity analyses.
From the overall population OS curve, a hazard ratio (HR; calculated from SARAH) was applied to estimate the OS for non-curative patients, and the OS for patients downstaged to a TCI was calculated with a HR from data from a prospective study comparing the outcomes of intermediate and advanced HCC patients that received a TCI versus those that received non-curative treatmentsCitation68. The proportion of patients downstaged to a TCI, as well as the mean time to receive a TCI, were estimated from SARAH (6.9% and 1.5% downstaged after SIRT and sorafenib, respectively; 15.8 months to receive a TCI, on average, for all patients)Citation68.
Because SIRT with Y-90 resin microspheres is a one-off treatment, a time-to-discontinuation (TTD) curve was only estimated for sorafenib. This was calculated by fitting an exponential curve to the median TTD (5.5 months) adopted by the Brazilian National Committee for Technology Incorporation (CONITEC) in the appraisal of sorafenib (Supplementary Figure S2)Citation71. This was considered the most relevant value for analysis in the healthcare setting in Brazil, although other estimates of sorafenib use in the real-world were tested in sensitivity analysesCitation72.
Subsequent treatments
Patients not downstaged to receive a TCI were assumed to receive one or more of the following treatments: sorafenib, lenvatinib, regorafenib, and best supportive care (BSC). Due to the lack of relevant, local data in Brazil, data on the proportion of patients receiving each treatment was taken from a clinician survey in the UK (Supplementary Table S3)Citation68. Patients downstaged to a TCI received either liver surgery, liver transplantation, or ablation, as per the local guidelines in Brazil, other international guidelines and SARAH, and the proportion of patients receiving each treatment were taken from the SARAH trial (Supplementary Table S4)Citation16,Citation17,Citation30. Clinicians in Brazil acknowledged the limitations for sourcing local data due to the sparse use of SIRT in Brazil at the time of model development, and therefore these assumptions were considered reasonable.
Utilities
For the base case analysis, utility estimates included in the NICE TA668 AG were used ()Citation60. Rather than using estimates derived from the ITT SARAH population, these estimates were derived from the per protocol population which were deemed by the NICE AG to be more reflective of the HRQoL associated with SIRTCitation60,Citation68. As there were limited utility data available from the SARAH RCT for patients downstaged to receive TCI due to low patient numbers, the same utility estimate as used for the pre-progression SIRT health state was applied, potentially resulting in conservative cost-effectiveness estimates for SIRT with Y-90 resin microspheres (tested in scenario analyses).
Table 1. Utilities used in the model.
Adverse events
The reported incidence of Grade 3–4 treatment-related AEs that affected ≥5% of the population in the SARAH RCT were used to calculate AE costsCitation30. The costs of the AEs were applied as a one-off cost at the beginning of treatment, and incidence rates over the entire study period were used. This approach was also used within the NICE TA668 AG model. Adverse event incidence data are reported in Supplementary Table S5.
Resource use
For patients receiving SIRT with Y-90 resin microspheres, resource use for the work-up phase included angiography, a clinical examination, computed tomography (CT) scan, laboratory tests (including the measurement of albumin and bilirubin), single photon emission CT (SPECT), and other material, medicines, and general hospital costs. Patients assessed for SIRT with Y-90 resin microspheres typically undergo a single work-up, although a small number may receive a second work-up: the model considered that patients would undergo 1.05 work-ups on averageCitation68. Resource use for the treatment phase included anesthesia, CT scan, resources for conducting the SIRT procedure, SPECT and other material, medicines, and general hospital costs. The number of treatment procedures was taken from the real-world experience in a hospital in Brazil (Albert Einstein Hospital [at the time of model development]; 1.01 treatments on average; n = 101)Citation73. The model also included routine follow-up consisting of one consultation per month, and CT scans and laboratory tests every 3 months, all for the rest of the patients’ lives. For patients receiving sorafenib, treatment initiation costs included an initial consultation with the oncologist, a CT scan, and laboratory tests (Supplementary Table 6). Follow-up consultations, scans, and tests were assumed to be the same as for SIRT with Y-90 resin microspheres.
Costs
Initial treatment costs with SIRT with Y-90 resin microspheres comprised the device costs, cost of the work-up, and the treatment procedure (Supplementary Table S7). The cost of Y-90 resin microspheres was fixed at $21,500 (USD) and a USD to BRL exchange rate was used to convert the cost to local currency. The exchange rate at the time of model development was used (1 USD = 5.1913 BRL; 16/06/2021, the time of model development for submission to ANS). The total cost, per work-up, was R$5,317.26 and the total cost per treatment (including the device acquisition cost) was R$123,291.58 in the base case. The value of the exchange rate was tested in sensitivity analyses.
The price of sorafenib, per pack (60 × 200 mg per tablet) and without taxes, was R$7,756.52Citation74. The indicated dose of sorafenib is 800 mg daily; however, in alignment with the literature and HTA reports, including cost-effectiveness analyses in cancer, as well as clinician experts in Brazil, relative dose intensity (RDI) was applied to reflect the impact of dose reductions and interruptions on sorafenib acquisition costsCitation64–66,Citation75–77. Patient-level data from SARAH were used to calculate the RDI for sorafenib, estimated as actual dose divided by the planned dose (i.e. 800 mg daily), and expressed as a percentageCitation78. The value of RDI was 86%Citation68. Discontinuation of sorafenib treatment was modeled with the sorafenib TTD curve. The cost of treatment initiation with sorafenib was R$1,396.59.
