Abstract
Aims
To capture the economic and healthcare resource utilization (HCRU) burden in older adults due to respiratory syncytial virus (RSV) infection.
Methods
An electronic literature search of PubMed, Embase, the Cochrane Library, PsycINFO, and EconLit was conducted for studies of the cost and HCRU outcomes of RSV infection in adult patients, with no language or country restrictions. The search dates for the primary studies were January 1, 2002–May 18, 2022. The methodological quality of included studies was assessed using a modification of the Critical Appraisal Skills Programme (CASP) checklist for economic studies and the Drummond checklist.
Results
Forty-two studies were identified that reported cost or HCRU data associated with RSV infections, with geographic locations across North America, South America, Europe, Asia, and Oceania. Generally, hospitalization costs were highest in the United States (US). Driving factors of increased cost included older age, comorbidities, and length of stay. US studies found that the national direct cost burden of RSV hospitalizations was $1.3 billion for all adults and $1.5–$4.0 billion for adults aged ≥60 years (estimates for other countries were not identified). Studies estimating incremental costs for RSV cases versus controls and costs pre- and post-RSV infection demonstrated higher costs for RSV cases. Hospitalizations accounted for the majority of total costs.
Evidence Limitations and Gaps
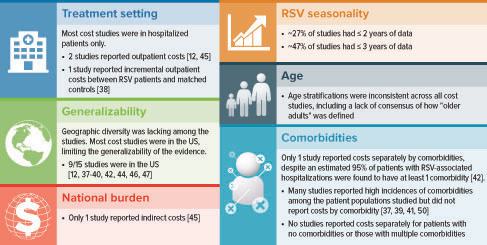
The variability in definitions of cost outcomes, age groups, study seasons, and geographic locations was prohibitive of a meta-analysis and comparisons across studies. Cost and HCRU data were limited per country outside the US, per comorbidity, and in settings other than the inpatient setting. Only one study reported indirect costs, and only the US had national cost burden data.
Conclusion
Despite several data gaps, the economic burden of RSV infections on healthcare systems and payers was found to be substantial, globally, underscoring the need for RSV preventive strategies for reducing this burden.
Introduction
Respiratory syncytial virus (RSV) is a seasonal virus that commonly affects infants and children, but the virus poses a risk for severe disease in adults as wellCitation1,Citation2. Most people experience an RSV infection as an infant or young child and have some immunity to further exposures during their lifespan, but the immunity from childhood exposure does not provide complete or sustained immunityCitation3. In older adults, changes in the immune system and lung function regarding clearance of microbes lead to an inflammatory state that impairs responses to infection and prolongs inflammation after an infection has clearedCitation4. Therefore, older adults in the general population or in long-term care facilities (LTCFs) are susceptible to severe RSV infectionCitation1. In addition, adults with comorbidities such as chronic heart or lung disease, functional disability, frailty, and compromised immune systems are susceptible to severe RSV disease and are more likely to require hospitalization than healthy older adultsCitation1,Citation2,Citation5–9. After older adult patients are diagnosed with RSV infection, their return to pre-RSV respiratory functioning and ability to perform activities of daily living may take several monthsCitation10. Additionally, at hospital discharge, a substantial proportion of older adults or adults with comorbidities require discharge to a skilled nursing facility, rehabilitation facility, or assisted living facility not needed before RSV infectionCitation11–14.
The annual incidence of RSV infection is estimated to be 3–7% in healthy older adults and 4–10% in high-risk adults based on prospective cohort studies conducted in the United States (US) and EuropeCitation2,Citation15. Among adults aged ≥60 years, RSV infection accounted for approximately 470,000 RSV-associated hospitalizations (attack rate: 0.15%) and 33,000 RSV-associated in-hospital deaths (in-hospital case fatality rate: 7.13%) in the community setting of high-income countries in 2019Citation8. In low- or middle-income countries, there is a paucity of data on the burden of RSV disease in older adults; however, available data from prospective cohort studies in India and Thailand suggest a significant burden that increases with age and for persons with certain underlying conditions, such as cardiac and pulmonary diseasesCitation16,Citation17. Although a significant global RSV burden has been documented, the actual burden due to RSV may be even higher. A study estimating the incidence of RSV-associated hospitalization found that it may be 2.2-times that reported across 12 studies in high-income countries in adults ≥65 years of ageCitation18. This conclusion was obtained by adjusting for both the clinical specimen and clinical testing methods. In low- or middle-income countries, more limited access to care and more restricted care-seeking behaviors have been speculated to further underestimate the burden of RSV disease in older adultsCitation19.
RSV circulates year-round but typically peaks in a seasonal pattern, during winter in temperate climates and during the rainy season in tropical climatesCitation20–24. The COVID-19 pandemic impacted the circulation of RSVCitation25. For example, in the 3 years immediately before the COVID-19 pandemic, the US RSV season spanned from October to March and peaked in December. The latest US RSV season (2022–2023) spanned from June to January and peaked in NovemberCitation25.
Although RSV infections have decreased among adults during the COVID-19 pandemicCitation26,Citation27, including adults aged ≥50 yearsCitation26, this decline is likely due to older adults’ adherence to public health infection prevention measures; whether it will be sustained as SARS-CoV-2 infections become endemic is currently unknownCitation26. More recently, in the US, following the COVID-19 pandemic, the reported peak rate of RSV hospitalization in older adults increased approximately twice as much as and occurred 2 months earlier than it did in older adults during the pre-COVID-19 pandemic years, 2017–2018 and 2019–2020Citation28.
A recent systematic literature review (SLR) reported that in approximately 33% of laboratory tests, RSV infection is under-detected in adults tested for RSVCitation29. This estimate may be conservativeCitation30. Under-diagnosis from lack of testing in adults also contributes to the under-ascertainment of RSV infection in adults, although, to our knowledge, this has yet to be quantified in the current literature and may be changing as RSV vaccines become availableCitation29,Citation31.
The costs of management of acute respiratory infections (ARI) among older adults has been estimated in a recent meta-analysisCitation32. However, this study did not report respiratory pathogen-specific management costs. With RSV vaccines for adults aged ≥60 years under development, there will be a need to better understand the cost and healthcare resource burden of RSV infection in adults. This information will be critical for policy decision-makers to assess the value and cost-effectiveness of vaccination strategies among those at risk for severe outcomes due to age and underlying comorbidities. Our objectives in this global SLR were to summarize the economic burden of RSV infection in adults and highlight any critical gaps in the existing literature. It was not designed to capture RSV hospitalization rates, which has been a focus of other recent SLRsCitation8,Citation9,Citation19,Citation29,Citation33,Citation34.
Methods
Study selection, data extraction, and quality assessment
Based on a prespecified protocol (available upon request) and in accordance with the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) statement, a literature search of PubMed, Embase, the Cochrane Library, PsycINFO, and EconLit was conducted for studies of the economic and health-related quality-of-life (HRQoL) outcomes of RSV infection in adult patients (HRQoL findings will be examined in a forthcoming article). The search strategy incorporated Medical Subject Heading or Emtree terms and free-text terms for sources published in the past 20 years, with no language or country restrictions. Search dates for primary studies were January 1, 2002–May 18, 2022. The search included conference abstracts from select organizations () published between 2019 and 2022 that were indexed in Embase or available as abstract books or online, as well as from bibliographies of relevant SLRs.
Figure 1. PRISMA diagram. Abbreviations. ICU, intensive care unit; MV, mechanical ventilation; PRISMA, Preferred Reporting Items for Systematic Reviews and Meta-Analyses. aConferences and years indexed in Embase were as follows: American Thoracic Society (2019–2021 abstracts); CHEST annual meeting (2019–2021 abstracts); Infectious Diseases Society of America (IDWeek) (2019–2021 abstracts); ISPOR (2019–2021 abstracts); CHEST Congress (2019–2020 abstracts). bTwo publicationsCitation14,Citation84 reported on the same population.
