Abstract
Objectives
Paediatric growth hormone deficiency (pGHD) manifests as growth failure associated with inadequate growth hormone (GH) production. Daily injections of recombinant human GH (dGH) [somatropin] is the current standard of care, which has been shown to be well tolerated and effective, but associated with suboptimal adherence, leading to reduced effectiveness. Somatrogon, a once-weekly injectable long-acting human GH, has demonstrated clinical non-inferiority and significantly lower life interference (i.e. treatment burden) vs. somatropin in two Phase 3 studies. This work evaluated cost-effectiveness and cost-utility of somatrogon vs dGHs from an Irish payer perspective.
Methods
A Markov model was developed for patients starting somatrogon or dGHs treatment at 3–12 years and continuing up to achievement of near adult height (NAH), with growth driven by trial-based height velocity (HV) and treatment-specific adherence. Patients could discontinue treatment at the end of Year 1 (4%). DGH adherence (95.3%–65% over treatment duration) and adherence-growth relationship were based on published evidence. Higher Year 1 adherence of 4%, tapering over time, for somatrogon vs. dGHs was based on clinical consultation. Treatment costs, monitoring costs and costs due to different wastage types (device setting and adherence) were sourced from local data. Health utilities based on height and injection frequency were derived from published literature. Scenario analysis, deterministic and probabilistic sensitivity analysis were performed.
Results
Somatrogon treatment led to 1.87–3.66 cm greater NAH gain and 0.21–0.50 higher quality adjusted life years (QALYs) vs. dGHs, across the base case and scenarios evaluated. Somatrogon treatment was associated with cost savings of €5,699–€21,974 and lower cost per cm gained vs. dGHs (€197–€527), per patient. Somatrogon was cost-effective vs. dGHs, with the result consistent across the sensitivity analyses conducted.
Conclusion
Somatrogon weekly injections were estimated to result in higher NAH, higher QALYs, lower overall costs and lower costs per cm gained than dGHs, in pGHD.
Introduction
Paediatric growth hormone deficiency (pGHD) is a rare disease, with estimated prevalence of 1 in 3,480–4,000 children in the United KingdomCitation1 and United StatesCitation2 and up to 1:29,000 in BelgiumCitation3. PGHD is caused by the disruption of growth hormone (GH) secretion due to abnormalities in the pituitary gland or hypothalamus, which results in GH deficiency manifesting in short stature and altered body composition, with higher accumulation of body fatCitation4,Citation5. While variations in pGHD diagnostic criteria exists among practitioners, poor height velocity (HV) and failure of two growth hormone stimulatory tests are commonly used to diagnose pGHDCitation4,Citation6,Citation7.
Children diagnosed with pGHD are commonly treated with daily growth hormones (dGHs), administered by daily subcutaneous injectionCitation6, up until 18–19 years of ageCitation6,Citation8, with end of treatment based on personalized assessment driven by attainment of near adult height (NAH) and bone maturationCitation7. Daily GHs have been proven well tolerated and effective for long-term use in treatment of pGHDCitation4,Citation6,Citation9,Citation10 and prescribed in a starting dosage of 0.16–0.24 mg/kg/week, with potential individualization of subsequent dosingCitation6. A mean initial dose of 0.20–0.22 mg/kg/week was observed in the full KIGS cohort analysis (Citation11 – largest and longest running international database of children treated with GHs), and a mean dose of 0.224 mg/kg/week and 0.322 mg/kg/week over the treatment duration was observed in the NordiNet IOS (Europe) and ANSWER (United States) real-world studiesCitation12.
Adherence is an essential prerequisite for any therapy to be effective, with the risk of non-adherence associated with dGHs being highCitation7, given the need of timely and accurately dosed injections every day, which poses a significant burden on children and parentsCitation13–16. Poor adherence associated with dGHs is well documented in the literatureCitation7,Citation17, with multiple causing factors identifiedCitation18–20, including social circumstances, injection problems (e.g. needle or injection phobia), lack of education about benefits of treatment (children and parents), and “injection fatigue” since pGHD is a chronic condition potentially treated over many yearsCitation19–21.