The costs of sorafenib as a subsequent treatment were implemented as described above for initial costs. The costs of lenvatinib and regorafenib as subsequent treatments are described in the Supplementary Materials. The cost of BSC was assumed to be zero. For TCIs, the costs are provided in Supplementary Table S11. The costs of AEs are reported in Supplementary Table S12.
Sensitivity analyses
One-way sensitivity analyses (OWSA) were conducted to test the effects of parameter uncertainty in the model. The model parameters were varied using 95% confidence intervals (CIs) as lower and upper limits, if available. If these were not available, standard probability distributions were assigned to model parameters to estimate lower and upper limits, calculated as the 2.5th and 97.5th percentile, respectively, assuming a standard deviation (SD) of 10% of the corresponding base case values. In the case of parametric curves, the lower and upper bounds of curve fit parameters were calculated with variance–covariance matrices within a multinormal distribution. The results of the OWSA were displayed in tornado diagrams. Probabilistic sensitivity analyses (PSA) were also conducted. Mean LYs, QALYs, costs, and ICERs were calculated with 5,000 Monte Carlo simulations, and the results were displayed in incremental cost-effectiveness scatter plots as well as cost-effectiveness acceptability curves (CEACs). Mean values, ranges (lower and upper bounds), and distributions used for each parameter are reported in Supplementary Tables S13, S14, and S15. Several scenario analyses were conducted to test key model assumptions, such as the time horizon, discount rates, curve selection, or proportion of patients undergoing a second treatment with SIRT with Y-90 resin microspheres, and exchange rate, among others.
Results
In the base case, LYs and QALYs were higher for SIRT with Y-90 resin microspheres versus sorafenib (0.27 and 0.20 incremental LYs and QALYs, respectively), and costs were slightly higher for SIRT with Y-90 resin microspheres (R$15,864 incremental costs; ). This yielded an ICER of R$77,602 per QALY, which equates to an ICER of US$14,948 per QALY. To generate these results, the base case model assumed a median OS of 9.9 months for patients receiving SIRT without a subsequent TCI (Supplementary Figure S3) and, for those receiving a TCI (6.9%), the mean time to TCI was 16 months. For sorafenib, those patients without a subsequent TCI had a median OS of 10.3 months (Supplementary Figure S4), and the mean time to TCI for those patients receiving a TCI (1.5%) was also 16 months.
Table 2. Base case cost-effectiveness results.
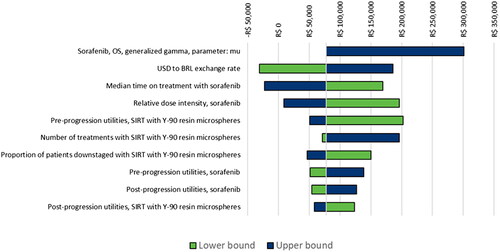
The OWSA indicated that the ICER was most influenced by some of the parameters defining the sorafenib OS curve, the exchange rate, the median time on treatment and RDI with sorafenib, and utilities (). The model results were less sensitive to variables such as the proportion of patients downstaged (with both SIRT with Y-90 resin microspheres and sorafenib), OS HR for curative versus non-curative patients, or OS HR for non-curative versus all patients.
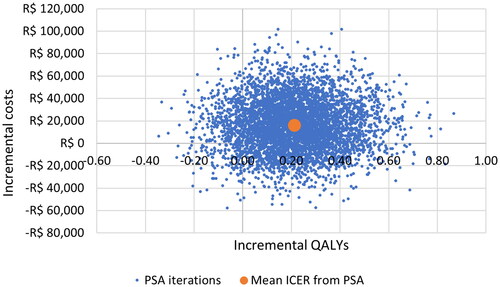
In the PSA, estimates most commonly fell in the north-east quadrant of the cost-effectiveness plane, indicating that overall SIRT with Y-90 resin microspheres is more expensive but also more effective than sorafenib (). The mean ICER from the PSA was R$75,864 per QALY, which equates to US$14,614 per QALY.
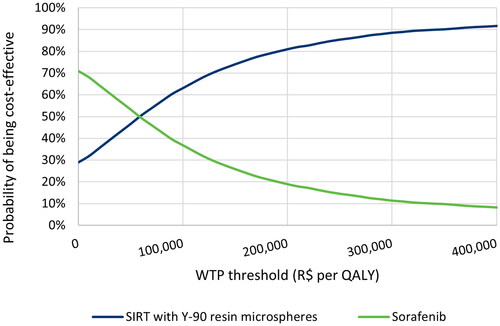
The CEAC presented in shows that at a willingness-to-pay (WTP) threshold of R$100,000 the probability of SIRT with Y-90 resin microspheres to be cost-effective was 63%. This probability increased to 72% and 88% with a WTP threshold of R$135,761 per QALY (approximately 3-times the per capita gross domestic product [GDP] in Brazil; GDP per capita in 2019: US $8,717.19) and R$280,235 per QALY (the highest identified ICER approved as of 2017), respectivelyCitation79–82.
