Abstract
Objective
This study aimed to determine the potential cost-savings for implementing continuous vital sign monitoring in a hospital’s medical-surgical units.
Methods
A cost-savings analysis was designed to calculate potential cost-savings for an average-sized U.S. community hospital (153 total beds) over a 1-year time horizon. Analysis parameters were extracted from national databases and previous studies that compared outcomes for patients receiving continuous vital sign monitoring (SpO2, HR, and RR) or standard of care (intermittent vital sign measurements) in medical-surgical units based on a targeted literature review. Clinical parameters and associated costs served as analysis inputs. The analysis outputs were costs and potential cost-savings using a 50% and 100% adoption rate of continuous monitoring technologies across the medical-surgical unit.
Results
Potential annual cost-savings for in-hospital medical-surgical stays were estimated at $3,414,709 (2022 USD) and $6,829,418 for a 50% and 100% adoption rate, respectively. The cost-savings for an adoption rate of 100% equated to a ∼14% reduction in the overall annual cost of medical-surgical unit stays for an average-sized hospital. The largest contribution to potential cost-savings came from patients that avoided serious adverse events that require transfer to the intensive care unit; this resulted in annual cost-savings from reduced average length of stay between $1,756,613 and $3,513,226 (50% and 100% adoption rate, respectively). Additional cost-savings can be attained from reductions in in-hospital cardiac arrest-associated hospitalizations and decreased rapid response team activation.
Conclusions
Our findings demonstrate that there is the potential for cost-savings of over $6.8 million dollars per year in an average-sized US community hospital by improving patient outcomes through implementation of continuous monitoring technologies in medical-surgical units. Continuous vital sign monitoring technologies that increase patient mobility and facilitate recovery may further contribute to cost-savings and should be considered for economic analyses. Future research is needed to explore these health-related outcomes.
Introduction
Hospital medical-surgical units provide care to patients for a variety of conditions such as cancer, stroke, orthopedic injuries, and post-operative care following surgical procedures. Medical-surgical unit patients may experience subtle or rapid deterioration and require unplanned interventions including activation of rapid response teams (RRT), transfer to the intensive care unit (ICU), or additional procedures; these unplanned adverse events may contribute to increased costs and hospital length of stays (LOS)Citation1–3. Patients that undergo unrecognized deterioration have a higher risk of morbidity and mortality and increase the toll on healthcare staffing and resourcesCitation1,Citation4,Citation5.
Unrecognized deterioration may take place due to numerous causes such as opioid-induced respiratory depression (OIRD), sepsis, and cardiac or respiratory failureCitation6,Citation7. OIRD and sepsis have recently been emphasized as patient safety prioritiesCitation8,Citation9. To address these issues, the Center for Medicare and Medicaid Services (CMS) includes sepsis in hospital quality measures and has drafted a new rule to reduce opioid-related harm; the “Hospital-Harm—Opioid-Related Adverse Events” is a new quality measure included in the hospital Inpatient Quality-Reporting (IQR) ProgramCitation9.
While the underlying cause for unrecognized deterioration may vary, the literature is unequivocal in that a significant proportion of these adverse events can be prevented by improved monitoring of vital signsCitation10,Citation11. Bates et al. recently reported that adverse events could be identified in nearly one in four hospital admissions, and approximately one-fourth of these events are preventableCitation12. Additionally, up to 76% of adverse events that require ICU admission from the medical-surgical unit and 36% of all ICU readmissions are preventableCitation1,Citation4. While several methods are employed to attenuate preventable adverse events, the early detection of abnormal vital signs has been demonstrated as an important indicator of deterioration and can improve patient outcomes by enabling earlier interventionCitation1,Citation2,Citation13,Citation14.
Respiratory rate (RR), heart rate (HR), blood pressure, arterial blood oxygen saturation (SpO2), and temperature are vital signs that inform clinicians of a patient’s condition at a single point in time or as a trend. Each measurement differs in its ability to identify clinical deterioration, and published studies report variable results on which parameters are most predictive of dangerous changes in a patient’s condition. Churpek et al. found respiratory rate and heart rate were predictors of cardiac arrest while blood pressure and oxygen saturation were notCitation3. Fieselmann et al. concluded that a respiratory rate greater than 27 breaths per minute was a predictor of cardiopulmonary arrest, whereas heart rate was not predictiveCitation15. Jones et al. demonstrated that tachycardia was responsible for 19% of RRT activations, whereas increased respiratory rates were responsible for 14%Citation16. The utility of SpO2 measurements was highlighted by the COVID-19 pandemic where decreases in SpO2 were predictive of ICU admissionCitation17. In fact, each individual parameter may be most predictive in certain disease states (driven by the underlying pathophysiologic changes), suggesting that a multiparameter continuous monitoring strategy may be most efficacious. Overall, respiratory rate and heart rate may be most predictive of deterioration with additional vital signs, patient age, and clinical characteristics having variable but important predictive valuesCitation18.