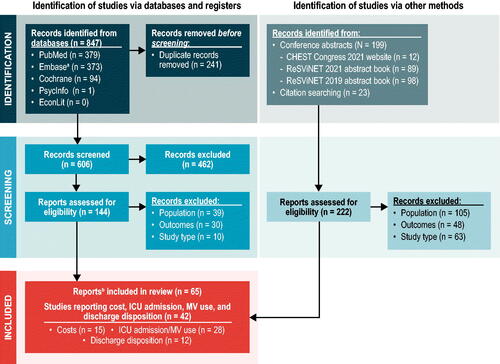
Unique titles and abstracts (level 1) and full texts (level 2) were independently reviewed by two reviewers, with consultation involving a third, senior-level researcher when consensus was not reached. Studies were included that reported economic outcomes for adults (aged ≥18 years) with or without comorbidities. There were no inclusion restrictions for interventions. Economic outcomes of interest were direct and indirect costs of RSV infection; healthcare resource utilization (HCRU) related to RSV infection, including intensive care unit (ICU) admission and mechanical ventilation (MV) use and the requirement for a higher level of care after hospital discharge. A broad range of study designs were included (Supplementary Table A1), but general reviews were excluded. For ICU admission and MV use, only studies with ≥100 patients with RSV infection were included.
Study details and data were extracted into tables and independently verified by a second reviewer not involved in the data extraction. Data extracted from the economic studies included study type and details; patient population characteristics (age, comorbidities); patient setting (inpatient, outpatient, any setting); follow-up period; cost outcomes; and proportions of patients needing ICU admission, MV, or a higher level of care at discharge. All cost outcomes were converted and inflated to 2022 US$ to compare costs. Cost conversions were calculated by multiplying reported costs by the corresponding inflation factor from the US Bureau of Labor Statistics Consumer Price Index Inflation Calculator (Supplementary Table A2). When currency year was not reported, it was assumed that it was the same as the publication year.
We included studies that reported HCRU even when the study’s purpose was about clinical outcomes instead of economic outcomes. Therefore, the quality assessment of included studies was evaluated using a modification of the Critical Appraisal Skills Programme (CASP) checklist for economic studiesCitation35 and the Drummond checklistCitation36, which took into account the primary research aims as well as the cost and HCRU aims.
Results and discussion
A total of 828 sources were reviewed for eligibility, and 65 sources were included (55 articles and 10 conference abstracts or study registry entries) (). Eligible studies were identified across North America, South America, Europe, Asia, and Oceania. This article summarizes 42 economic burden studies: 15 presented the cost burden of adult RSV infectionCitation12,Citation37–50, 28 presented ICU admission and/or MV useCitation2,Citation11,Citation13,Citation14,Citation39,Citation41,Citation43,Citation48,Citation50–69, and 12 presented the need for a higher level of care at dischargeCitation2,Citation11–14,Citation53,Citation54,Citation56,Citation70–73. Quality assessment of these 42 studies is presented in the Supplementary Material. Economic models were excluded from this manuscript because many of the cost inputs in the models were influenza costs, not RSV.
Cost studies
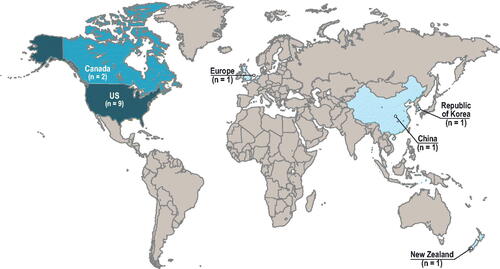
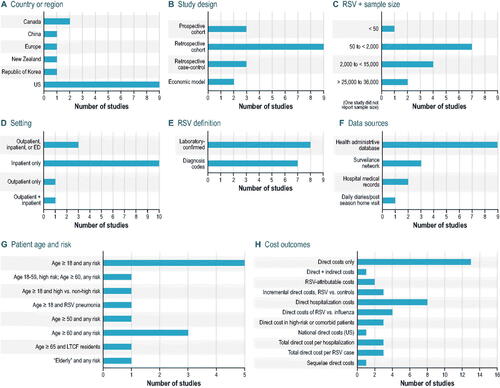
In this SLR, 15 studies reported the cost associated with RSV infection in adults in a primary study of general populationsCitation12,Citation37–50. The studies were conducted across one region and five different countries, with the majority being in the US: one in EuropeCitation45 and nine in the USCitation12,Citation37–40,Citation42,Citation44,Citation46,Citation47, two in CanadaCitation43,Citation49, one in ChinaCitation41, one in New ZealandCitation48, and one in the Republic of KoreaCitation50 ().
Of the 15 studies, 12 were retrospective and three were prospectiveCitation42,Citation43,Citation45. For the studies that specified, RSV diagnosis was confirmed by either laboratory assessment (n = 8) or use of International Classification of Diseases (ICD) codes (n = 7) (). In Bosco et al.Citation40, the study evaluated excess cardiorespiratory hospitalizations (ICD codes) during a time of high RSV circulation, and patients specifically did not have a diagnosis of RSV infection because the study aimed to evaluate sequelae of RSV infection. Five studies reported comorbidities, with the highest incidences being cardiovascular disease (CVD) and chronic pulmonary diseaseCitation37,Citation39,Citation41,Citation42,Citation50. Two studies reported costs for high-risk patients, characterized as older adults with chronic cardiopulmonary conditions and those who were immunosuppressedCitation12,Citation46.
Figure 3. Design features of cost studies (n = 15). Abbreviations. ED, emergency department; LTCF, long-term care facilities; RSV, respiratory syncytial virus; US, United States.
identifies the cost studies by author (year) according to the types of cost outcomes reported.
Figure 4. Cost studies: Venn diagram of cost types, treatment settings, and outcome types. Abbreviations. Flu, influenza; RSV, respiratory syncytial virus; US, United States. aConference abstract.
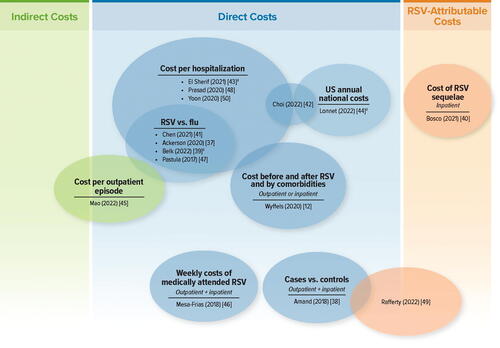
Broad comparisons across the studies were difficult because of the wide variation in cost outcomes, period over which costs were collected, cost year, country, types of care setting, and patient characteristics, as well as the overall lack of consistency in metrics across studies (e.g. costs per hospitalization, costs per RSV case, pre- and post-RSV costs over a fixed period). Therefore, the studies were grouped into sections where they report similar cost outcomes (e.g. hospitalization costs or incremental costs versus control cohorts).
Direct national cost burden
Two studies estimated the national direct cost burden due to RSV hospitalizations in the US for two different age groups; no studies outside the US estimated national cost burden ().
Table 1. Direct US national cost burden.
Choi et al.Citation42 used the mean total cost of RSV hospitalization, RSV incidence rate/100,000 persons, and 2019 US population estimates to obtain an estimate of $1.3 billion in RSV hospitalization costs annually in the US for persons aged ≥18 years. Mean hospitalization costs were highest among the youngest age group (18–49 years), but the greatest national cost burden was for those aged ≥65 years ($810,932,993) (). This is a result of the RSV incidence for persons aged ≥65 years being 17-times that of 18–49-year-olds and 3-times that of 50–64-year-olds.
Lonnet et al.Citation44 used RSV hospitalization costs from 2008–2017 to obtain an estimate of $1.5–$1.8 billion or $3.3–$4.0 billion for persons aged ≥60 years, depending on RSV incidence estimates. The lower estimate agrees with Choi et al.Citation42, but Choi et al.Citation42 used only used two data years in their estimate versus the 10 data years in Lonnet et al.Citation44.