Results of the scenario analyses are reported in . Given the wide variety of model approaches and assumptions tested, the results reinforced the base case results indicating that SIRT with Y-90 resin microspheres was highly likely to be cost-effective compared with sorafenib. In spite of the lack of a specific WTP threshold in Brazil, interestingly all scenarios but one resulted in an ICER below the WTP threshold of 3-times the GDP and all ICERs were well below the maximum accepted ICER in Brazil.
Table 3. Results from scenario analyses with sorafenib as the comparator.
Discussion
Recently, researchers showed that patients with HCC were willing to trade time alive to avoid certain treatment-related AEs and maintain their HRQoL, emphasizing how crucial safety and HRQoL associated with treatments are for these patientsCitation41. In addition, the study demonstrated that reducing hospital stay is important for HCC patients, a need that has not been fully addressed with current treatment optionsCitation41. All this highlights the necessity of alternative treatment options for this patient population, as none of the current treatment options fully meet patients’ and physicians’ needs. In this environment, SIRT with Y-90 resin microspheres is an alternative treatment option that may help overcome some of the limitations of current treatments, hence the importance of demonstrating its value. This was acknowledged by the ANS approval of SIRT in 2022, which led to the mandatory coverage by private payers in Brazil of intermediate- and advanced-stage unresectable HCC patients, without extrahepatic disease for whom TACE is inadequate, with or without thrombosis or portal vein invasionCitation56. The study presented here assessed the economic value of SIRT with Y-90 resin microspheres in these patients, resulting in an ICER of R$77,602 per QALY, equal to US$14,948 per QALY, in the base case. These are the model and results reviewed and assessed by ANS.
A number of sensitivity analyses were conducted to test the robustness of these results. One-way sensitivity analyses identified the parameters with the largest influence on the ICER: sorafenib OS curve, USD to BRL exchange rate, RDI of sorafenib and some utility values. These results were aligned with results from the NICE TA688 AG model and the health economics literatureCitation60,Citation75. In addition, the best sources of data available in the literature (i.e. pivotal RCTs) were used to derive these parameters (e.g. sorafenib OS curve, RDI of sorafenib, utilities) and other key model values (e.g. SIRT with Y-90 resin microspheres survival curves, sorafenib PFS curves), meaning that the results from the base case analysis represented the best possible estimates of cost-effectiveness of SIRT. In terms of the USD to BRL exchange rate, market fluctuations are expected to affect the cost-effectiveness of SIRT with Y-90 resin microspheres in the future, as demonstrated by the OWSA. The exchange rate used in the model, however, remains relevant considering the 3-month average at the time of manuscript write-up (5.2598 on 17 January 2023), and scenario analyses also showed it has not had any substantial impact on results in the last few months. The PSA confirmed the robustness of the analyses and reinforced that SIRT with Y-90 resin microspheres are highly likely to be cost-effective compared with sorafenib at potentially relevant WTP thresholds (i.e. no explicit WTP threshold exists in Brazil, so the threshold of three times the GDP [R$135,761 per QALY] and the maximum accepted ICER in Brazil [R$280,235 per QALY] as of 2017 were considered relevant)Citation79–82. Scenario analyses further underlined that SIRT with Y-90 resin microspheres is cost-effective compared with sorafenib: in spite of the lack of a specific WTP threshold in Brazil, all scenarios but one resulted in an ICER below potentially relevant WTP thresholdsCitation79–82.
This study had a number of strengths. The model was developed following Brazilian Guidelines for Economic Evaluation and ISPOR Modeling Good Research Practices, and the inputs and assumptions were reviewed and validated by local clinicians in Brazil, which represents an important strength of this studyCitation57,Citation58. The approach to modeling survival curves, reproducing what the NICE TA688 AG did and following NICE DSU guidelines, represents another key strengthCitation60,Citation69. The OWSA demonstrated that the average number of SIRT interventions, per patient, was an important model driver. The base case value (1.01 treatments on average) was derived from a local source in Brazil (Albert Einstein Hospital), which can be considered a strength as it reflects local practice. It is true, however, that further research is required to confirm this value. Nevertheless, a substantial number of scenario analyses were performed to evaluate the impact of parameter uncertainty, including the average number of SIRT interventions, and all supported the robustness of the results.
This analysis also had several limitations. First, the treatment landscape for HCC is evolving rapidly and, since the submission of this model to ANS, other systemic therapies such as lenvatinib and atezolizumab plus bevacizumab have become available for this patient population in Brazil. However, to the authors’ knowledge no head-to-head RCTs exist for SIRT versus these new treatments, and further work needs to be conducted to indirectly compare all these treatment options and estimate their cost-effectiveness. Utility estimates in the economic model were initially mapped from the EORTC QLQ-C30 to the EQ-5D, and then mean health state values were estimated with multivariable regression models using UK general population weightsCitation60. Following ISPOR Good Practices, these utilities were then adjusted with age-related population norms. Due to a lack of data specific to Brazil, adjustment factors were derived from the general population in Argentina in the base case. Not using Brazil-specific population norms represents a limitation of the analysis, although sensitivity analyses demonstrated the impact of population norms on model results is minimal. Similarly, the lack of availability of local data in Brazil on a number of other inputs represents a limitation. For instance, data on subsequent treatments were taken from a clinician survey in the UK, which may differ from actual practice in Brazil. Despite all these assumptions, clinicians in Brazil validated the data used and acknowledged the challenges associated with capturing current local practices. Also, the data used in this model have been validated by NICE and, consequently the assumptions mentioned above were deemed reasonable. Nevertheless, and because of the uncertainty around subsequent treatments, the influence of these on model results was tested in sensitivity analyses.