In the medical-surgical ward, vital signs are measured intermittently every 4–6 h as the standard of careCitation19,Citation20. However, due to patient complexity and staffing resources, compliance with scheduled routine vital sign measurement may be as low as ∼62%, with some patients waiting 9 h or more between assessmentsCitation21. The ability for nursing staff to recognize early deterioration is linked to the quality and frequency of vital sign measurementsCitation21,Citation22. It has been shown that episodes of hypotension and hypoxemia may be prolonged, severe, and undetected by intermittent monitoringCitation23,Citation24. To address this gap in timely detection of deterioration, continuous vital sign monitoring systems are an essential component for earlier detection of deterioration in a patient’s condition.
Continuous vital sign monitoring has been shown to significantly improve health outcomes in medical-surgical unit patientsCitation20,Citation25–27. Compared to routine vital sign monitoring, continuous monitoring may significantly decrease patient total LOS, number of days spent in the ICU, and incidence of cardiac and respiratory arrestCitation20. Advancements in continuous monitoring technologies allow health institutions to refine patient monitoring, reduce nurse workloads, and improve patient outcomesCitation25,Citation28. However, early continuous vital sign monitoring systems were cumbersome for patients, as tethering wires restricted ambulation, decreased patient compliance, and reduced monitoring qualityCitation29. Patient mobilization is essential for enhanced recovery after surgery (ERAS) pathways and to reduce hospital complications such as venous thromboembolism and pneumoniaCitation30–32. Recent technologic advances have improved patient comfort, compliance, and mobility through wireless and wearable devices with accuracy that matches or exceeds current standard of care for vital sign monitoringCitation33.
Establishing economic evidence that continuous monitoring technologies result in cost-savings will support widespread adoption in the capital constrained healthcare systemCitation34–36. In this regard, analyses on the potential cost-savings for the implementation of continuous monitoring technology are currently lackingCitation37. Furthermore, the emergence of technologies that increase patient mobility emphasize the need for evaluating the economic impacts of these technologies that monitor similar physiologic parameters. Herein we present cost-savings estimates for the utilization of continuous vital sign monitoring systems compared to standard of care vital sign monitoring in general medical-surgical units. The current analysis modeled the potential cost-savings for implementing continuous vital sign monitoring systems, such as the PortraitTM Mobile system (GE HealthCare), in an average sized (153 total beds) U.S.-based community hospital with 4,715 annual discharges from the medical-surgical unitCitation38,Citation39.
Methods
Analysis overview
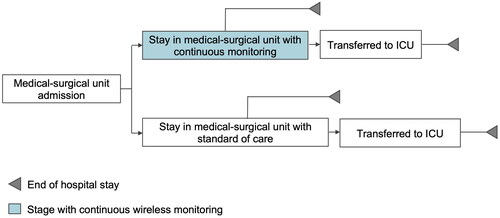
To determine the estimated cost-savings of implementing continuous vital sign monitoring, we developed a cost-savings analysis comparing the use of continuous RR, SpO2, and HR monitoring in general medical-surgical units to standard of care intermittent vital sign monitoring. Using established best practicesCitation40, our analysis calculated potential cost-savings for an average-sized U.S. community hospital over a 1-year time horizon. The average-sized US-based hospital was assumed to have 153 total beds and 4,715 annual patient discharges from medical-surgical units based on data reported by American Hospital Association and the Healthcare Cost and Utilization ProjectCitation38,Citation39. Cost-savings were based on the following clinical parameters: (i) LOS in medical-surgical unit for patients not transferred to the ICU; (ii) LOS in medical-surgical unit for patients transferred to the ICU; (iii) LOS in the ICU for patients transferred from medical-surgical units; (iv) frequency of RRT activation; and (v) incidence of in-hospital cardiac arrest (IHCA) (). The clinical parameters for this analysis were extracted from previous studies comparing continuous monitoring against the standard of careCitation20,Citation27. Using these inputs, cost-savings were determined as the difference between the estimated costs of standard of care and continuous vital sign monitoring.
Analysis inputs, data sources, and assumptions
Clinical parameter estimates
A targeted literature review was conducted to identify relevant studies for clinical parameter inputs; studies were assessed based on their validity, robust sample size, generalizability (e.g. device type and clinical setting), and reported outcomes. The analysis includes the following clinical data derived from published literature: (i) average LOS in medical-surgical unit patients without ICU stay; (ii) average LOS in medical-surgical unit prior to ICU transfer, per discharge; (iii) average LOS in ICU after transfer from medical-surgical unit, per discharge; (iv) ICU transfer rate; (v) incidence of RRT activation events per 1,000 discharges; and (vi) incidence of IHCAs per 1,000 discharges ()Citation20,Citation27.
Table 1. Key analysis input parameters.
The cost-savings analysis was developed with data extracted from the studies identified by the targeted literature review and represent 8,379 patient dischargesCitation20,Citation27. In a controlled clinical trial, Brown et al. demonstrated that continuous monitoring results in significant reductions (p < 0.05) in LOS in the medical-surgical unit, LOS in ICU, and IHCA incidence compared to standard of careCitation20. ICU transfers were defined as direct, unplanned transfers to the hospital’s ICU for patients that spent at least 12 h in the general medical-surgical unit. Additionally, using a prospective observational study, Weller et al. found significant reductions (p < .05) in RRT activation rate following the implementation of continuous monitoringCitation27.