The clinical benefit of adherence is well established in the pGHD literature, with multiple studies across different countries that have quantified the strong association between poor adherence and poorer clinical outcomesCitation17,Citation22–25. A national study of 177 pGHD patients in New Zealand demonstrated a statistically significant reduction in HV standard deviation scores (HVSDS) for medium adherence patients (missing two doses of dGH per week) and low adherence patients (missing at least three doses per week) compared to high adherence patients (missing at most one dose per week)Citation23. Similar findings were observed in three studies; a retrospective study in Italy, which demonstrated that HV was directly related to treatment adherenceCitation25, a prospective study in Turkey demonstrating that patients with excellent and good adherence to dGHs had better HV and HVSDS after the first year of therapy vs. fair and poor adherence patients (HV at 12 months of 9.1 cm/year vs. 7.6 cm/year)Citation22, and a UK study that showed lower HV in pGHD patients missing more than two dGHs injections per week vs up to one per week (4.6 cm/year vs. 6.6–7.8 cm/year)Citation26. Two further studies quantified a loss of height gain of 0.11 SD for a single dose missed per week (Australia)Citation7, and an average 1.8 cm gain over one year for adherent patients compared with nonadherent patientsCitation27. Similar impact has been reported in observational multi-countryCitation28 and nationalCitation29,Citation30 studies where dGH treatment delivery by EasyPod device was investigated, with an increase in HV of 1,1 cm/year for each 10% change in adherence. In addition, the direct relationship between adherence, HV and final height was recently confirmed by clinical experts consulted by CADTHCitation31.
Somatrogon is a long-acting growth hormone (LAGH), newly developed to be administered by weekly injection to address the burden of daily injections to treat pGHD, and which could potentially improve compliance. Somatrogon was investigated in a 12 months open-label randomized, active controlled, phase 3 noninferiority study that compared the efficacy and safety of once-weekly somatrogon (0.66 mg/kg/week) vs. once-daily somatropin (0.24 mg/kg/week) in treatment naïve children with pGHDCitation32. Somatrogon demonstrated noninferiority vs. somatropin (HV at 12 months of 10.10 cm/year vs. 9.78 cm/year, respectively) with a comparable safety profile. In a separate randomized cross-over study comparing 12 weeks of somatrogon vs. 12 weeks of somatropin, somatrogon demonstrated significantly lower life interference (i.e. treatment burden), greater convenience and a more favourable treatment experienceCitation33.
While somatrogon demonstrated non-inferiority over somatropin, long-term implications beyond the trial duration of weekly injections on patients’ quality of life (QoL), treatment adherence and healthcare costs need to be quantified. Therefore, the objective of the current study is to perform cost-effectiveness and cost-utility analysis of somatrogon vs. dGHs, the current standard of care, from the Irish healthcare perspective.
Methods
Previous pGHD economic models published in the last 20 years were searched on PubMed to conceptualize the economic model and guide decision of model structure and modelling assumptions. Further targeted searches were conducted with respect to the National Institute for Health and Care Excellence (NICE), Scottish Medicines Consortium (SMC), Canadian Agency for Drugs and Technologies in Health (CADTH), Spanish Agency for Health Technology Assessment (AETS), Institute for Clinical and Economic Review (ICER) and National Centre for Pharmacoeconomics (NCPE). Ten studies have been identified, four of them conducted a cost-minimization analysis using a simple cost-calculator structureCitation34–37, while the remaining six studies focussed on cost-effectiveness analysis (CEA)Citation8,Citation37–39or cost-utility analysis (CUA)Citation1,Citation40,Citation41. Focussing on the CEAs/CUAs, most studies adopted a cohort approach and employed as a model structure either a decision treeCitation39, a multi-level equation derived from a databaseCitation38, or a Markov model with either two health states (alive and death)Citation1 or three health states (alive not treated, alive treated, death)Citation40. Only one study was identified which pursued a patient level simulation using a hybrid model structure with a decision-tree followed by a Markov model after the first year of treatmentCitation8. The impact of treatment adherence was explored in five modelsCitation1,Citation8,Citation35,Citation39,Citation40, lifetime time horizon was adopted in three of the analyses identifiedCitation1,Citation40,Citation41 and three studies considered treatment wastageCitation8,Citation35,Citation42.