Importantly, the whole range of benefits that could be generated by the adoption of SIRT using Y-90 resin microspheres in Brazil has not been captured by this study. For example, for those patients who do go on to achieve long-term disease control with TCIs following SIRT with Y-90 resin microspheres, a substantial economic benefit can be expected in terms of reduced disease management costs and reduced costs due to lower premature mortality, lower lost productivity and reduced use of resources. This phenomenon was confirmed in this analysis and is in line with the results published by Pollock et al. who developed a cost model to compare the costs of systemic treatment with sorafenib or lenvatinib versus SIRT from the payer perspective in France, Italy, Spain, and the UKCitation83. In relation to sorafenib, SIRT with Y-90 resin microspheres achieved cost savings in all four countries, in which the costs of the microspheres and procedure were more than offset by reductions in the costs of drugs and particularly the lower costs of treating AEs; cost savings ranged from 5.4% to 24.9% of total expenditure from the payer perspectiveCitation83. The results from the current model are also aligned with the majority of economic evaluations available in the literature concluding that SIRT using Y-90 resin microspheres is cost-effective, including studies in the UK, US, and ItalyCitation50,Citation67,Citation84–87. In Italy, interestingly, two studies demonstrated that SIRT is highly likely to be cost-effective or even dominant for advanced HCC patients based on real-world data collected locallyCitation84,Citation87. One study in the UK, on the other hand, indicated that SIRT is likely to be less costly and less effective than sorafenib; however, the authors concluded that SIRT represents value for money for the whole advanced HCC population and that results are likely to look more favorable for the subgroup of patients with low tumor burden and good liver functionCitation88.
Furthermore, even though traditionally work-up and administration of SIRT would be scheduled as separate appointments, practice using SIRT with Y-90 resin microspheres is evolving to a single appointment for both procedures, either on the same day or on the same hospital admission, reducing the burden of treatment to patients as well as the costs to the healthcare systemsCitation83. This is facilitated by the newly introduced Order-Map-Treat program, which allows ordering of the Y-90 resin microspheres prior to the treatment day, followed by the mapping and treatment occurring on the same dayCitation89. The logistical, economic, and environmental benefits of this program have been demonstrated in a study conducted in the UK, and there is no reason to think Brazil could not benefit from this similarly in the futureCitation90. Additionally, the FLEXdose Delivery Program facilitates the same-day treatment by allowing the patient-specific dose to be drawn up from the shipping vial on the day, providing flexibility in timing and dosing strategy that may change from work-up to treatmentCitation91. The vial can also be split to achieve the optimum dosing per patient (sparing healthy liver parenchyma while maximizing dose to tumor[s]), and multiple liver tumors (single lobe or bilobar disease) can be treated super-selectively in a single session.
In Brazil, approximately 24.5% of the population have access to healthcare through private plans, meaning that only 24.5% of the population can potentially benefit from having access to SIRT. The adoption of SIRT in the public health system would not only help realize all the potential benefits that SIRT with Y-90 resin microspheres can bring, but also resolve this particular situation of inequitable access to healthcare.
Conclusions
The results from these analyses indicate that SIRT with Y-90 resin microspheres is cost-effective compared with sorafenib from the payer perspective in Brazil.
Transparency
Author contributions
IA, VKB, and SS conceptualized the study; IA analyzed the data and developed the model; LSP, FN, DB, FLG, ALA, and JMM validated the approach, local data, and assumptions; all authors interpreted the results; IA drafted the manuscript; all authors revised the manuscript and approved its final version before being submitted for publication. No authors meeting the authorship criteria have been omitted. All named authors meet the International Committee of Medical Journal Editors (ICMJE) criteria for authorship for this article, take responsibility for the integrity of the work as a whole, and have given their approval for this version to be published.
Reviewer disclosures
Peer reviewers on this manuscript have received an honorarium from JME for their review work but have no other relevant financial relationships to disclose.
Previous presentations
This work was presented at the HTAi 2022 Annual Meeting in Utrecht, Netherlands.
Supplemental Material
Download MS Word (192.9 KB)Acknowledgements
None reported.
Declaration of funding
This study was funded by Sirtex Medical United Kingdom Ltd.
Declaration of financial/other relationships
IA, VKB, and SS are full-time employees of Sirtex at the time of manuscript preparation. ALA has provided paid health economics consulting services for Sirtex. FN, DB, GL, and JMM received consultancy fees from Sirtex. LSP declares no financial or other interests.
Data availability statement
The data used in this study which were derived from published sources, including data from published Kaplan-Meier curves from the NICE TA688 appraisal, can be made available upon request.
References
- Crissien AM, Frenette C. Current management of hepatocellular carcinoma. Gastroenterol Hepatol (NY). 2014;10(3):153–161.
- Kumar M, Panda D. Role of supportive care for terminal stage hepatocellular carcinoma. J Clin Exp Hepatol. 2014;4(Suppl 3): s 130–9.
- Sun VC-Y, Sarna L. Symptom management in hepatocellular carcinoma. Clin J Oncol Nurs. 2008;12(5):759–766.