Costs of hospital services
A targeted literature review was conducted to identify relevant studies assessing hospital service costs. The analysis includes the following costs derived from published literature and national database estimates: (i) medical-surgical unit cost per patient per dayCitation39; (ii) ICU cost per patient per dayCitation41; (iii) RRT activation duration and costCitation9; and (iv) IHCA-associated hospitalization cost per patientCitation42. For RRT activation costs, the 2022 Physician Fee Schedule from the Centers for Medicare & Medicaid Service associated with Current Procedural Terminology (CPT) code 99291 was used; this code is used to report critical care services provided by a physician in the first 30–74 min of an event which corresponds to the mean time of critical care service (30.53 min) in an RRT eventCitation43. Foreign currency (i.e. the cost of IHCA reported in CAD) was converted into equivalent USD using purchasing power parities, and all cost values were inflated to 2022 USD using the U.S. consumer price indexCitation44. It is likely that this estimated RRT cost underrepresents true expense due to the requirements for multiple additional non-physician personnel to be on staff and available to respond to emergencies as well as in cases where the time of an RRT event exceed the average response times used to calculate our findings.
Outcomes, analysis, and analysis assumptions
The economic outcomes of our analysis show cost-savings generated from key patient health parameters using continuous monitoring: (i) reduced medical-surgical unit stay prior to discharge; (ii) reduced medical-surgical unit stay prior to ICU transfer; (iii) reduced ICU transfer and ICU stay; (iv) avoided RRT activation; and (v) prevented IHCA events.
We reported the cost-savings for 50% and 100% adoption rates to calculate potential cost-savings at different technology usage levels. A 50% and 100% were chosen to model a broad range of cost avoidance outcomes and facilitate adaption of this analysis to various localized implementation scenarios. The cost-savings provided for these adoption rates are the result of the improved patient outcomes and are reported in 2022 USD. Our analysis assumes discharge from the hospital following their medical-surgical unit or ICU stay, which is consistent with methods in similar literatureCitation20.
Cost-savings were calculated as the difference of each cost component between the standard of care (intermittent vital signs measurement) and continuous monitoring-implemented scenarios; this method was also used to determine cost-savings for the total hospital LOS. Using established best practices, the cost-savings analysis was developed using Microsoft Excel (version 16.66.1)Citation40.
Results
Estimated cost breakdown and cost-savings are presented in for medical-surgical unit stay without ICU transfer, medical-surgical unit stay prior to ICU transfer, ICU stay, RRT activation, and IHCA hospitalization. Annual estimated costs and cost-savings are provided using a 50% and an 100% adoption of continuous monitoring within an individual medical-surgical unit. Annual cost-savings are based on an average of 4,715 patient annual discharges, which is representative of an average sized U.S.-based hospital medical-surgical unitCitation38,Citation39. Unit costs per patient discharge are also provided for use in estimating potential cost-savings in hospital medical-surgical units of varying sizes.
Table 2. Economic outcomes associated with the implementation of continuous monitoring in the medical-surgical unit (MSU) of a community hospital.
The annual estimated cost-savings for in-hospital stays in an average community medical-surgical unit are $3,414,709 (2022 USD) for an adoption rate of 50% compared to $6,829,418 with an adoption rate of 100%. The largest contribution to cost-savings was the reduction in LOS for patients that avoided a transfer to the ICU and accounted for 51% of the cost savings; this results in potential annual cost-savings between $1,756,613 and $3,513,226 (50% and 100% adoption rate, respectively). In addition, cost-savings can be accrued through continuous monitoring-induced reductions in IHCA-associated hospitalizations resulting in potential annual cost-savings of $128,335 and $256,671, respectively. Potential cost-savings from decreased RRT activation ranges from $20,637 to $41,275; however, as noted previously, this figure likely underrepresents actual savings and is a conservative estimate due to lack of economic data describing the full extent of costs associated with RRT activation.
Discussion
Our findings reveal that implementing continuous vital sign monitoring in an average-size medical-surgical unit can result in large cost-savings for that unit and ultimately, for the associated hospital system. Importantly, cost-savings in our analysis all stem from clinical improvements in patient health parameters; the largest contributing factors were reductions in medical-surgical unit LOS without ICU transfer, medical-surgical unit LOS prior to ICU transfer, and ICU stay. Our findings indicate that cost-savings can occur in excess of $6,800,000 (2022 USD) per year, which equates to a ∼12% reduction in the overall annual cost of hospital stays in medical-surgical units for an average sized U.S.-based hospital.
This report provides administrative and clinical decision makers the ability to determine real world cost-savings for their specific institution by subtracting the costs of implementing a continuous monitoring device of their choosing (i.e. capital costs, one-time noncapital costs, and annual operational costs) from the savings achieved through reduced unrecognized deterioration and improved patient outcomes. Our cost-savings analysis considers the clinical and financial benefits of continuous vital sign monitoring using RR, SpO2, and HRCitation18,Citation20,Citation27, but does not account for the costs of implementing and using continuous monitoring. Thus, the net savings of adopting continuous monitoring technologies will depend on the acquisition costs of the monitors, the costs of implementation, training, and maintenance of the equipment and expertise needed to improve patient outcomes and provide more efficient care. We chose this approach since there is a great variety of available and emerging continuous monitoring systems with distinct costs and varying pricing models that would prohibit an analysis that accounts for all of these optionsCitation37,Citation45. Furthermore, the costs of implementing continuous monitoring technologies may change over time as these technologies are further developed. The costs associated with continuous monitoring systems may also benefit through economies of scale, that is adding additional monitoring to existing infrastructures may have lower cost than the initial system installation and setup. This contrasts with the cost avoidance from improved patient outcomes which is directly proportional to the number of patients monitored as observed in our analysis. A full discussion on the novelty of our current findings, the conservative nature of this analysis, and the limitations of our research follow.