Incremental costs
Three US studies and one Canadian study presented incremental costs for patients with RSV, either comparing costs pre- and post-RSV diagnosisCitation12,Citation46 or costs of RSV cases compared with those of a control groupCitation38,Citation49 (). Older patient populations had a significantly higher incidence of hospitalization, which was the driving factor for increased incremental costsCitation38,Citation46.
Table 2. Incremental costs for RSV patients versus controls.
Mesa-Frias et al.Citation46, a US-based study, found increases in mean weekly all-cause costs when comparing pre-RSV diagnosis in weeks 2–8 and post-RSV diagnosis in week 1 across patient age groups, with the highest increase ($18,062) among patients aged ≥85 years. Mesa-Frias et al.Citation46 reported a significant number of cardiovascular comorbidities in this age group, which may have resulted in more complex and costly RSV cases. Mesa-Frias et al.Citation46 also found increases in all-cause costs at 1 week pre-RSV diagnosis compared with 2–8 weeks pre-RSV diagnosis, which could be attributed to the latency between onset of symptoms, which resulted in HCRU, and diagnosis confirmation via ICD codesCitation46. Accounting for cost increases pre-RSV diagnosis, the total incremental cost of RSV from 1 week pre-RSV diagnosis to week 8 post-RSV diagnosis was found to be as high as $19,007 among those aged ≥85 yearsCitation46.
Wyffels et al.Citation12 reported mean total all-cause healthcare costs, comparing the 180-day period pre- and post-RSV diagnosis in patients with versus without high risk (chronic cardiac/pulmonary disease or immunocompromised) for RSV complications in an outpatient or hospitalized setting (see Costs among high-risk patients and patients with comorbidities). The largest incremental difference in total costs pre- and post-180 days from RSV diagnosis was incurred by hospitalized non-high-risk patients ($22,969), while the incremental difference in total costs among hospitalized high-risk patients was less than half thatCitation12. A similar result was seen in the outpatient setting, with incremental costs pre- and post-180 days from RSV diagnosis of $1,932 and $2,487 for high-risk outpatients and non-high-risk outpatients, respectively. It is possible that the incremental costs for high-risk patients were lower than for non-high-risk patients due to higher mortality ratesCitation12. The mortality rates for hospitalized high-risk patients were 4.2% during inpatient stay and 13.4% within 30 days of diagnosis versus 0.0% and 8.1%, respectively, for non-high-risk patients. Another possible explanation is that there may be an overlap in the healthcare resources that high-risk patients used pre- and post-RSV index, resulting in higher overall resource use but a smaller incremental cost, but this is yet to be explored in the literature. For more details on healthcare costs for high-risk patients, see the section Costs among high-risk patients and patients with comorbidities.
One US and one Canadian study calculated total incremental all-cause costs for patients within 12 months of RSV diagnosis versus a control groupCitation38,Citation49. The studies differed greatly in the costs reported, with Rafferty et al.Citation49 reporting costs up to almost 12-times those reported by Amand et al.Citation38. In both studies, the highest mean incremental all-cause cost per RSV case was incurred by older age groups: in Rafferty et al.Citation49, 65–79-year-olds incurred an incremental cost increase of $136,416 versus controls, and in Amand et al.Citation38, 75–84-year-olds incurred an incremental cost increase of $31,381 versus controls. These higher incremental costs among the older population could be attributed to the potential for RSV-related complications triggering additional healthcare visitsCitation49. Rafferty et al.Citation49 noted several key differences between the two studies that could explain the markedly different costs: the US has higher healthcare costs than Canada, which affected the costs of the controls in Amand et al.Citation38; Rafferty et al.Citation49 gathered costs across five RSV seasons, while Amand et al.Citation38 used one RSV season; Rafferty et al.Citation49 used laboratory confirmation to diagnose RSV while Amand et al.Citation38 used ICD-10 codes; and Rafferty et al.Citation49 used a different methodology to match RSV patients with controls, including consideration for comorbidities and geographic location. For both studies, incremental cost increases 1 year after RSV diagnosis may be indicative of sequelae or other long-term health impactsCitation38,Citation49.
Rafferty et al.Citation49 also reported shorter-term incremental costs, with all-cause cost increases up to $27,753 within 30 days of RSV diagnosis. Short-term incremental costs were the highest among adults aged 50–64 years. RSV infection is not as well recognized among this age group compared with children and older adults, which may result in a lack of testing and a delay in treatment. These patients may have had more serious RSV cases due to this delay, resulting in a higher incremental cost than other age groupsCitation49.
Hospitalization costs
Four studies reported all-cause direct costs per RSV hospitalization within the USCitation37,Citation39,Citation42,Citation47 (). Cost estimates varied substantially, ranging from as low as $8,049Citation42 to as high as $58,117Citation47. Outside of the US, RSV hospitalization costs were reported in one study each from CanadaCitation43, ChinaCitation41, New ZealandCitation48, and the Republic of KoreaCitation50.
Table 3. Hospitalization costs.
Of the four US studies, the lowest cost estimates were from Choi et al.Citation42, with mean all-cause direct costs ranging from $8,049–$12,125 per RSV hospitalization, depending on the age group. The highest costs were incurred by the youngest age group of patients, aged 18–49 years. This was attributed to the concept that younger patients may have a higher threshold of illness severity for hospital admittance and would wait until they were sicker or had more complications before seeking hospitalizationCitation42. Belk et al.Citation39 reported all-cause direct costs of $27,757 per RSV hospitalization among elderly patients. (“Elderly” was not defined.) Ackerson et al.Citation37 also reported costs for older adults in a study population with a mean age of 78.4 years. Both Ackerson et al.Citation37 and Belk et al.Citation39 reported high incidence of comorbidities, including chronic heart conditions and chronic pulmonary conditions.
The analysis in Pastula et al.Citation47 captured the broadest timeline of data, with costs extracted across 15 years (1997–2012). The breadth of the data allowed for larger trends to be identified, and the findings are more easily generalized to the total US population than results of smaller studiesCitation47. Additionally, a wider range of seasonal severity was captured, which could explain the difference in costs reported compared with the other two studies, which collected costs across 3.5 yearsCitation37 and 3 yearsCitation39. However, both Ackerson et al.Citation37 and Belk et al.Citation39 examined more recent costs from 2011–2015 and from 2016–2018, respectively, and Pastula et al.Citation47 did not report comorbidities.
Estimates of the cost burden in Canada were most similar to that in the US. El Sherif et al.Citation43 reported a mean direct medical cost of $18,209/RSV case for hospitalized patients of all ages, with the highest cost, $21,487, reported in the oldest age group (≥80 years), which is similar to that in multiple estimates in the USCitation37,Citation42. Overall, cost burden for US patients hospitalized due to RSV infection was substantially higher than that for patients in all other countries, with Canada having the most similar costs to the US and China having the least similar.
Among studies reporting inpatient costs, China had the lowest cost burden, with a median cost of $3,123.44 per hospitalization for adults aged ≥18 yearsCitation41. The Republic of Korea had the second lowest costs per hospitalization, with median medical costs up to $3,314.48 for patients ≥65 yearsCitation50.
Multiple care settings costs
Two studies reported costs for patients in multiple care settingsCitation45,Citation49 ().
Table 4. Multiple care settings costs.
In a European all-care-settings study that followed a community-based cohort, the direct cost to the patient was $14.28 and to the healthcare payer was $6.71/RSV caseCitation45. This was the only study to report costs in all care settings (home, ambulatory, emergency department [ED], hospital) and to report societal costs ($37.54/RSV episode). However, the cohort was characterized as relatively healthy, with no hospitalizations during the observational period of two consecutive RSV seasonsCitation45.