- Rich NE, Yopp AC, Singal AG. Medical management of hepatocellular carcinoma. J Oncol Pract. 2017;13(6):356–364.
- Dimitroulis D, Damaskos C, Valsami S, et al. From diagnosis to treatment of hepatocellular carcinoma: an epidemic problem for both developed and developing world. World J Gastroenterol. 2017;23(29):5282–5294.
- Fan S, Eiser C, Ho M. Health-Related quality of life in patients with hepatocellular carcinoma: a systematic review. Clin Gastroenterol Hepatol. 2010;8(7):559–564.e10.
- Zabora J, BrintzenhofeSzoc K, Curbow B, et al. The prevalence of psychological distress by cancer site. Psychooncology. 2001;10(1):19–28.
- Kohn CG, Singh P, Korytowsky B, et al. Humanistic and economic burden of hepatocellular carcinoma: systematic literature review. Am J Manag Care. 2019;25(2):SP61–SP73.
- Shih W-MJ, Hsiao P-J, Chen M-L, et al. Experiences of family of patient with newly diagnosed advanced terminal stage hepatocellular cancer. Asian Pac J Cancer Prev. 2013;14(8):4655–4660.
- Gondek K, Lang K, Danchenko N, et al. Economic costs of hepatocellular carcinoma in the United States. J Clin Oncol. 2008;26(15_suppl):6555–6555.
- Scalone L, Fagiuoli S, Ciampichini R, et al. The societal burden of chronic liver diseases: results from the COME study. BMJ Open Gastroenterol. 2015;2(1):e000025–e000025.
- European Association for the Study of the Liver. Electronic address: [email protected]; European Association for the Study of the Liver. EASL clinical practice guidelines: management of hepatocellular carcinoma. J Hepatol. 2018;69(1):182–236.
- The Global Cancer Observatory (International Agency for Research on Cancer - World Health Organization). Brazil fact sheet [Internet]. 2021. [cited 2021 Jun 24]. Available from: https://gco.iarc.fr/today/data/factsheets/populations/76-brazil-fact-sheets.pdf.
- Fernandes GDS, Campos D, Ballalai A, et al. Epidemiological and clinical patterns of newly diagnosed hepatocellular carcinoma in Brazil: the need for liver disease screening programs based on real-world data. J Gastrointest Cancer. 2021;52(3):952–958.
- Vogel A, Cervantes A, Chau I, et al. Hepatocellular carcinoma. ESMO clinical practice guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2021;29(Suppl 4):iv238–iv255.
- Chagas AL, Mattos AA, de Carrilho FJ, et al. Brazilian society of hepatology updated recommendations for diagnosis and treatment of hepatocellular carcinoma. Arq Gastroenterol. 2020;57(suppl 1):1–20.
- Sociedade Brasileira de Oncologia Clínica. Carcinoma hepatocelular. Diretrizes 2021 - Actualização. 2021; [cited 2021 Jul 8]. Available from: https://www.scielo.br/j/ag/a/jnWVcf9QdnNQNgsYQ8KxjBk/
- Díaz LA, Barrera MF. Clasificación Barcelona Clinic Liver Cancer (BCLC) de carcinoma hepatocelular. Gastroenterol latinoam. 2015;6(1):63–68.
- Llovet JM, Brú C, Bruix J. Prognosis of hepatocellular carcinoma: the BCLC staging classification. Semin Liver Dis. 1999;19(3):329–338.
- Oliveri RS, Wetterslev J, Gluud C. Transarterial (chemo)embolisation for unresectable hepatocellular carcinoma. Cochrane Database Syst Rev. 2011;(3):CD004787.
- Forner A, Llovet JM, Bruix J. Chemoembolization for intermediate HCC: is there proof of survival benefit? J Hepatol. 2012;56(4):984–986.
- Ahmed S, de Souza NN, Qiao W, et al. Quality of life in hepatocellular carcinoma patients treated with transarterial chemoembolization. HPB Surg. 2016;2016;2016:6120143.
- Hinrichs JB, Hasdemir DB, Nordlohne M, et al. Health-related quality of life in patients with hepatocellular carcinoma treated with initial transarterial chemoembolization. Cardiovasc Intervent Radiol. 2017;40(10):1559–1566.
- Steel JL, Eton DT, Cella D, et al. Clinically meaningful changes in health-related quality of life in patients diagnosed with hepatobiliary carcinoma. Ann Oncol. 2006;17(2):304–312.
- Pitton MB, Kloeckner R, Ruckes C, et al. Randomized comparison of selective internal radiotherapy (SIRT) versus drug-eluting bead transarterial chemoembolization (DEB-TACE) for the treatment of hepatocellular carcinoma. Cardiovasc Intervent Radiol. 2015;38(2):352–360. Apr
- Megías Vericat JE, García Marcos R, López Briz E, et al. Trans-arterial chemoembolization with doxorubicin-eluting particles versus conventional trans-arterial chemoembolization in unresectable hepatocellular carcinoma: a study of effectiveness, safety and costs. Radiologia. 2015;57(6):496–504.
- Marelli L, Stigliano R, Triantos C, et al. Transarterial therapy for hepatocellular carcinoma: which technique is more effective? A systematic review of cohort and randomized studies. Cardiovasc Intervent Radiol. 2007;30(1):6–25.