There is limited published data regarding the economic impact of implementing continuous monitoring, even though the clinical benefits are well describedCitation37. To date, only Slight et al. have performed a cost-savings analysis for implementing continuous monitoring across an entire medical-surgical unit floorCitation46, and our approach has several advantages. The Slight et al. study incorporates the costs of one specific continuous monitoring device for all of its economic outputsCitation46. Our approach allows hospital decision makers the ability to assess the cost-savings of implementing a device of their choosing by subtracting the costs of the device from the currently reported cost-savings. Further, our report provides values in 2022 USD and is the first to provide cost-savings estimates based on the most recently reported data regarding clinical parameters of medical-surgical unit LOS without ICU transfer, medical-surgical unit LOS prior to ICU transfer, and ICU stay, RRT and reductions for in-hospital cardiac arrest. Other reports have also shown significant cost-savings when implementing continuous monitoring technologies; however, these reports limit broad implementation in a medical-surgical unit due to patient selection (i.e. only patients that received opioids) or methodology (i.e. comparing pre- and post-optimization of continuous monitoring usage)Citation26,Citation47. Studies outside of the U.S. have also demonstrated that continuous monitoring improves patient outcomes for general medical-surgical units and gastroenterological patientsCitation48,Citation49. Overall, our findings agree with the reported cost-savings data regarding continuous monitoring devices—that there is a large potential for cost-savings through implementing these technologies in medical-surgical unitsCitation26,Citation46,Citation47,Citation49.
While the purpose of the current cost-savings analysis was to capture potential cost-savings across the broad spectrum of medical-surgical unit health services, prior reports have shown that continuous monitoring can also ameliorate the occurrence of specific adverse events and confer improved patient outcomes and cost-savingsCitation6,Citation46,Citation50–52. For instance, Slight et al. found that implementing continuous monitoring technologies is associated with a reduction in the incidence of pressure ulcers and a decrease in associated hospital costsCitation46. Continuous monitoring has also been shown useful for detecting OIRD; a recent study found that continuous monitoring technologies detected at least one episode of respiratory depression in 46% of patients receiving opioids during in-patient careCitation6. Implementing continuous monitoring technologies improves patient outcomes associated with OIRD and attenuates their accompanying increase in hospital costsCitation6,Citation50–52. Importantly, our approach most likely captures a large portion of the underlying cost-savings that occur from these continuous monitoring-induced improvements (e.g. decreased LOS and ICU transfer) as these adverse events are included within the sample populations that comprise the clinical parameters for the current analysis.
Notably, our estimates are conservative in nature, especially regarding the range of adoption rates. We estimated potential cost-savings using a 50% and 100% adoption rate for use of continuous monitoring in medical-surgical unit patients. When implemented by an institution, these technologies are expected to be used by the majority of medical-surgical unit patients since continuous monitoring is well received by both patients and healthcare providers but 100% monitoring may not be realistic in various institutionsCitation26,Citation53–55. Nonetheless, our results demonstrate that cost-savings can occur even at lower adoption rates, and more widespread use would further increase the potential for cost-savings.
Our estimates for cost-savings may also vary according to the patient population treated at a respective hospital. Data from Brown et al. contributed the largest proportion of the cost-savings estimates provided by our analysis; the mean age of the studied population in Brown et al. was ∼49Citation20. Notably, elderly patients have higher rates of comorbidities, higher ICU admissions, and increased rates of mortalityCitation56. Given that the patient demographics of hospital admissions could affect the baseline costs for treatment, it is plausible that continuous monitoring could result in even greater cost-savings in hospitals that typically treat older adult populations or patients with more severe conditions; however, this assumes that the relative reduction rates in adverse events are similar among different patient cohorts receiving continuous vital sign monitoring. It is currently not known if continuous monitoring may have variable efficacy for detecting adverse events in specific patient cohorts, and this point warrants future study.
Recent innovations in wireless, wearable technologies have increased monitoring parameter sensitivity and specificity that is likely to improve patient well-being and increase the potential for downstream cost-savings. One such wireless, wearable continuous monitoring system, PortraitTM Mobile, (GE HealthCare, Chicago, IL) was demonstrated to have high sensitivity for respiratory rate and significantly fewer artifacts than capnography; the reported accuracy of this device was also higher than currently available devicesCitation33. Advancements in device sensitivity and specificity will also increase the ability to detect adverse events and decrease false alarms that contribute to alarm fatigueCitation57. Modern technologies such as PortraitTM Mobile further address alarm fatigue by enabling configuration of alarm level and time delay thresholds tailored to the baseline values for individual patients or for specific medical-surgical units. These advancements in monitoring capabilities will further supplement the patient monitoring provided by clinicians and patients themselves, and successful monitoring will still require the insights of the clinicians providing care for their patients. A majority of patients report feeling safer with continuous monitoring during hospitalizationCitation58, and interviews with nursing staff have indicated the importance of maintaining frequent nurse-patient interactionsCitation55.