In contrast, much higher costs were reported in a Canadian studyCitation49. The greatest mean all-cause cost per RSV case was incurred by patients aged 65–79 years during the observational period of 1 year ($146,290)Citation49. This age group also had the largest incremental cost increase when compared with controls (see Incremental costs). Patients aged 50–64 years had the second-greatest costs ($121,036). As previously noted, lack of recognition of RSV infection in these populations may have led to delays in receiving treatment and testing, resulting in more severe casesCitation49. Costs included hospitalization, ED visits, and physician billing, and costs were collected over a 365-day period.
Costs among high-risk patients and patients with comorbidities
One study reported costs for patients with cost breakouts by comorbidityCitation42, one study reported costs for immunocompromised (IC) patientsCitation47, and two studies reported mean all-cause costs for patients categorized as high riskCitation12,Citation46 (). “High risk” in both studies was defined as adult patients aged ≥65 years with chronic cardiac disease and/or chronic pulmonary disease and those who were immunosuppressed, as classified by the Centers for Disease Control and PreventionCitation74. Additionally, Wyffels et al.Citation12 included patients who had prior pneumonia on or within 180 days before the date of RSV diagnosis.
Table 5. Costs among high-risk patients and patients with comorbidities.
Mesa-Frias et al.Citation46 limited high-risk patients to the youngest age group and compared their increases in direct medical costs with patients aged 60–64, ≥65, and ≥85 years. The study found that high-risk patients aged 18–59 years had the second highest weekly direct all-cause costs post-RSV diagnosis in week 1 ($10,170), as well as the second-greatest increase in mean weekly all-cause costs post-RSV diagnosis in week 1 versus pre-diagnosis in weeks 2–8 ($8,349), after any-risk patients aged ≥85 years.
Wyffels et al.Citation12 reported mean total all-cause healthcare costs pre- and post-180 days from RSV diagnosis for high-risk patients in both inpatient and outpatient settings. Unsurprisingly, the highest total costs were incurred by hospitalized high-risk patients, with a mean total all-cause healthcare cost of $49,108 post-180 days from RSV diagnosisCitation12. Outpatient high-risk patients incurred total all-cause post-180 days RSV diagnosis costs of $19,330Citation12.
Pastula et al.Citation47 reported mean total direct RSV hospitalization costs for IC patients, which were up to 2.5-times the costs incurred by non-immunocompromised (non-IC) patients: IC patients aged 20–44 years had mean total hospitalization costs of $86,186.76, while non-IC patients of the same age group incurred costs of $32,425.32. The highest mean hospitalization costs ($88,675.80) were among IC patients aged 45–59 years, who reported the highest MV use and longest length of stay (LOS)Citation47. For all patient age groups, IC patients had higher cost of hospitalization due to RSV infection compared with non-IC patients as well as patients with influenzaCitation47. IC patients compared with non-IC patients had an increased death rate during hospitalization (7.3% vs 5.6%) and longer mean LOS (7.3 days vs 5.4 days)Citation47.
Choi et al.Citation42 was the only study to report direct medical costs by comorbidity. Considering costs by comorbidity, patients with CVD incurred an additional $3,237 in costs when compared with patients without CVD. Patients with chronic liver disease had increased costs of $5,883, and patients with chronic kidney disease saw a cost increase of $936. Surprisingly, there was no substantial difference in costs among patients who were immunosuppressed and those who were not. Additionally, costs were lower in patients with chronic lung conditions and those with diabetes, with costs decreasing by $1,569 and $1,537, respectively. Choi et al.Citation42 did not provide insight into the cause for these cost decreases but stated that the main predictors for cost increases were LOS, ICU admission, use of antibiotics, and the presence of four or more comorbidities. As these were simple comparisons of patients with and without a specific comorbidity and most patients (95%) had at least one comorbidity, it may be possible that the groups without chronic lung conditions and without diabetes have a higher prevalence of other expensive comorbidities, such as chronic liver disease.
Sequelae costs
Only one study reported RSV sequelae costs, as considered through RSV-attributable cardiorespiratory hospitalization costs (). It is possible that a primary diagnosis is actually a sequelae of RSV, but the downstream effects of RSV infection are unrecognized without prior testing to identify RSV infection. Due to a lack of routine testing, this may result in an underestimation of the true cost burden of RSV sequelae. Therefore, Bosco et al.Citation40 estimated cardiorespiratory hospitalizations attributable to RSV infection and influenza infection (separately and together) during times when these viruses were circulating, based on surveillance data. The cohort studied were residents of LTCFs who were enrolled in Medicare. National cost-burden estimates for cardiorespiratory hospitalizations attributable to both RSV infection and influenza infection were also reported.
Table 6. Sequelae costs.
Bosco et al.Citation40 found no difference in the mean cost per cardiorespiratory hospitalization when comparing RSV and influenza sequelae, with an average of $10,136/hospitalization across all age groups. This finding is dissimilar to that in three US studies that compared RSV and influenza hospitalization costsCitation37,Citation39,Citation47, which trended toward sizeable direct cost increases in RSV infection when compared with influenza infection (no formal statistical analysis was done).
The national burden of RSV-attributable cardiorespiratory hospitalizations for those in LTCF on Medicare totaled $59.74 million, and the national burden of cardiorespiratory hospitalizations attributable to influenza for those in LTCF on Medicare was $66.62 million due to higher case numbers of influenzaCitation40. These national cost estimates are limited to residents of LTCF on Medicare. The full national burden of RSV-attributable cardiorespiratory hospitalizations is likely to be much higher when including adults of all ages, those in other places of residence, and those with non-Medicare insurance coverage.
RSV infection costs compared with influenza infection costs
RSV infection costs compared with influenza infection costs were presented in three US studiesCitation37,Citation39,Citation47, one Chinese study of hospitalized patientsCitation41, and one European study of outpatientsCitation45 (). Cost comparisons among the US studies trended toward relatively higher costs for RSV infection than for influenza, with RSV hospitalization costs ranging from $713 to $38,800 more than influenza hospitalizations. The higher cost was attributed to the higher incidence of congestive heart failure, chronic pulmonary disease, bronchitis, respiratory failure, and LOS for patients with RSV infectionCitation37,Citation39. In contrast, comparisons among the Chinese and European studies trended toward relatively higher influenza costs (up to $480 increase per hospitalization for RSV infection; up to $45 for healthcare payers per RSV episode in all treatment settings for a relatively healthy cohort)Citation41,Citation45.
Table 7. RSV costs compared with influenza costs.
For two studies of hospitalized patients with RSV or influenza infection, HCRU outcomes were notably different and likely accounted for the higher cost of one virus over the other in China and the US. The Chinese study reported incidence of ICU admittance that was twice as high for patients with influenza-related pneumonia than for patients with RSV infectionCitation41. In the US study, the higher cost of RSV hospitalization versus influenza hospitalization was attributed to patients with RSV infection incurring more than double the mechanical ventilator use than patients with influenza infection, as well as patients with RSV infection having an average LOS nearly double that of patients with influenza infectionCitation47. The greater cost of RSV hospitalization was seen in all three age groups studied (20–44, 45–59, and ≥60 years)Citation47.
HCRU studies
This SLR identified 33 studies that reported HCRU, specifically 28 studies reporting ICU admission rates and/or use of MV and 12 reporting discharge to a higher level of care. Of these, 30 < > used laboratory tests to confirm RSV infection in patientsCitation2,Citation11,Citation13,Citation14,Citation41,Citation43,Citation48,Citation50–68,Citation70–73, two used diagnosis codesCitation12,Citation39, and one included patients during an RSV outbreak separate from an influenza outbreak or non-RSV and non-influenza seasonsCitation69. The majority of the studies were retrospective, with six being prospectiveCitation2,Citation13,Citation43,Citation53,Citation55,Citation57. Study locations were primarily in the US, Europe, and Asia, with the US being the most represented country.