- Kolligs FT, Bilbao JI, Jakobs T, et al. Pilot randomized trial of selective internal radiation therapy vs. chemoembolization in unresectable hepatocellular carcinoma. Liver Int. 2015;35(6):1715–1721.
- Fateen W, Khan F, O'Neill RJ, et al. Healthcare costs of transarterial chemoembolization in the treatment of hepatocellular carcinoma. J Hepatocell Carcinoma. 2017;4:123–130.
- Vilgrain V, Pereira H, Assenat E, et al. Efficacy and safety of selective internal radiotherapy with yttrium-90 resin microspheres compared with sorafenib in locally advanced and inoperable hepatocellular carcinoma (SARAH): an open-label randomised controlled phase 3 trial. Lancet Oncol. 2017;18(12):1624–1636.
- Gill J, Baiceanu A, Clark PJ, et al. Insights into the hepatocellular carcinoma patient journey: results of the first global quality of life survey. Future Oncol. 2018;14(17):1701–1710.
- Llovet JM, Ricci S, Mazzaferro V, et al. Sorafenib in advanced hepatocellular carcinoma. N Engl J Med. 2008;359(4):378–390.
- Brunocilla PR, Brunello F, Carucci P, et al. Sorafenib in hepatocellular carcinoma: prospective study on adverse events, quality of life, and related feasibility under daily conditions. Med Oncol. 2013;30(1):345.
- Cabrera R. Multicenter, randomized pilot study of the effect of sorafenib dosing schedule on tolerability and drug delivery. Hepatology. 2017;66(SI):731A–7732A.
- Brose MS, Frenette CT, Keefe SM, et al. Management of sorafenib-related adverse events: a clinician’s perspective. Semin Oncol. 2014;41(Suppl 2):S1–S16.
- Pereira H, Bouattour M, Dioguardi Burgio M, et al. Health-related quality of life in locally advanced hepatocellular carcinoma treated by either radioembolisation or sorafenib (SARAH trial). Eur J Cancer. 2021;154:46–56.
- Kudo M, Finn RS, Qin S, et al. Lenvatinib versus sorafenib in first-line treatment of patients with unresectable hepatocellular carcinoma: a randomised phase 3 non-inferiority trial. Lancet. 2018;391(10126):1163–1173.
- Finn RS, Qin S, Ikeda M, et al. Atezolizumab plus bevacizumab in unresectable hepatocellular carcinoma. N Engl J Med. 2020 May 13;382(20):1894–1905.
- de Castro T, Jochheim LS, Bathon M, et al. Atezolizumab and bevacizumab in patients with advanced hepatocellular carcinoma with impaired liver function and prior systemic therapy: a real-world experience. Ther Adv Med Oncol. 2022;14:17588359221080298.
- D’Alessio A, Fulgenzi CAM, Nishida N, et al. Preliminary evidence of safety and tolerability of atezolizumab plus bevacizumab in patients with hepatocellular carcinoma and Child-Pugh a and B cirrhosis: a real-world study. Hepatology. 2022;76(4):1000–1012.
- Lo SH, Sharma R, Costentin CE, et al. Patient preferences for advanced hepatocellular carcinoma treatment: a multicountry stated preference study. Future Oncol. 2021;17(32):4275–4287.
- Miller JC, Blaszkowsky LS, Kalva SP. Selective internal radiation therapy—tackling the tumor, sparing the organ. US Oncol Rev. 2008;4(1):68–71. Available from: https://touchoncology.com/wp-content/uploads/sites/2/2015/07/private_articles_18385_pdf_kalva.pdf
- Sundram FX, Buscombe JR. Selective internal radiation therapy for liver tumours. Clin Med (Lond). 2017;17(5):449–453
- Chow PKH, Gandhi M, Tan S-B, et al. SIRveNIB: selective internal radiation therapy versus sorafenib in Asia-Pacific patients with hepatocellular carcinoma. J Clin Oncol. 2018;36(19):1913–1921. Jul
- Helmberger T, Golfieri R, Pech M, et al. Clinical application of trans-arterial radioembolization in hepatic malignancies in Europe: first results from the prospective multicentre observational study CIRSE Registry for SIR-Spheres Therapy (CIRT). Cardiovasc Intervent Radiol. 2021;44(1):21–35.
- Loffroy R, Ronot M, Greget M, et al. Short-term safety and quality of life outcomes following radioembolization in primary and secondary liver tumours: a multi-Centre analysis of 200 patients in France. Cardiovasc Intervent Radiol. 2021;44(1):36–49.
- Frantz S, Matsuoka L, Vaheesan K, et al. Multicenter evaluation of survival and toxicities of hepatocellular carcinoma following radioembolization: analysis of the RESiN registry. J Vasc Interv Radiol. 2021;32(6):845–852.
- Gramenzi A, Golfieri R, Mosconi C, et al. Yttrium-90 radioembolization vs sorafenib for intermediate-locally advanced hepatocellular carcinoma: a cohort study with propensity score analysis. Liver Int. 2015;35(3):1036–1047.
- de la Torre MA, Buades-Mateu J, de la Rosa PA, et al. A comparison of survival in patients with hepatocellular carcinoma and portal vein invasion treated by radioembolization or sorafenib. Liver Int. 2016;36(8):1206–1212.