Historically, continuous monitoring technologies were limited to in-bed monitoringCitation20,Citation46 and/or had multiple wired connections and bulky devices which resulted in impeded mobility and patient compliance as low as 16%Citation24,Citation29,Citation59. In contrast, 93% of patients believe that present-day wearable continuous monitoring technologies are “comfortable” when surveyed following their use post-surgeryCitation58. In addition, wearable devices with smaller footprints now allow for the portable measurement of vital signs which can increase the mobility of patientsCitation33. Continuous monitoring devices that promote patient mobility are critical, as low in-hospital mobility is associated with poor patient outcomes compared to patients with high mobilityCitation60. In particular, patient mobilization is a key component of ERAS pathways and provides clinical benefits such as a reduced risk of venous thromboembolism and hospital-acquired pneumoniaCitation30–32. These technological advancements will likely lead to further improvements in patient outcomes by facilitating early mobilization and detection of adverse events which will translate to further improvement of outcomes and additional cost-savings.
Cost-savings can also be increased by improved implementation practices such as personnel training and clinical protocols. A recent report by Dykes et al. shows that concerted efforts to optimize continuous monitoring significantly improves patient health outcomesCitation26,Citation61. Dykes et al. demonstrated that a targeted program designed to optimize continuous monitoring results in further reductions in hospital LOS, ICU transfer rate, and ICU LOS compared to pre-optimization levelsCitation26. Importantly, Dykes et al. utilized the same technology as Brown et al. which served as the source for several key clinical data inputs in the currently reported analysisCitation20. Utilizing the clinical parameter values for the optimized continuous monitoring cohort in Dykes et al. in our current analysis would generate an estimated cost reduction of $7,515,758 per medical-surgical unit annually, which is $686,339 more savings compared to our estimates when using an 100% adoption rate. However, this study did not report clinical parameter values for patients that did not receive continuous monitoring (i.e. standard of care) and therefore was not used for our analysis. Nonetheless, this observation reveals how advancements in continuous monitoring will accelerate cost-savings in the years to come.
It is also notable that in the United States health system, continuous monitoring technologies in the medical-surgical unit are currently not separately reimbursable and will have their costs covered through the diagnosis related group (DRG) system of CMS and private payers. It is thus essential that the health-related economic outcomes associated with these technologies be validated to provide decision makers with the information required to optimally allocate capital to address their patient populations most pressing needs and to justify the additional costs that hospitals may bear. It is also critical to note that savings will be most readily realized with monitoring technologies that are associated with high caregiver acceptance, patient compliance, strong technical performance in parameter measurements, and continuous connectivity of the monitoring system to the hospital IT infrastructure. Considerable variation exists among monitoring system performance which may directly impact patient outcomes and potential costsCitation62.
Limitations
Our study has several limitations. First, our clinical parameters are derived from data collected at two medical-surgical units, which may not be representative of all the hospitals in the U.S. Variations in healthcare practice may change the LOS and health outcomes experienced by patients, which would affect the cost-savings presented in the current report. The underlying health condition of patients may also vary across hospital systems which could impact the costs and potential cost-savings of continuous monitoring. For instance, it is plausible that localities with aging populations could result in higher admissions of patients that have a higher incidence of comorbidities that complicate care plans, increase the rate of unplanned ICU transfers, and increase the risk of mortalityCitation56. The studies included in our analysis had patient cohorts with mean ages of ∼49 years of age for Brown et al. and ∼60 years of age for Weller et al. Citation20,Citation27. Brown et al. reported a mean Charlson score (a metric for comorbidities) of ∼1.8 for the patients included in their studyCitation20,Citation27. Variations in the age and pre-existing comorbidities of the patient populations being treated could alter the baseline hospital costs for treatment and subsequent estimations of cost-savings that may occur when implementing continuous monitoring technology. It is currently unknown whether continuous vital sign monitoring is more advantageous for specific patient cohorts and how the patient characteristics may impact its efficacy; these considerations warrant future research. Nonetheless, the clinical parameters used to generate our findings represent observation from 8,379 patients and serves as a robust sample size for drawing conclusions.
As previously discussed, we did not include the cost of implementing continuous monitoring devices due to the multitude of available monitoring device technologies, their associated costs, and the variance in cost differences that occur between hospital systems. While this is a limitation, our approach allows for decision makers to apply our findings toward the economic evaluation of implementing a technology of their choosing. In addition, the costs associated with implementing continuous vital sign monitoring are likely to change over time as these technologies are further developed. Our cost-savings analysis only considers cost-savings for medical-surgical units LOS for patients without ICU transfer, medical-surgical units LOS prior to ICU transfer, ICU LOS, RRT activation, and IHCA-associated costs. Notably, the cost inputs used for our analyses are derived from large databases (e.g. HCUP) and the aggregate nature of these values should confer generalized applicability to U.S.-based hospitals. In the case of RRT, the estimated costs may underrepresent actual costs for additional non-physician personnel to ensure timely response to emergencies and in cases where RRT events exceed the time utilized for our estimations. Our analysis also does not factor in the cost-savings from other patient benefits. For instance, wireless wearable continuous monitoring technologies will allow earlier ambulation, reduced risk of venous thromboembolism, improved pulmonary function, and decreased pressure ulcersCitation46,Citation47,Citation63. In addition, interviews with healthcare workers suggest that continuous monitoring may decrease workload and provide reassuranceCitation54. Continuous monitoring has also been shown particularly important for detecting OIRD; OIRD increases hospital costs and continuous monitoring has been shown to result in improved patient outcomes that would ameliorate increased costsCitation6,Citation50.