An economic model to conduct CEA and CUA for somatrogon vs. dGHs was developed, based on the reviewed pGHD economic literature and the desire to capture the impact of somatrogon on adherence and patients’ QoL (in terms of reduced treatment burden) due to reduced injections frequency vs. dGHs. Cost-minimization analysis (CMA) was not considered since, as described in the literatureCitation43–45, it is rarely appropriate given the need of assuming a priori that there are no differences between treatments, other than costs. This assumption can be tested and quantified in the CEA/CUA framework, which is therefore preferred.
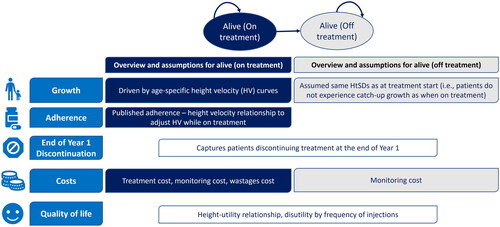
Similar to previous economic models, a Markov cohort model was developed with two health states () (Alive and on treatment and Alive and off treatment) and one year cycle lengthCitation1,Citation40. The modelled cohort includes pre-pubertal children (3–11 years for girls and 3–12 years for boy) with either isolated GHD or GH insufficiency as part of multiple pituitary hormone deficiency and treatment naïve, aligned with somatrogon phase 3 trial populationCitation32. Each yearly age band is run separately in the model, given that height SDs (HtSDs) gain in the first year of therapy (and consequent years) depends on the starting ageCitation8,Citation46.
The analysis time horizon includes treatment up to 18 years of age, with death not considered given that no excess mortality is associated with pGHD and the mortality rate up to the end of adolescence is limited (10–12 per 100,000 person-years in a recent study of 4,324 GHD childrenCitation47) However, note that this modelling choice is conservative for treatments leading to higher NAH, given that higher HtSDs are associated with higher utility among adultsCitation8,Citation40. Therefore, structural uncertainty is explored in scenario analysis adopting a lifetime horizonCitation1,Citation40,Citation41 and including a Death health state.
At the model start, pGHD children either start off on treatment with either somatrogon or with a dGH, with the modelled growth dependent on starting age, treatment received and treatment adherence. Patients may discontinue treatment at the end of first year, as in previous studiesCitation39,Citation41,Citation48, after which patients are assumed to remain on treatment until the end of the time horizon. Patients off-treatment are assumed to revert to the initial HtSDs, thus capturing that these patients will still experience growth but will not experience catch-up growth. This assumption was previously usedCitation1, and it is based on a middle-ground across two considerations: (1) off-treatment patients might experience a fraction of catch-up growth; (2) off-treatment patients might fall back below their initial HtSDs.
The economic model considers treatment costs (accrued while on treatment) and monitoring costs (stratified by on and off treatment) and includes different types of treatment wastages, in line with previously published modelsCitation8,Citation35,Citation42: (1) device setting wastage that occurs due to dosing increments of the device being higher than the prescribed dosage; (2) last dose wastage due to the remaining product in the cartridge not worthy a second injection; (3) storage wastage due to the expiration of the product (commonly after 21–28 days); (4) preparation wastage due to the inherent preparation and use of the device; (5) adherence wastage which is intended to capture paid but not used medicine due to missed injections and is calculated as yearly proportion of missed doses multiplied by the overall daily/weekly treatment costs.