Supplementary Tables A10–A15 present the details of the studies, for example, the number of adults with RSV infection, the mean/median age or age categories of the adults by the RSV infection determination method, and setting (hospital vs. outpatient). Studies that report the percentages of patients with specific comorbidities report only the most frequent comorbidities and not the overall percentage of patients having any comorbidities.
ICU admittance
All the studies reporting ICU admission rates used laboratory testing to confirm the RSV diagnosis (Supplementary Table A10), except for Zhou et al.Citation69, which relied on ICD diagnosis codes for ARI during an RSV outbreak (Supplementary Table A11). In the mostly retrospective studies of adults hospitalized with RSV infection, the percentages of patients who were admitted to the ICU ranged from 4–33.5% (Supplementary Table A10). Among the US studies, ICU admission ranged from 13–33.5%Citation2,Citation11,Citation14,Citation54,Citation59,Citation61–63,Citation65. Within the US studies and all studies combined, ICU admission rates appeared to not be associated with age (no formal statistical analysis was done); however, most of the studies had high mean/median ages. There appears to be no clear trend in ICU admission with comorbidity across the studies. This informal analysis was based on categorizing the extent of comorbidities in the population by the highest single specific comorbidity reported, whereby studies were assigned to have high (≥50%), middle (>30%–< 50%), and low (≤30%) levels of comorbidity, since only two studies reported the overall level of comorbidities in patients with RSV infectionCitation55,Citation67.
MV Use
Mechanical ventilation can be invasive, involving endotracheal intubationCitation75, or non-invasive, which includes high-flow nasal oxygen, continuous positive airway pressure, or bilevel intermittent positive airway pressureCitation76. Most of the studies in this review reporting MV rates did not specify whether the mechanism was invasive or noninvasive (Supplementary Tables A12 and A13); those that did are shown in Supplementary Table A14.
Among all the studies with laboratory confirmation of RSV diagnosis (Supplementary Table A12), the percentage of patients requiring MV ranged from 3.8–23.1%Citation2,Citation11,Citation14,Citation43,Citation50,Citation51,Citation53–56,Citation58,Citation61,Citation63–68. In most of the studies, the patients were either aged ≥50 years or the mean or median age was >60 years. The percentage of patients needing MV spanned a wide range, but overall MV was needed in a minority of patients.
Additionally, one US study found that 9.8% of 13,841 patients hospitalized with RSV infection, as determined by International Classification of Diseases, Tenth Revision, Clinical Modification (ICD-10-CM) diagnosis codes, required any MVCitation39.
Discharged to a higher level of care
The percentage of adults hospitalized with RSV infection who were discharged to a level of care not previously needed before RSV-related hospitalization ranged from 5–46% (Supplementary Table A15)Citation2,Citation11–14,Citation53,Citation54,Citation56,Citation70–73. The high of 46% was for patients with healthcare-associated RSV infection, and this was significantly higher than the 17% of patients with community-onset RSV infection who required care at a skilled nursing or rehabilitation facility following discharge (p = 0.04)Citation71. There was no pattern of patient subgroups needing greater care based on age or comorbidity. Because most of the studies were in the US, there is no clear pattern based on geography, except that the second highest rate of requirement for higher level care was reported by a 12-country study, in which 36.1% of patients required professional care at home or discharge to institutional careCitation53. None of the studies estimated the cost for discharging patients with RSV infection to a higher level of care.
Evidence limitations and gaps
Study design and reporting limitations
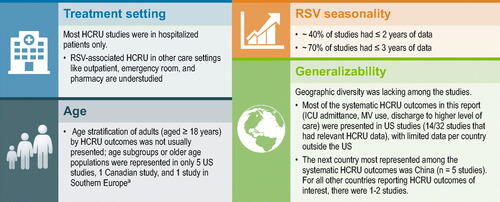
Several limitations exist due to either study design or reporting limitations. In general, overall rates of comorbidities were not typically reported, limiting our ability to analyze the studies by severity of underlying illness. Among the cost studies, about half identified the RSV-positive population by ICD diagnosis codes, without laboratory confirmation. Without a diagnostic test result to confirm the presence of RSV infection, it is unclear whether the physician made the RSV diagnosis based on local RSV infection trends or clinical parameters, when another virus may have been responsible for the infection. Studies may have excluded some cost types because of limitations in the data, resulting in an underestimation of the cost of RSV infection (e.g. Rafferty et al.Citation49 does not include pharmacy or laboratory costs). Despite the known association of older age with greater risk of more severe RSV disease, most studies presented HCRU for those aged ≥18 years and did not present HCRU by age subgroups. In studies that reported MV, invasive MV was usually not distinguished, so its use could not be determined. Only one study presented costs associated with sequelae associated with RSV infectionCitation40.
Gaps in the economic literature
The gap analysis of RSV-associated costs revealed a lack of available direct-cost estimates for many countries, with most studies conducted in the US (), and only one study reporting indirect costsCitation45. Cost studies were variable in their measurement by age groups and were mostly direct costs for hospitalized patients ( and Supplementary Figure A2).
Figure 5. Economic gap analysis. Abbreviations: RSV, respiratory syncytial virus; US, United States. a Excluding transplant studies and economic models. b Belgium, United Kingdom, and the Netherlands. c Sequelae costsCitation40 or incremental costs of RSV when compared to a control groupCitation49. d Numbers indicate total number of studies in which studied age ranges exactly match those specified here. Studies with outcomes for multiple age groups are counted more than once. e Bosco et al.Citation40 determined RSV-attributable cardiorespiratory hospitalizations (based on ICD coding) during RSV peaks.
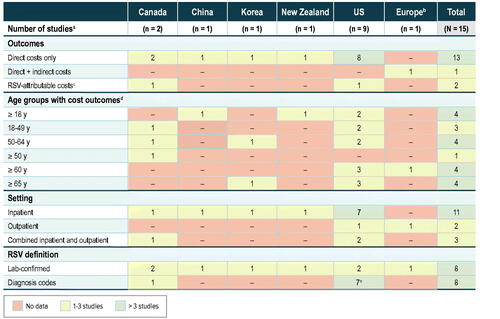
Owing to the limited number of studies available and lack of uniformity across them, it was difficult to create a high-level overview of baseline patient characteristics to compare costs across studies and across patient groups. The main limitation in the economic literature was the lack of uniformity in metrics across the studies. Costs were reported either for a specified period (e.g. 30 days, 8 weeks, 1 year) or for a medical incident (e.g. per hospitalization, per RSV case). Studies that reported costs for medical incidents did not provide an average or estimated duration of each incident (e.g. how many days a hospitalization lasts); therefore, it was not possible to create a conversion factor between periods and medical incidents to compare the costs across similar timeframes. Only two studies compared pre- and post-RSV-infection costsCitation12,Citation46. Comparing pre- and post-RSV-infection costs among the same cohort to estimate incremental cost differences due to RSV infection is informative for identifying patients at risk of incurring higher costs. Because of data limitations, pharmacy and laboratory costs were not included, resulting in an underestimation of the costs of RSV infectionCitation49. Despite a high incidence of comorbidities that are associated with higher risk of developing serious RSV infection among the patient populations in the studies in this SLR, only one study reported costs by comorbidityCitation42 and only two studies reported costs by high-risk versus non-high-risk patient categoriesCitation12,Citation46. Additional gaps are highlighted in .
Gaps in the HCRU literature
As with the cost studies, the gap analysis of HCRU in patients with RSV infection revealed a lack of available estimates for many countries; most studies were conducted in the US ( and Supplementary Figure A2). One of the knowledge gaps identified in a meeting of experts at the Centers for Disease Control and Prevention on 16–17 May 2016 is the understanding of the short- and long-term outcomes of RSV infection, including the impact on those adults who may need nursing home or long-term facility-related careCitation77. While the rates of discharge to a higher level of care following an RSV-associated hospitalization were reported in several studies, the cost associated with that higher level of care has not been quantified.