Our analyses also do not include the cost-savings that may benefit patients due to improved health outcomes such as lower risk for long-term disability and societal benefits such as increased time to return-to-workCitation64. While preliminary results indicate the feasibility for savings to occur in the aforementioned areas, the currently available data regarding continuous monitoring and the respective outcomes is not robust enough to reliably make such analyses.
Conclusion
Our findings demonstrate that, for a medical-surgical unit in an average-sized U.S. community hospital, there is a potential for cost-savings of over $6.8 million dollars per year by improving health outcomes through the implementation of continuous monitoring in the medical-surgical unit. This study provides the most up-to-date analysis on the economic impact for implementing continuous monitoring in hospital medical-surgical units based on the currently available evidence. Nonetheless, future studies are required to determine how emerging continuous monitoring technologies can provide further benefits for both patients and hospitals. Our findings will help equip decision-makers with informative evidence for improving patient healthcare while ensuring the financial health of their institution.
Transparency
Author contributions
All authors participated in the design of this study. The analysis was performed by JB, AS, WJ, HH, and HY. The writing for this manuscript was reviewed and edited by all authors. All authors approved of the final version of this manuscript.
Reviewer disclosures
Peer reviewers on this manuscript have no relevant financial or other relationships to disclose.
Acknowledgements
Writing and editorial support was provided by Yu-Shan at Boston Strategic Partners, Inc. (funded by GE HealthCare).
Declaration of financial/other relationships
JB and AS are employees of GE HealthCare. WJ, HH, and HY are employees of Boston Strategic Partners. ME is a member of Mary Erslon, LLC. FO is a member of Heuristica, LLC.
Additional information
Funding
References
- Vlayen A, Verelst S, Bekkering GE, et al. Incidence and preventability of adverse events requiring intensive care admission: a systematic review. J Eval Clin Pract. 2012;18(2):485–497.
- Calzavacca P, Licari E, Tee A, et al. The impact of Rapid Response System on delayed emergency team activation patient characteristics and outcomes–a follow-up study. Resuscitation. 2010;81(1):31–35.
- Churpek MM, Yuen TC, Edelson DP. Predicting clinical deterioration in the hospital: the impact of outcome selection. Resuscitation. 2013;84(5):564–568.
- Sauro KM, Soo A, de Grood C, et al. Adverse events after transition from ICU to hospital ward: a multicenter cohort study. Crit Care Med. 2020;48(7):946–953.
- Young MP, Gooder VJ, McBride K, et al. Inpatient transfers to the intensive care unit: delays are associated with increased mortality and morbidity. J Gen Intern Med. 2003;18(2):77–83.
- Khanna AK, Bergese SD, Jungquist CR, et al. Prediction of opioid-induced respiratory depression on inpatient wards using continuous capnography and oximetry: an international prospective, observational trial. Anesth Analg. 2020;131(4):1012–1024.
- Burke JR, Downey C, Almoudaris AM. Failure to rescue deteriorating patients: a systematic review of root causes and improvement strategies. J Patient Saf. 2022;18(1):e140–e155.
- The Joint Commission. Pain assessment and management standards for critical access hospitals 2018. [cited 2023 Jan 12]. https://www.jointcommission.org/-/media/tjc/documents/standards/r3-reports/r3_15_pain_assess_mgmt_cah_6_22_18_final.pdf.
- Centers for Medicare & Medicaid Service. FY 2023 Hospital Inpatient Prospective Payment System (IPPS) and Long Term Care Hospitals (LTCH PPS) Proposed Rule - CMS-1771-P2022 [cited 2023 Jan 10]. https://public-inspection.federalregister.gov/2022-08268.pdf?mkt_tok=NzEwLVpMTC02NTEAAAGD3kqtT2R1iNStDVI043sInTvZRPNeHskNEhx5LNQMhTBJvcSj44O6HOeJqCay1tY3mkShz1yb2iv7CrcLS8AYSylPua10sufe57fRm-ghdwm7.
- DeVita MA, Smith GB, Adam SK, et al. “Identifying the hospitalised patient in crisis”—a consensus conference on the afferent limb of rapid response systems. Resuscitation. 2010;81(4):375–382.
- Tirkkonen J, Skrifvars MB, Tamminen T, et al. Afferent limb failure revisited–a retrospective, international, multicentre, cohort study of delayed rapid response team calls. Resuscitation. 2020;156:6–14.
- Bates DW, Levine DM, Salmasian H, et al. The safety of inpatient health care. N Engl J Med. 2023;388(2):142–153.