- Shaya FT, Breunig IM, Seal B, et al. Comparative and cost effectiveness of treatment modalities for hepatocellular carcinoma in SEER-Medicare. Pharmacoeconomics. 2014;32(1):63–74. Jan
- Edeline J, Crouzet L, Campillo-Gimenez B, et al. Selective internal radiation therapy compared with sorafenib for hepatocellular carcinoma with portal vein thrombosis. Eur J Nucl Med Mol Imaging. 2016;43(4):635–643.
- Gaia S, Tabone M, Cantamessa A, et al. 257 Sorafenib versus radioembolization with y90: clinical evaluation and survival in patients with advanced hepatocellular carcinoma and cirrhosis. J Hepatol. 2013;58:S109–S110.
- Cantamessa A, Gaia S, Tabone M, et al. Sorafenib versus Y90-Radioembolization: a preliminary assessment of tolerability and survival in advanced Mono-lobar hepatocellular carcinoma. Dig Liver Dis. 2014;46:e64. Available from:
- Martelletti C, Gesualdo M, Carucci P, et al. The Piedmont-Aosta valley oncology network experience in locally advanced HCC with intrahepatic neoplastic portal vein thrombosis: y 90-radioembolization versus sorafenib. Dig Liver Dis. 2020;52:e53.
- Palmer DH, Hawkins NS, Vilgrain V, et al. Tumor burden and liver function in HCC patient selection for selective internal radiation therapy: SARAH post-hoc study. Future Oncol. 2020;16(1):4315–4325.
- Agência Nacional de Saúde Suplementar. Rol de procedimentos e eventos em saúde. Anexo II. Diretrizes de utilização para cobertura de procedimentos na saúde suplementar. RN 465/2021 e suas alterações [Internet]. [cited 2023 Jan 17]. Available from: https://www.gov.br/ans/pt-br/arquivos/assuntos/consumidor/o-que-seu-plano-deve-cobrir/Anexo_II_DUT_2021_RN_465.2021_tea.br_RN473_RN477_RN478_RN480_RN513_RN536_RN537_RN538_RN539_RN540_RN541_RN542.pdf.
- Ministério da Saúde (Brasil). Diretrizes metodológicas: diretriz de avaliação econômica. Brasília: Ministério da Saúde; 2014.
- Caro JJ, Briggs AH, Siebert U, et al. Modeling good research practices--overview: a report of the ISPOR-SMDM modeling good research practices task force-1. Value Heal [Internet]. 2012;15(6):796–803. Available from:
- Sirtex. Questionnaire for clinician validation in Brazil. Data on file. 2021.
- Walton M, Wade R, Claxton L, et al. Selective internal radiation therapies (SIRT) for treating hepatocellular carcinoma [ID1276]. Assessment Report [Internet]. 2019. Available from: https://www.nice.org.uk/guidance/ta688/documents/assessment-report.
- Briggs A, Claxton K, Sculpher M. Decision modelling for health economic evaluation. New York (NY): Oxford University Press; 2006.
- National Institute for Health and Care Excellence. Guide to the Methods of Technology Appraisal [Internet]. London; 2013. [cited 2021 May 14]. Available from: https://www.nice.org.uk/process/pmg9/resources/guide-to-the-methods-of-technology-appraisal-2013-pdf-2007975843781.
- Scottish Medicine Consortium. 2nd Re-submission: sorafenib 200mg film-coated tablets (Nexavar®) SMC No. (482/08) [Internet]. 2015. [cited 2021 Apr 9]. p. 1–12. Available from: https://www.scottishmedicines.org.uk/media/2325/sorafenib_nexavar_2nd_resubmission_final_dec_2015_for_website.pdf.
- National Institute for Health and Care Excellence. Sorafenib for treating advanced hepatocellular carcinoma. Technology appraisal guidance [TA474] [Internet]. 2017. [cited 2021 Apr 9]. Available from: https://www.nice.org.uk/guidance/ta474.
- National Institute for Health and Care Excellence. Lenvatinib for untreated advanced hepatocellular carcinoma. Technology appraisal guidance [TA551] [Internet]. 2018 [cited 2021 Apr 9]. Available from: https://www.nice.org.uk/guidance/ta551.
- National Institute for Health and Care Excellence. Regorafenib for previously treated hepatocellular carcinoma [ID1519] (rapid review TA514) [Internet]. 2018. [cited 2021 Apr 9]. Available from: https://www.nice.org.uk/guidance/ta555/documents/committee-papers.
- Muszbek N, Remak E, Evans R, et al. Cost-utility analysis of selective internal radiation therapy with Y-90 resin microspheres in hepatocellular carcinoma. Future Oncol. 2021;17(9):1055–1068.
- National Institute for Health and Care Excellence. Selective internal radiation therapies for treating hepatocellular carcinoma. Technology appraisal guidance [TA688] [Internet]. 2021. [cited 2021 May 17]. Available from: https://www.nice.org.uk/guidance/ta688.
- Latimer NR. NICE DSU Technical Support Socument 14: survival analysis for economic evaluations alongside clinical trials - extrapolation with patient-level data [Internet]. 2013. Available from: http://nicedsu.org.uk/wp-content/uploads/2016/03/NICE-DSU-TSD-Survival-analysis.updated-March-2013.v2.pdf.