Daily GHs were modelled as a basket of available treatments in Ireland, with the same efficacy, since essentially they are all the same molecule (somatropin)Citation8, but a weighted average cost across available daily GHs, to account for differences in unit costs and product formulation (e.g. device size, storage life) that drives overall dGHs treatment cost and wastages costCitation1,Citation8. In fact, comparing somatrogon vs. a specific dGH would not be representative of the real-world landscape and would bias the results depending on whether the dGH with the lowest or highest cost per mg is chosen.
A very limited number of serious adverse events (SAEs) was observed in the somatrogon trial (5 out of 224 patients experienced SAEs) and the number of SAEs was comparable in the two trial armsCitation32, thus AEs were not considered in the model. This is consistent with previous pGHD economic models comparing different dGHs or dGHs vs. no treatment. Only Christensen et al. considered AEs as a scenario assuming that all patients treated with dGHs would require an additional endocrinologist visit per year due to AEsCitation40,Citation41,Citation49.
The model structure, assumptions and inputs choices when multiple sources were available in the literature were discussed with clinical experts from different countries (Canada, Italy, Spain, United States) and adapted based on the feedback received. Furthermore, the additional advice received from clinical experts consulted by CADTH is publicly available and has been considered in the current analysisCitation31.
The manuscript was developed in alignment with the Consolidated Health Economic Evaluation Reporting Standards (CHEERS) 2022 guidelines (Supplementary Table 7)Citation50.
Model inputs
Clinical inputs
The clinical inputs are summarized in , with patient characteristics and distribution across age bands derived from the somatrogon phase 3 clinical trialCitation32. The proportion of patients discontinuing at the end of Year 1 (4%) was based on previous economic modelsCitation48,Citation50, with higher values of 20%–30% reported in the literatureCitation39,Citation41. Based on consultation with clinical experts it has been confirmed that 4%–20% is a plausible range of end of Year 1 discontinuation, with Spanish experts highlighting that the lower estimates would be more applicable to their settings, while Canadian experts suggesting that end of Year 1 discontinuation would be much higher than 1% but much lower than 20% (CADTH evaluated 10% in scenario analysis)Citation31. Therefore, alternative values of 10% and 20% were considered in scenario analysis.
Table 1. Clinical inputs.
Height and BMI were sourced from WHO Child Growth StandardsCitation51 for ages of 2–4 years and from British 1990 growth referenceCitation52 for ages of 4–20 years (assumed to be applicable to Ireland) and used to extrapolate growth and derive age specific BMI, respectively. Year 1 HV was obtained from the somatrogon phase 3 trialCitation32, stratified by treatment and by age bands (3–7 and 8–12 years old). From Year 2 of treatment onwards, height velocity was extrapolated applying the yearly reduction in HtSDs gain observed in the literature (average of 40.54%)Citation46, assumed to be constant over time and the same for somatrogon and dGHs, with the resulting HV curves summarized in the Supplementary Figures 3 and 4). Based on clinical experts’ consultationCitation31 and somatrogon non-inferiority trial design, the impact of assuming the same HV for somatrogon and dGHs was explored in scenario analysis.
Treatment adherence with dGH was derived from a recent real-world study, where a 2.2% yearly decrease in adherence was observedCitation25. Somatrogon adherence is not currently available in the real world, thus consultations with clinical experts were conducted, which recommended that a 4% increase in Year 1 adherence for somatrogon vs. dGHs is plausible given the weekly injection schedule and the results of the phase 3 randomized controlled cross-over trial, the “Treatment Burden” studyCitation33 (which showed treatment burden was reduced, and the overall treatment experience was preferred with weekly somatrogon, supporting improved adherence with somatrogon), with the incremental difference vs. dGHs tapering over the treatment duration. Scenario analysis with lower (2%) and higher (6%) Year 1 incremental adherence for somatrogon was considered due to the input uncertainty and clinical experts’ opinion, highlighting that a 5% adherence difference between dGHs and somatrogon in Year 1 is reasonable and might not be tapering over timeCitation31.
Finally, the relationship between adherence and HV was derived from the same adherence source for dGHsCitation25, supported by internal and external clinical expertsCitation31, and was assumed the same between dGHs and somatrogon, since no evidence is available demonstrating differences in the adherence-HV relationship between dGHs and somatrogon. A similar adherence-HV relationship was observed in other studiesCitation22,Citation26 and included in the scenario analyses.