- Mann KD, Good NM, Fatehi F, et al. Predicting patient deterioration: a review of tools in the digital hospital setting. J Med Internet Res. 2021;23(9):e28209.
- McGloin H, Adam SK, Singer M. Unexpected deaths and referrals to intensive care of patients on general wards. Are some cases potentially avoidable? J R Coll Physicians Lond. 1999;33(3):255–259.
- Fieselmann JF, Hendryx MS, Helms CM, et al. Respiratory rate predicts cardiopulmonary arrest for internal medicine inpatients. J Gen Intern Med. 1993;8(7):354–360.
- Jones D, Duke G, Green J, et al. Medical emergency team syndromes and an approach to their management. Crit Care. 2006;10(1):R30.
- Zhao Z, Chen A, Hou W, et al. Prediction model and risk scores of ICU admission and mortality in COVID-19. PLoS One. 2020;15(7):e0236618.
- Churpek MM, Yuen TC, Winslow C, et al. Multicenter comparison of machine learning methods and conventional regression for predicting clinical deterioration on the wards. Crit Care Med. 2016;44(2):368–374.
- Ghosh E, Eshelman L, Yang L, et al. Description of vital signs data measurement frequency in a medical/surgical unit at a community hospital in United States. Data Brief. 2018;16:612–616.
- Brown H, Terrence J, Vasquez P, et al. Continuous monitoring in an inpatient medical-surgical unit: a controlled clinical trial. Am J Med. 2014;127(3):226–232.
- Eddahchouri Y, Koeneman M, Plokker M, et al. Low compliance to a vital sign safety protocol on general hospital wards: a retrospective cohort study. Int J Nurs Stud. 2021;115:103849.
- Howell MD, Ngo L, Folcarelli P, et al. Sustained effectiveness of a primary-team-based rapid response system. Crit Care Med. 2012;40(9):2562–2568.
- Turan A, Chang C, Cohen B, et al. Incidence, severity, and detection of blood pressure perturbations after abdominal surgery: a prospective blinded observational study. Anesthesiology. 2019;130(4):550–559.
- Sun Z, Sessler DI, Dalton JE, et al. Postoperative hypoxemia is common and persistent: a prospective blinded observational study. Anesth Analg. 2015;121(3):709–715.
- Weenk M, Koeneman M, van de Belt TH, et al. Wireless and continuous monitoring of vital signs in patients at the general ward. Resuscitation. 2019;136:47–53.
- Dykes PC, Lowenthal G, Lipsitz S, et al. Reducing ICU utilization, length of stay, and cost by optimizing the clinical use of continuous monitoring system technology in the hospital. Am J Med. 2022;135(3):337–341 e1.
- Weller RS, Foard KL, Harwood TN. Evaluation of a wireless, portable, wearable multi-parameter vital signs monitor in hospitalized neurological and neurosurgical patients. J Clin Monit Comput. 2018;32(5):945–951.
- Boatin AA, Wylie BJ, Goldfarb I, et al. Wireless vital sign monitoring in pregnant women: a functionality and acceptability study. Telemed J E Health. 2016;22(7):564–571.
- Watkinson PJ, Barber VS, Price JD, et al. A randomised controlled trial of the effect of continuous electronic physiological monitoring on the adverse event rate in high risk medical and surgical patients. Anaesthesia. 2006;61(11):1031–1039.
- Stolbrink M, McGowan L, Saman H, et al. The Early Mobility Bundle: a simple enhancement of therapy which may reduce incidence of hospital-acquired pneumonia and length of hospital stay. J Hosp Infect. 2014;88(1):34–39.
- Kehlet H, Wilmore DW. Multimodal strategies to improve surgical outcome. Am J Surg. 2002;183(6):630–641.
- Baxter R, Squiers J, Conner W, et al. Enhanced recovery after surgery: a narrative review of its application in cardiac surgery. Ann Thorac Surg. 2020;109(6):1937–1944.
- Jarvela K, Takala P, Michard F, et al. Clinical evaluation of a wearable sensor for mobile monitoring of respiratory rate on hospital wards. J Clin Monit Comput. 2022;36(1):81–86.
- Mylotte D, Osnabrugge RLJ, Windecker S, et al. Transcatheter aortic valve replacement in Europe: adoption trends and factors influencing device utilization. J Am Coll Cardiol. 2013;62(3):210–219.
- Barasa EW, Molyneux S, English M, et al. Setting healthcare priorities in hospitals: a review of empirical studies. Health Policy Plan. 2015;30(3):386–396.
- Hatz MH, Schreyogg J, Torbica A, et al. Adoption decisions for medical devices in the field of cardiology: results from a European survey. Health Econ. 2017;26 Suppl 1(Suppl 1):124–144.
- Leenen JPL, Leerentveld C, van Dijk JD, et al. Current evidence for continuous vital signs monitoring by wearable wireless devices in hospitalized adults: systematic review. J Med Internet Res. 2020;22(6):e18636.
- American Hospital Association Fast Facts on U.S. Hospitals, 2021. 2021. [cited 2022 Oct 27]. https://www.aha.org/statistics/fast-facts-us-hospitals.