Figure 7. HCRU gap analysis. Abbreviations: HCRU, healthcare resource utilization; ICU, intensive care unit; MV, mechanical ventilation; RSV, respiratory syncytial virus; US, United States. aAustralia, Argentina, Brazil, Canada, France, Germany, Japan, Malaysia, Mexico, Republic of Korea, South Africa, US. bCyprus, Italy, Portugal. c One US studyCitation69 reported outcomes during an RSV surge for patients hospitalized with ARI. This study is not represented in this table. d Two studies had cohorts slightly different from ≥18 years: Hill-Ricciuti et al.Citation71 included patients aged >18 years, and Falsey et al.Citation2 included high-risk adults aged ≥21 years, nine of whom were part of an 132 hospitalized cohort (age range not reported). e “Elderly” was not defined in Belk et al.Citation39. f In one US studyCitation11, data were presented for age subgroups 60–74 years, ≥60 years, and ≥75 years.
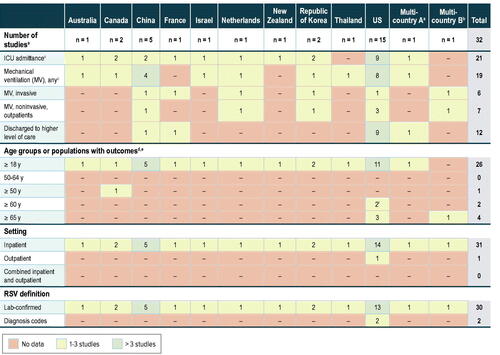
Because adults are not routinely tested for RSV, studies evaluating HCRU during RSV seasons versus periods of lower RSV and influenza infection rates are informative for broader understanding of morbidity from RSV infection even though the number of RSV cases is not confirmed in these studies. A difficulty in these studies is that winter viral seasons often overlap, necessitating assumptions about RSV impact during RSV peaks. Additional gaps are highlighted in .
Figure 8. Evidence limitations and gaps in the HCRU literature (for systematic HCRU outcomes only). Abbreviations: HCRU, healthcare resource utilization; ICU, intensive care unit; MV, mechanical ventilation; RSV, respiratory syncytial virus; US, United States. aBelgium, United Kingdom, and the Netherlands.
Conclusion
In adults, RSV infection represents a substantial economic burden, despite the under-ascertainment of RSV infections due to a lack of routine testing and limitations of current diagnostic testingCitation18,Citation29,Citation30. There is evidence of under-detection of RSV infection, as well as a lack of cost and HCRU studies, in individuals aged <50 years and those aged 50–64 yearsCitation29. The degree of under-ascertainment in those aged ≥65 years is greater for RSV than for influenzaCitation78.
Globally, the population aged ≥65 years is rapidly increasing in high-, middle-, and lower-middle-income countriesCitation79. A US study found that the population of persons aged ≥55 years continued to grow in the first half of 2020, despite the high mortality rate from COVID-19Citation80, indicating that the cost burden of RSV infection could continue to rise in the absence of an RSV vaccine and as transmission mitigation efforts for COVID-19 wane. RSV infection is also a significant concern in nursing homes because of high transmission rates among a concentrated, highly susceptible populationCitation40. Based on economic models of RSV vaccination in adults aged ≥60 years, the current national direct medical costs of RSV infections are high and are estimated to be $1.70–$3.35 billion in the US, $196.43 million in the United Kingdom, and $14.61 million in the Netherlands (adjusted to 2022 USD, see Supplementary Table A2)Citation81,Citation82.
Among large studies (RSV-positive cases, n ≥ 100), the percentages of hospitalized adults with RSV infection who require ICU admission and/or MV were substantial, with many studies reporting rates of 10–20%Citation53.
Given the substantial data gaps and variability across studies, further research is needed to provide additional cost and HCRU data for certain age groups; countries, including middle- and low-income countries; cost in outpatient care settings; indirect costs associated with RSV infection; and costs and HCRU in patients at risk for severe outcomes due to underlying comorbidities. It is important for these studies to account for under-reporting of RSV incidence and costsCitation83. Studies suggest that there are health and cost impacts beyond the acute phase of RSV infectionCitation38,Citation40,Citation49, highlighting the need to assess the long-term economic and health impacts in future studies. Despite these data gaps, the economic burden of RSV infections was found to be substantial, underscoring the need for RSV preventive strategies for reducing this burden.
Transparency
Declaration of financial/other relationships
AC, MG, and SW are full-time employees of RTI Health Solutions. Their compensation is unconnected to the studies on which they work. PG and CP are employees of Moderna, Inc. and hold shares and/or stock options in the company.
Author contributions
AC, SW, CP, and PG were involved in the conception and design of this study. MG, AC, SW, CP, and PG were involved in the analysis and interpretation of the data. MG led the drafting of the paper, with all authors involved in revising it critically for intellectual content. All authors gave final approval of the version to be published, and all authors agree to be accountable for all aspects of the work.
Reviewer disclosures
Peer reviewers on this manuscript have no relevant financial or other relationships to disclose.
Previous presentations
The manuscript, in whole or in part, has not been published nor is currently under consideration for publication by any other journal. Preliminary analyses were presented at RSVVW’23 (7th Respiratory Syncytial Virus Foundation [ReSViNET] conference), in Lisbon, Portugal on 22–24 February 2023, and will also be presented at the Communicable Diseases & Immunisation Conference (CDIC 2023) in Perth, Western Australia on 19–21 June 2023.
Supplemental Material
Download MS Word (2.7 MB)Acknowledgements
The authors thank John Forbes of RTI-HS for medical editing assistance. Moderna, Inc. provided funding for publication support in the form of manuscript writing, styling, and submission.
Additional information
Funding
References
- Falsey AR, Walsh EE. Respiratory syncytial virus infection in adults. Clin Microbiol Rev. 2000;13(3):371–384.
- Falsey AR, Hennessey PA, Formica MA, et al. Respiratory syncytial virus infection in elderly and high-risk adults. N Engl J Med. 2005;352(17):1749–1759.
- Tin Tin Htar M, Yerramalla MS, Moïsi JC, et al. The burden of respiratory syncytial virus in adults: a systematic review and meta-analysis. Epidemiol Infect. 2020;148:e48.
- Boe DM, Boule LA, Kovacs EJ. Innate immune responses in the ageing lung. Clin Exp Immunol. 2017;187(1):16–25.
- Coultas JA, Smyth R, Openshaw PJ. Respiratory syncytial virus (RSV): a scourge from infancy to old age. Thorax. 2019;74(10):986–993.
- Jansen AG, Sanders EA, Hoes AW, et al. Influenza- and respiratory syncytial virus-associated mortality and hospitalisations. Eur Respir J. 2007;30(6):1158–1166.
- Walsh EE, Peterson DR, Falsey AR. Risk factors for severe respiratory syncytial virus infection in elderly persons. J Infect Dis. 2004;189(2):233–238.
- Savic M, Penders Y, Shi T, et al. Respiratory syncytial virus disease burden in adults aged 60 years and older in high-income countries: a systematic literature review and meta-analysis. Influenza Other Respir Viruses. 2022;17(1):e13031.
- Shi T, Vennard S, Jasiewicz F, et al. Disease burden estimates of respiratory syncytial virus related acute respiratory infections in adults with comorbidity: a systematic review and meta-analysis. J Infect Dis. 2022;226(Suppl 1):S17–s21.
- Branche A, Granieri E, Walsh E, et al. 733. Incidence and evaluation of the change in functional status associated with respiratory syncytial virus infection in hospitalized older adults. Open Forum Infect Dis. 2018;5(suppl_1):S263–S263.
- Tseng HF, Sy LS, Ackerson B, et al. Severe morbidity and short- and mid- to long-term mortality in older adults hospitalized with respiratory syncytial virus infection. J Infect Dis. 2020;222(8):1298–1310.
- Wyffels V, Kariburyo F, Gavart S, et al. A real-world analysis of patient characteristics and predictors of hospitalization among US medicare beneficiaries with respiratory syncytial virus infection. Adv Ther. 2020;37(3):1203–1217.