Utility inputs
Quality adjusted life years were calculated based on patients’ HtSDs for each year in the time horizon, and the utility-HtSDs relationship for the adult population used in previous economic analysesCitation1,Citation8,Citation40,Citation49 () was used, given the lack of paediatric specific utilities. Life interference caused by frequency of treatment administration has been captured through added utility for reduced frequency of injections, based on available literature in adults with diabetes mellitus or haemophilia, which reported an added utility of 0.015–0.043Citation53–58, depending on the study country and the extent of frequency reduction considered. In the base case (), an annual added utility of 0.023Citation53 for the difference in weekly injections vs. daily injections was considered, which is a more conservative value, as it was the lowest value among the studies where weekly vs. daily injections were compared.
Table 2. related utilities by HTSDs scores.
Cost inputs
Yearly dGH treatment costs were calculated based on dGHs market shares specific to the Irish marketCitation59, brand and device specific unit costs and device size (). The weekly cost per kg for dGHs ranged from €5.13–€6.58 vs. €4.90 for somatrogon, with illustrative yearly cost for an average child of 15 kg, 30 kg and 50 kg summarized in Supplementary Table 8.
Table 3. Treatment formulation, recommended dosage and unit costs.
Among the different wastages that the economic model could capture, only device setting wastage (related to discrepancy between injection device’s dose setting and prescribed dose) and adherence wastage were considered, since significant data gaps were identified for the preparation wastage, while storage wastage and last dose wastage are expected to be similar across the treatments.
A dose of 0.66 mg/kg/week for somatrogon and 0.24 mg/kg/week (0.034 mg/kg/day) for dGHs was used in the analysis, consistent with the Phase 3 trialCitation32 and within the dGHs dosing range of 0.175–0.245 mg/kg/week (0.025–0.035 mg/kg/day) recommended by the HPRACitation60. While the dGHs dose of 0.24 mg/kg/week is close to the upper bound of the HPRA recommended range, using the mid value of 0.21 mg/kg/week or the lower value of 0.175 mg/kg/week is equivalent to a 12.5%–27.1% dGHs dose reduction, which is comparable to missing one to two injections per week (doses missed: 1/7 = 14.2%, 2/7 = 28.6%). There is extensive evidence in the literature that missing more than 1 injection per week leads to reduce height-velocity compared to fully adherent patientsCitation22,Citation23,Citation25,Citation27, thus assuming that a lower dGHs dose compared to the dose used in the Phase 3 trial would have no impact on the effectiveness of dGHs cannot be assumed. Furthermore, the model explicitly accounts for the adherence-HV relationships. Therefore, a dGHs dose of 0.24 mg/kg/week was used in the base case, while 0.224 mg/kg/week (from RWECitation12) and 0.21 mg/kg/week (from HRPACitation60) was considered in scenario analyses.
Monitoring costs () are based on resource use frequency from previous pGHD economic modelsCitation1,Citation40,Citation49, with the same frequency assumed between dGHs and somatrogon, given the lack of evidence supporting differences in monitoring frequencies.
Table 4. Monitoring costs.
Analyses
The base case analysis focussed on pGHD patients starting treatment at 3–12 years of age () and either receiving dGHs (0.24 mg/kg/week) or somatrogon (0.66 mg/kg/week), up to 18 years of age. The costs and health outcomes were discounted at 4%Citation61 () and the analysis considered an Irish healthcare payer perspective, which included treatment costs, wastage costs (due to adherence and device setting) and monitoring costs. Extensive scenario analysis was conducted to quantify the impact of alternative sources or assumptions (Supplementary Table 5).
The outcomes of interest for the analysis were total and incremental costs, quality-adjusted life years (QALYs), near adult height (NAH – cm) and incremental cost-effectiveness ratio (ICER) per QALY, which was compared vs. the willingness-to-pay threshold (WTP) in Ireland of €20,000 and €45,000Citation61.