- Agency for Healthcare Research and Quality Healthcare Cost and Utilization Project (HCUPnet) 2022. [cited 2022 Oct 12]. https://datatools.ahrq.gov/hcupnet
- Husereau D, Drummond M, Petrou S, et al. Consolidated health economic evaluation reporting standards (CHEERS)–explanation and elaboration: a report of the ISPOR health economic evaluation publication guidelines good reporting practices task force. Value Health. 2013;16(2):231–250.
- Halpern NA, Goldman DA, Tan KS, et al. Trends in critical care beds and use among population groups and medicare and medicaid beneficiaries in the United States: 2000–2010. Crit Care Med. 2016;44(8):1490–1499.
- Fernando SM, McIsaac DI, Rochwerg B, et al. Frailty and associated outcomes and resource utilization following in-hospital cardiac arrest. Resuscitation. 2020;146:138–144.
- Fiero ML, Rosenblatt S, Zhang B, et al. Providing early attending physician expertise via telemedicine to improve rapid response team evaluations. Pediatr Crit Care Med. 2020;21(5):e221–e227.
- U.S. Inflation Calculator U.S. Inflation Calculator 2022. [cited 2022 Oct 10]. https://www.usinflationcalculator.com/.
- Grennan M, Swanson A. Transparency and negotiated prices: the value of information in hospital-supplier bargaining. J Pol Econ. 2020;128(4):1234–1268.
- Slight SP, Franz C, Olugbile M, et al. The return on investment of implementing a continuous monitoring system in general medical-surgical units. Crit Care Med. 2014;42(8):1862–1868.
- Khanna AK, Jungquist CR, Buhre W, et al. Modeling the cost savings of continuous pulse oximetry and capnography monitoring of United States general care floor patients receiving opioids based on the PRODIGY trial. Adv Ther. 2021;38(7):3745–3759.
- Subbe CP, Duller B, Bellomo R. Effect of an automated notification system for deteriorating ward patients on clinical outcomes. Crit Care. 2017;21(1):52.
- Vroman H, Mosch D, Eijkenaar F, et al. Continuous vital sign monitoring in patients after elective abdominal surgery: a retrospective study on clinical outcomes and costs. J Comp Eff Res. 2023;12(2):e220176.
- Khanna AK, Saager L, Bergese SD, et al. Opioid-induced respiratory depression increases hospital costs and length of stay in patients recovering on the general care floor. BMC Anesthesiol. 2021;21(1):88.
- Stites M, Surprise J, McNiel J, et al. Continuous capnography reduces the incidence of opioid-induced respiratory rescue by hospital rapid resuscitation team. J Patient Saf. 2021;17(6):e557–e561.
- McGrath SP, McGovern KM, Perreard IM, et al. Inpatient respiratory arrest associated with sedative and analgesic medications: impact of continuous monitoring on patient mortality and severe morbidity. J Patient Saf. 2021;17(8):557–561.
- Downey CL, Brown JM, Jayne DG, et al. Patient attitudes towards remote continuous vital signs monitoring on general surgery wards: an interview study. Int J Med Inform. 2018;114:52–56.
- Weenk M, Bredie SJ, Koeneman M, et al. Continuous monitoring of vital signs in the general ward using wearable devices: randomized controlled trial. J Med Internet Res. 2020;22(6):e15471.
- Prgomet M, Cardona-Morrell M, Nicholson M, et al. Vital signs monitoring on general wards: clinical staff perceptions of current practices and the planned introduction of continuous monitoring technology. Int J Qual Health Care. 2016;28(4):515–521.
- Fuchs L, Chronaki CE, Park S, et al. ICU admission characteristics and mortality rates among elderly and very elderly patients. Intensive Care Med. 2012;38(10):1654–1661.
- McGrath SP, Taenzer AH, Karon N, et al. Surveillance monitoring management for general care units: strategy, design, and implementation. Jt Comm J Qual Patient Saf. 2016;42(7):293–302.
- Leenen JPL, Dijkman EM, van Dijk JD, et al. Feasibility of continuous monitoring of vital signs in surgical patients on a general ward: an observational cohort study. BMJ Open. 2021;11(2):e042735.
- Taenzer AH, Pyke JB, McGrath SP, et al. Impact of pulse oximetry surveillance on rescue events and intensive care unit transfers: a before-and-after concurrence study. Anesthesiology. 2010;112(2):282–287.
- Zisberg A, Shadmi E, Sinoff G, et al. Low mobility during hospitalization and functional decline in older adults. J Am Geriatr Soc. 2011;59(2):266–273.
- Dykes PC, Lowenthal G, Faris A, et al. An implementation science approach to promote optimal implementation, adoption, use, and spread of continuous clinical monitoring system technology. J Patient Saf. 2021;17(1):56–62.
- Breteler MJM, KleinJan EJ, Dohmen DAJ, et al. Vital signs monitoring with wearable sensors in high-risk surgical patients: a clinical validation study. Anesthesiology. 2020;132(3):424–439.
- Koenders N, Weenk M, van de Belt TH, et al. Exploring barriers to physical activity of patients at the internal medicine and surgical wards: a retrospective analysis of continuously collected data. Disabil Rehabil. 2021;43(13):1883–1889.
- Kamdar BB, Suri R, Suchyta MR, et al. Return to work after critical illness: a systematic review and meta-analysis. Thorax. 2020;75(1):17–27.