- Descamps A, Lenzi N, Galtier F, et al. In-hospital and midterm post-discharge complications of adults hospitalised with respiratory syncytial virus infection in France, 2017-2019: an observational study. Eur Respir J. 2022;59(3):2100651.
- Walsh E, Lee N, Sander I, et al. RSV-associated hospitalization in adults in the USA: a retrospective chart review investigating burden, management strategies, and outcomes. Health Sci Rep. 2022;5(3):e556.
- Korsten K, Adriaenssens N, Coenen S, et al. Burden of respiratory syncytial virus infection in community-dwelling older adults in Europe (RESCEU): an international prospective cohort study. Eur Respir J. 2021;57(4):2002688.
- Kumar R, Dar L, Amarchand R, et al. Incidence, risk factors, and viral etiology of community-acquired acute lower respiratory tract infection among older adults in rural North India. J Glob Health. 2021;11:04027.
- Praphasiri P, Shrestha M, Patumanond J, et al. Underlying cardiopulmonary conditions as a risk factor for influenza and respiratory syncytial virus infection among community-dwelling adults aged ≥ 65 years in Thailand: findings from a two-year prospective cohort study. Influenza Other Respir Viruses. 2021;15(5):634–640.
- Li Y, Kulkarni D, Begier E, et al. Adjusting for case under-ascertainment in estimating RSV hospitalisation burden of older adults in high-income countries: a systematic review and modelling study. Infect Dis Ther. 2023;12(4):1137–1149.
- Shi T, Denouel A, Tietjen AK, et al. Global disease burden estimates of respiratory syncytial virus-associated acute respiratory infection in older adults in 2015: a systematic review and meta-analysis. J Infect Dis. 2020;222(Suppl 7):S577–s583.
- Griffiths C, Drews SJ, Marchant DJ. Respiratory syncytial virus: infection, detection, and new options for prevention and treatment. Clin Microbiol Rev. 2017;30(1):277–319.
- Janet S, Broad J, Snape MD. Respiratory syncytial virus seasonality and its implications on prevention strategies. Hum Vaccin Immunother. 2018;14(1):234–244.
- Li Y, Wang X, Broberg EK, et al. Seasonality of respiratory syncytial virus and its association with meteorological factors in 13 european countries, week 40 2010 to week 39 2019. Euro Surveill. 2022;27(16):2100619.
- Obando-Pacheco P, Justicia-Grande AJ, Rivero-Calle I, et al. Respiratory syncytial virus seasonality: a global overview. J Infect Dis. 2018;217(9):1356–1364.
- Taleb SA, Al Thani AA, Al Ansari K, et al. Human respiratory syncytial virus: pathogenesis, immune responses, and current vaccine approaches. Eur J Clin Microbiol Infect Dis. 2018;37(10):1817–1827.
- Hamid S, Winn A, Parikh R, et al. Seasonality of respiratory syncytial virus - United States, 2017-2023. MMWR Morb Mortal Wkly Rep. 2023;72(14):355–361.
- Falsey AR, Cameron A, Branche AR, et al. Perturbations in respiratory syncytial virus activity during the SARS-CoV-2 pandemic. J Infect Dis. 2022;227(1):83–86.
- Li ZJ, Yu LJ, Zhang HY, et al. Broad impacts of coronavirus disease 2019 (COVID-19) pandemic on acute respiratory infections in China: an observational study. Clin Infect Dis. 2022;75(1):e1054–e1062.
- Centers for Disease Control and Prevention. RSV-NET interactive dashboard. 2022. https://www.cdc.gov/rsv/research/rsv-net/dashboard.html
- McLaughlin JM, Khan F, Begier E, et al. Rates of medically attended RSV among US adults: a systematic review and meta-analysis. Open Forum Infect Dis. 2022;9(7):ofac300.
- Onwuchekwa C, Moreo LM, Menon S, et al. Underascertainment of respiratory syncytial virus infection in adults due to diagnostic testing limitations: a systematic literature review and meta-analysis. J Infect Dis. 2023; jiad012.
- Widmer K, Zhu Y, Williams JV, et al. Rates of hospitalizations for respiratory syncytial virus, human metapneumovirus, and influenza virus in older adults. J Infect Dis. 2012;206(1):56–62.
- Zhang S, Wahi-Singh P, Wahi-Singh B, et al. Costs of management of acute respiratory infections in older adults: a systematic review and meta-analysis. J Glob Health. 2022;12:04096.
- Nguyen-Van-Tam JS, O'Leary M, Martin ET, et al. Burden of respiratory syncytial virus infection in older and high-risk adults: a systematic review and meta-analysis of the evidence from developed countries. Eur Respir Rev. 2022;31(166):220105.
- Youssef Y, Chmaisse A, Boutros C, et al. The burden of respiratory syncytial virus (RSV) infection in the Middle east and North africa (MENA) region across age groups: a systematic review. Vaccine. 2021;39(29):3803–3813.
- Critical Appraisal Skills Programme. CASP economic evaluation checklist. 2018. https://casp-uk.net/images/checklist/documents/CASP-Economic-Evaluation-Checklist/CASP-Economic-Evaluation-Checklist-2018_fillable_form.pdf
- Drummond M, Sculpher M, Claxton K, et al. Methods for the economic evaluation of health care programmes. Oxford: Oxford University Press; 2015.
- Ackerson B, An J, Sy LS, et al. Cost of hospitalization associated with respiratory syncytial virus infection versus influenza infection in hospitalized older adults. J Infect Dis. 2020;222(6):962–966.
- Amand C, Tong S, Kieffer A, et al. Healthcare resource use and economic burden attributable to respiratory syncytial virus in the United States: a claims database analysis. BMC Health Serv Res. 2018;18(1):294.
- Belk K, Clark L, Mallow P, et al. POSC190 hospital utilization for elderly patients diagnosed with respiratory syncytial virus (RSV) versus influenza in the US. Value Health. 2022;25(1):S138.
- Bosco E, van Aalst R, McConeghy KW, et al. Estimated cardiorespiratory hospitalizations attributable to influenza and respiratory syncytial virus among long-term care facility residents. JAMA Netw Open. 2021;4(6):e2111806.
- Chen L, Han X, Li Y, et al. Comparison of clinical characteristics and outcomes between respiratory syncytial virus and influenza-related pneumonia in China from 2013 to 2019. Eur J Clin Microbiol Infect Dis. 2021;40(8):1633–1643.
- Choi Y, Hill-Ricciuti A, Branche AR, et al. Cost determinants among adults hospitalized with respiratory syncytial virus in the United States, 2017-2019. Influenza Other Respir Viruses. 2022;16(1):151–158.
- El Sherif M, McNeil S, Andrew M, et al. Prevalence, severe outcomes, and costs associated with respiratory syncytial virus (RSV) in adults ≥ 50 years of age hospitalized with respiratory illness in Canada, 2012-2015; abstract no. E&E-17. 6th ReSViNET conference. Virtual; 2021.
- Lonnet G, Bracke B, Molnar D, et al. POSB60 evolution of direct medical costs associated with respiratory syncytial virus disease in older adults: a retrospective database analysis from 2008 to 2017. Value Health. 2022;25(1):S71–S72.
- Mao Z, Li X, Korsten K, et al. Economic burden and health-related quality of life of respiratory syncytial virus and influenza infection in european community-dwelling older adults. J Infect Dis. 2022;226(Suppl 1):S87–S94.
- Mesa-Frias M, Rossi C, Emond B, et al. Incidence and economic burden of respiratory syncytial virus among adults in the United States: a retrospective analysis using 2 insurance claims databases. J Manag Care Spec Pharm. 2022;28(7):753–765.
- Pastula ST, Hackett J, Coalson J, et al. Hospitalizations for respiratory syncytial virus among adults in the United States, 1997-2012. Open Forum Infect Dis. 2017;4(1):ofw270.