Deterministic sensitivity analysis
Each model parameter was varied, one at a time, in deterministic sensitivity analysis (DSA), to the lower and upper bounds. The lower and upper bounds were based on the 95% confidence intervals (CIs) of the literature estimate. If the input uncertainty estimate was not available in the literature, the lower and upper bounds were derived assuming ± 10% of the mean value used in base case.
Probabilistic sensitivity analysis
In the probabilistic sensitivity analysis (PSA), random samples of all model inputs were simultaneously used for each model run. This process was repeated 1,000 times and the PSA results derived as the average across all PSA runs. Utility and other binary values were assumed to follow beta distribution, to be bounded between 0 and 1. Event rates and costs were assumed to follow a gamma distribution, to be bounded to positive values only. For HtSDs and HV, the normal distribution was assumed. The assumed distributions followed modelling best practiceCitation62.
Results
Base case analysis
Results of the base case analysis are presented in . Treatment with somatrogon demonstrated lower costs, higher growth and higher QALYs vs. dGHs. Specifically, somatrogon treatment led to €17,548 cost savings, 2.85 cm higher NAH and 0.27 higher QALYs vs. dGHs, which resulted in a lower cost per cm gained (-€421 difference) and somatrogon being dominant (higher QALYs and lower costs), thus cost-effective, vs. dGHs. The predicted change in HtSDs at the end of treatment was 1.85 for somatrogon and 1.43 for dGHs (initial HtSDs was −2.86 – see ), with somatrogon treatment associated with 0.42 higher gain in HtSDs vs. dGHs. The incremental net monetary benefit was €22,978–€29,764 at the €20,000–€45,000 WTP threshold.
Table 5. Base case analysis results.
Scenario analysis
An extensive range of scenarios were evaluated, with the results summarized in the Supplementary Table 6. Across all scenarios, somatrogon led to cost savings of €5,699–€21,974, with the largest impact in incremental costs observed when the dGHs dose was lowered from 0.24 mg/kg/week to 0.21–0.224 mg/kg/week. Furthermore, somatrogon led to 1.87 − 3.66 cm gain in NAH, with the largest impact observed when somatrogon adherence was varied. In addition, somatrogon gained 0.21–0.50 QALYs vs. dGHs, with the weekly treatment added utility and the lifetime horizon being the key drivers of incremental QALYs. Finally, somatrogon remained dominant vs. dGHs across all scenarios investigated, with a INMB of €11,128–€28,637 at a WTP of €20,000 (INMB of €17,915–€40,110 at a WTP of €45,000).
In the base case, children are assumed to remain on treatment until 18 years of age, as in previous pGHD economic models (). Nevertheless, treatment duration might be shorter either due to attainment of satisfactory height or based on the limited HV observed beyond 16 years of age (Supplementary Figures 3 and 4). Therefore, shorter treatment durations were considered in scenarios analysis by shortening the end of treatment age to 15,16 or 17 years (Supplementary Table 6). Somatrogon remained dominant vs. dGHs across each of the 3 scenarios, with somatrogon cost savings reduced from €17,548 in the base case to €11,338–€15,461 and the incremental QALYs from 0.27 (base case) to 0.22–0.26.
Deterministic and probabilistic sensitivity analysis
Results of the DSA with respect to incremental total QALYs, incremental cost per cm gained and INMB (based on a WTP of €45,000 per QALY) are presented in , for the 15 most influential parameters to the outcomes. The added utility of weekly injections and cost discount rate were the major drivers of uncertainty across the outcomes of interest, with the proportion of discontinuers at the end of Year 1, the utility associated with a HtSDs score between −2.0 and −1.5 and the discount rates also ranking towards the top of the tornado diagrams. Compared to dGHs, somatrogon was cost-effective (INMB: €23,297–€40,923; incremental cost per cm gained: −€527–−€389, per patient) and led to higher QALYs (incremental total QALYs: 0.13–0.52) across all ranges of parameters tested. The same results were observed when the DSA was run with a WTP of €20,000, with the INMB varying between €20,103 and €27,937.