- Guyot P, Ades AE, Ouwens MJNM, et al. Enhanced secondary analysis of survival data: reconstructing the data from published Kaplan-Meier survival curves. BMC Med Res Methodol. 2012;12(1):9. Available from:
- Coordenação de Avaliação e Monitoramento de Tecnologias, Ministério da Saúde (Brasil). Sorafenibe para carcinoma hepatocelular (CHC) avançado irressecável [Internet]. 2018 [cited 2021 May 18]. Available from: http://conitec.gov.br/images/Relatorios/2018/Relatorio_Sorafenibe_CHC-Avancado.pdf.
- Ganten TM, Stauber RE, Schott E, et al. Sorafenib in patients with hepatocellular carcinoma-results of the observational INSIGHT study. Clin Cancer Res. 2017;23(19):5720–5728.
- Hospital Israelita Albert Einstein. Data on file. 2021.
- Câmara de Regulação do Mercado de Medicamentos (CMED) - Agência Nacional de Vigilância Sanitária (Anvisa). Listas de preços de medicamentos [Internet]. 2021. [cited 2021 May 19]. Available from: https://www.gov.br/anvisa/pt-br/assuntos/medicamentos/cmed/precos.
- Campioni M, Agirrezabal I, Hajek R, et al. Methodology and results of real-world cost-effectiveness of carfilzomib in combination with lenalidomide and dexamethasone in relapsed multiple myeloma using registry data. Eur J Health Econ. 2020;21(2):219–233. Mar
- Chirivella I, Bermejo B, Insa A, et al. Optimal delivery of anthracycline-based chemotherapy in the adjuvant setting improves outcome of breast cancer patients. Breast Cancer Res Treat. 2009;114(3):479–484.
- Delea TE, Amdahl J, Chit A, et al. Cost-effectiveness of lapatinib plus letrozole in her2-positive, hormone receptor-positive metastatic breast cancer in Canada. Curr Oncol. 2013;20(5):e371–e387.
- Hryniuk WM, Goodyear M. The calculation of received dose intensity. J Clin Oncol. 1990;8(12):1935–1937.
- Ribeiro RA, Neyeloff JL, Marcolino MA, et al. Cost-Effectiveness threshold in Brazil through a league-table approach: systematic review. Value Heal. 2017;20(9):A859–A860. Available from:
- Soarez PC, De Novaes HMD. Cost-effectiveness thresholds and the Brazilian Unified National Health System. Cad Saude Publica. 2017;33(4):e00040717.
- The World Bank. GDP per capita (current US$) - Brazil [Internet]. 2021. [cited 2021 Jun 22]. Available from: https://data.worldbank.org/indicator/NY.GDP.PCAP.CD?locations=BR.
- Marseille E, Larson B, Kazi DS, et al. Thresholds for the cost-effectiveness of interventions: alternative approaches. Bull World Health Organ. 2015;93(2):118–124. Available from: https://pubmed.ncbi.nlm.nih.gov/25883405
- Pollock RF, Colaone F, Guardiola L, et al. A cost analysis of SIR-Spheres yttrium-90 resin microspheres versus tyrosine kinase inhibitors in the treatment of unresectable hepatocellular carcinoma in France, Italy, Spain and the UK. J Med Econ. 2020;23(6):593–602.
- Lucà MG, Nani R, Schranz M, et al. Treatment of hepatocellular carcinoma: a cost analysis of yttrium-90 transarterial radioembolization versus sorafenib. Future Oncol. 2018;14(8):727–735.
- Palmer D, Ross P, Shah T, et al. Cost effectiveness of selective internal radiation therapy (SIRT) with Y-90 resin microspheres versus sorafenib in Barcelona Clinic Liver Cancer (BCLC) stage C hepatocellular carcinoma patients in the UK. Ann Oncol. 2017;28:v239–v240. Available from:
- Zori AG, Ismael MN, Firpi-Morell R, et al. Y90 radioembolization is a cost-effective bridging therapy for hepatocellular carcinoma: 1035. 2017;112:S574. Available from: https://journals.lww.com/ajg/Fulltext/2017/10001/Y90_Radioembolization_is_a_Cost_Effective_Bridging.1036.aspx
- Rognoni C, Ciani O, Sommariva S, et al. Real-world data for the evaluation of transarterial radioembolization versus sorafenib in hepatocellular carcinoma: a cost-effectiveness analysis. Value Health. 2017;20(3):336–344. Mar
- Claxton L, Walton M, Sharif-Hurst S, et al. The cost-effectiveness of selective internal radiation therapies compared with sorafenib for treating advanced unresectable hepatocellular carcinoma in the United Kingdom. Value Health. 2022;25(5):787–795.
- Sirtex Medical Inc. Order-Map-Treat Program [Internet]. 2020. [cited 2021 Sep 20]. Available from: https://www.sirtex.com/media/169579/apm-us-004-12-20_order-map-treat-brochure-us_finalpdf-1.pdf.
- Pollock RF, Shergill S, Carion PL, et al. Advances in delivery of Selective Internal Radiation Therapy (SIRT): economic and logistical effects of same-stay work-up and procedure in the treatment of unresectable liver tumors in England. Adv Ther. 2023;40(1):294–309.
- Sirtex Medical Inc. FLEXdose Delivery Program [Internet]. [cited 2021 Sep 10]. Available from: https://www.sirtex.com/us/clinicians/flexdose-delivery-program/.