- Prasad N, Newbern EC, Trenholme AA, et al. The health and economic burden of respiratory syncytial virus associated hospitalizations in adults. PLoS One. 2020;15(6):e0234235.
- Rafferty E, Paulden M, Buchan SA, et al. Evaluating the individual healthcare costs and burden of disease associated with RSV across age groups. Pharmacoeconomics. 2022;40(6):633–645.
- Yoon JG, Noh JY, Choi WS, et al. Clinical characteristics and disease burden of respiratory syncytial virus infection among hospitalized adults. Sci Rep. 2020;10(1):12106.
- Atamna A, Babich T, Froimovici D, et al. Morbidity and mortality of respiratory syncytial virus infection in hospitalized adults: comparison with seasonal influenza. Int J Infect Dis. 2021;103:489–493.
- Boattini M, Almeida A, Christaki E, et al. Severity of RSV infection in Southern european elderly patients during two consecutive winter seasons (2017-2018). J Med Virol. 2021;93(8):5152–5157.
- Falsey AR, Walsh EE, House S, et al. Risk factors and medical resource utilization of respiratory syncytial virus, human metapneumovirus, and influenza-related hospitalizations in adults. A global study during the 2017-2019 epidemic seasons (hospitalized acute respiratory tract infection [HARTI] study). Open Forum Infect Dis. 2021;8(11):ofab491.
- Goldman CR, Sieling WD, Alba LR, et al. Severe clinical outcomes among adults hospitalized with respiratory syncytial virus infections, New York city, 2017-2019. Public Health Rep. 2022;137(5):929–935.
- Lee N, Chan MC, Lui GC, et al. High viral load and respiratory failure in adults hospitalized for respiratory syncytial virus infections. J Infect Dis. 2015;212(8):1237–1240.
- Lee N, Lui GC, Wong KT, et al. High morbidity and mortality in adults hospitalized for respiratory syncytial virus infections. Clin Infect Dis. 2013;57(8):1069–1077.
- Lee N, Smith S, Zelyas N, et al. Burden of noninfluenza respiratory viral infections in adults admitted to hospital: analysis of a multiyear Canadian surveillance cohort from 2 centres. CMAJ. 2021;193(13):e439–e446.
- Minney-Smith CA, Selvey LA, Levy A, et al. Post-pandemic influenza a/H1N1pdm09 is associated with more severe outcomes than a/H3N2 and other respiratory viruses in adult hospitalisations. Epidemiol Infect. 2019;147:e310.
- Nam H, Ison MG. Respiratory syncytial disease in hospitalized adults: a retrospective cohort study. Open Forum Infect Dis. 2019;6(Supplement_2):S985–S986.
- Park SY, Kim T, Jang YR, et al. Factors predicting life-threatening infections with respiratory syncytial virus in adult patients. Infect Dis (Lond). 2017;49(5):333–340.
- Schmidt H, Das A, Nam H, et al. Epidemiology and outcomes of hospitalized adults with respiratory syncytial virus: a 6-year retrospective study. Influenza Other Respir Viruses. 2019;13(4):331–338.
- Sieling WD, Goldman CR, Oberhardt M, et al. Comparative incidence and burden of respiratory viruses associated with hospitalization in adults in New York city. Influenza Other Respir Viruses. 2021;15(5):670–677.
- Smithgall M, Maykowski P, Zachariah P, et al. Epidemiology, clinical features, and resource utilization associated with respiratory syncytial virus in the community and hospital. Influenza Other Respir Viruses. 2020;14(3):247–256.
- Vos LM, Oosterheert JJ, Hoepelman AIM, et al. External validation and update of a prognostic model to predict mortality in hospitalized adults with RSV: a retrospective dutch cohort study. J Med Virol. 2019;91(12):2117–2124.
- Walsh EE, Saimon L, Branche AR, et al. Incidence and clinical outcomes of respiratory syncytial virus infection among hospitalized adults, 2017-2019. In: 5th ReSViNET Conference, Ghana; 2019.
- Wong SS, Yu JW, Wong KT, et al. Initial radiographic features as outcome predictor of adult respiratory syncytial virus respiratory tract infection. AJR Am J Roentgenol. 2014;203(2):280–286.
- Wongsurakiat P, Sunhapanit S, Muangman N. Respiratory syncytial virus-associated acute respiratory illness in adult non-immunocompromised patients: outcomes, determinants of outcomes, and the effect of oral ribavirin treatment. Influenza Other Respir Viruses. 2022;16(4):767–779.
- Zhang Y, Zhao J, Zou X, et al. Severity of influenza virus and respiratory syncytial virus coinfections in hospitalized adult patients. J Clin Virol. 2020;133:104685.
- Zhou JA, Schweinle JE, Lichenstein R, et al. Severe illnesses associated with outbreaks of respiratory syncytial virus and influenza in adults. Clin Infect Dis. 2020;70(5):773–779.
- Belongia EA, King JP, Kieke BA, et al. Clinical features, severity, and incidence of RSV illness during 12 consecutive seasons in a community cohort of adults ≥ 60 years old. Open Forum Infect Dis. 2018;5(12):ofy316.
- Hill-Ricciuti AC, Walsh EE, Greendyke WG, et al. Burden of healthcare-associated (HA) respiratory syncytial virus (RSV) in hospitalized adults. Open Forum Infect Dis. 2021;8(Supplement_1):S494–S494.
- Nolen LD, Seeman S, Desnoyers C, et al. Respiratory syncytial virus and influenza hospitalizations in Alaska native adults. J Clin Virol. 2020;127:104347.
- Walker E, Ison MG. Respiratory viral infections among hospitalized adults: experience of a single tertiary healthcare hospital. Influenza Other Respir Viruses. 2014;8(3):282–292.
- Centers for Disease Control and Prevention. People at high risk for severe RSV infection. 2020. https://www.cdc.gov/rsv/high-risk/index.html
- Walter JM, Corbridge TC, Singer BD. Invasive mechanical ventilation. South Med J. 2018;111(12):746–753.
- Oxford Medical Information. Non-invasive ventilation (NIV). 2016. https://www.printfriendly.com/p/g/xN684V
- Kim L, Rha B, Abramson JS, et al. Identifying gaps in respiratory syncytial virus disease epidemiology in the United States prior to the introduction of vaccines. Clin Infect Dis. 2017;65(6):1020–1025.
- Zhou H, Thompson WW, Viboud CG, et al. Hospitalizations associated with influenza and respiratory syncytial virus in the United States, 1993-2008. Clin Infect Dis. 2012;54(10):1427–1436.
- Cheng X, Yang Y, Schwebel DC, et al. Population ageing and mortality during 1990-2017: a global decomposition analysis. PLoS Med. 2020;17(6):e1003138.
- Shiels MS, Almeida JS, García-Closas M, et al. Impact of population growth and aging on estimates of excess U.S. deaths during the COVID-19 pandemic, march to august 2020. Ann Intern Med. 2021;174(4):437–443.
- Herring WL, Zhang Y, Shinde V, et al. Clinical and economic outcomes associated with respiratory syncytial virus vaccination in older adults in the United States. Vaccine. 2022;40(3):483–493.
- Zeevat F, Luttjeboer J, Paulissen JHJ, et al. Exploratory analysis of the economically justifiable price of a hypothetical RSV vaccine for older adults in The Netherlands and the United Kingdom. J Infect Dis. 2022;226(Suppl 1):S102–S109.
- Rozenbaum MH, Judy J, Tran D, et al. Low levels of RSV testing among adults hospitalized for lower respiratory tract infection in the United States. Infect Dis Ther. 2023;12(2):677–685.
- Lee N, Walsh EE, Sander I, et al. Delayed diagnosis of respiratory syncytial virus infections in hospitalized adults: individual patient data, record review analysis and physician survey in the United States. J Infect Dis. 2019;220(6):969–979.