Figure 2. Results of sensitivity analysis: incremental QALYs-DSA (top-left), incremental cost per cm gained DSA (top-right), incremental net monetary benefit at €45,000 WTP-DSA (bottom-left), and incremental costs and incremental QALYs-PSA (on cost-effectiveness plane) (bottom-right). Abbreviations. DSA, deterministic sensitivity analysis; LI, lower interval; PSA, probabilistic sensitivity analysis; QALYs, quality-adjusted life years; UI, upper interval; WTP, willingness to pay threshold.
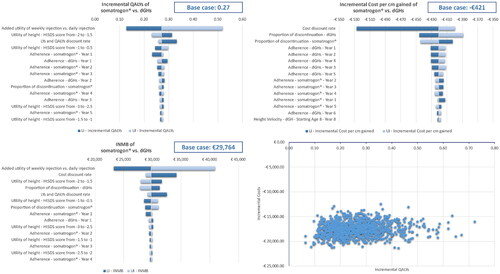
The PSA results are presented on the cost-effectiveness plane, where incremental QALYs and incremental costs of somatrogon vs. dGHs are shown (). Across all simulations somatrogon led to higher QALYs and lower costs than dGHs, (i.e. falling into the South-West quadrant of the cost-effectiveness plane). Compared with the base case, there was minimal deviation in the mean incremental costs (–€17,548 vs. –€17,559 [€17,469–€17,648 savings]), mean incremental height gain (2.85 cm vs. 2.83 cm [2.79 − 2.87]), mean incremental cost per cm gained (−€421 vs. –€420 [€419–€421 savings]) and mean incremental QALYs (0.27 vs. 0.27 [0.26–0.27]). Hence, the impact of the uncertainty associated with each parameter to the overall incremental benefit of somatrogon vs. dGHs was limited.
Discussion
In this study, somatrogon provided better long-term economic outcomes (with €17,548 cost savings and €421 lower cost per cm gained per patient) and health outcomes, in terms of growth and QALYs (with 2.85 cm gain in NAH and 0.27 additional QALYs) than dGHs. Better health outcomes with somatrogon were mostly associated with the higher adherence and the added utility of weekly vs. daily injections, while dGHs dosing was a key driver of somatrogon cost savings. Nevertheless, somatrogon remained dominant (i.e. lower costs and higher QALYs) across all scenarios investigated as well as when DSA and PSA were conducted.
This study is the first, to the best of our knowledge, to investigate the costs and health outcomes associated with somatrogon vs. dGHs in pGHD, and more broadly comparing a LAGH vs. dGHs from a cost-effectiveness perspective. Hence, we compared our findings with the results of a recent analysis that evaluated cost-effectiveness of somatropin delivered using the easypod device vs. other dGHs in the Italian population with pGHDCitation8. The model structure in Foo et al.Citation8 study was similar to the current analysis, with the impact of easypod detecting poor response earlier than other dGHs (6 months vs. 12 months) explicitly captured in the first model cycle. The final height predicted by Foo et al.Citation8 study (161.73 − 164.88 cm) is comparable with the current analysis (163.65 cm on dGHs and 166.50 cm on somatrogon). However, the base case difference in NAH between somatrogon and dGHs in the current analysis (2.85 cm) seems conservative, since Foo et al.Citation8 predicted a 3.02 cm difference between dGH delivered by the easypod and other dGHs, purely driven by the response assessment at 6 months vs. 12 months. A preliminary analysis of somatrogon vs. a specific dGH (Sandoz) was presented at a recent conference, from a German and UK perspectiveCitation63, with the health outcomes in this study well aligned with the current analysis in terms of final height (dGHs: 161.08 cm vs. 163.55 cm; somatrogon: 163.55 cm vs. 166.50 cm), incremental gain for somatrogon vs. dGHs (2.47 cm vs. 2.85) and incremental QALYs (0.54 vs. 0.50 from a scenario considering lifetime horizon [Scenario 3 in Supplementary Table 6], since a lifetime horizon was adopted in Mehl et al.Citation63 study). The cost outcomes are not comparable, since Mehl et al.Citation63 focussed on a different country (UK and Germany) and only compared somatrogon vs. a single dGH (Sandoz), which is not representative of the real-world landscape and biases the results depending on whether the dGH with the lowest or highest cost per mg is chosen.
As with all modelling studies, there are limitations that should be highlighted and discussed to properly interpret the results of this analysis. Firstly, the patient characteristics and Year 1 growth is based on the somatrogon phase 3 trial and has been assumed generalizable to the Irish settings. While Irish specific pGHD studies are not available for comparison, the somatrogon trial baseline characteristics (71.9% males and −2.86 initial HtSDs – ) are comparable with previous real-word studies from Ranke et al.Citation46 (global study: initial HtSDs between −3.46 and −3.09) and Savendal et al.Citation12 (European study 66% males and −2.55 initial HtSDs). Secondly, the Year 1 HV from the trial has been used for HV extrapolation, which is numerically higher for somatrogon than for dGHs, despite the trial design being a non-inferiority trialCitation32. However, the scenario analysis where the same HV for somatrogon and dGHs was used demonstrated a limited impact of this limitation (INMB relative change vs. dGHs of −1.55% at a WTP of €45,000). Thirdly, a 4% higher adherence with somatrogon in Year 1 vs. dGHs was assumed, based on the results of the “Treatment Burden” studyCitation33, since adherence can only be measured upon somatrogon approval and real world use (somatrogon was only approved and launched in Europe in 2022). However, scenario analysis demonstrated somatrogon benefit (higher health outcomes and lower costs) even when a 2% higher adherence in Year 1 vs. dGHs was assumed. Furthermore, the more optimistic scenario of a 6% higher adherence in Year 1 vs. dGHs resulted in a NAH benefit of 3.66 cm associated with somatrogon treatment, which is comparable to the difference among dGHs predicted in Foo et al.Citation8 study (3.02 cm). Thus, our current assumption of 4% higher adherence in Year 1 for somatrogon can be considered conservative. Fourthly, the height-utility relationship and added utility of weekly injections was derived from adult studies from proxy diseases (diabetes mellitus and haemophilia), given the lack of evidence sources in pGHD to provide a specific estimate. This limitation is common to previous pGHD economic analysesCitation8,Citation40. The base case value of the added utility (0.023Citation53) was chosen as the second lowest among the available studies (0.020Citation58 − 0.043Citation57) to remain on the conservative side. Fifthly, as highlighted in the methods section, AEs were not included in the economic analysis, since a very limited number of SAEs was observed in the somatrogon trial (5 out of 224 patients, 3 in somatrogon arm and 2 in the somatropin armCitation32) This is consistent with previous pGHD economic modelsCitation41,Citation49 and it is unlikely to have any impact on the economic analysis findings. Finally, the impact of growth hormones treatment on adult life, in terms of height and bone maturation (i.e. adult risk of osteoporosis) was not captured, given the limited amount of data availableCitation49,Citation64. Nevertheless, structural uncertainty was explored including the dead health state and simulating a lifetime horizon to capture the impact of higher NAH on the adult QoL, which demonstrated a higher QALY gain for somatrogon vs. dGHs than the base case (0.50 vs. 0.27).
Conclusion
Somatrogon weekly injections were estimated to result in higher NAH, higher QALYs, lower costs and lower costs per cm gained than dGHs, in paediatric growth hormone deficiency in the Irish healthcare setting.
Transparency
Declaration of funding
This study was funded by Pfizer.
Declaration of financial/other relationships
SR, PB and TK are employed by Evidera, a part of Thermo Fisher Scientific, which received funding from Pfizer Inc. to conduct this study. JL and MF are employees and may/may not be shareholders of Pfizer, Inc. A reviewer on this manuscript has disclosed that they are an investigator for clinical trials sponsored by Ascendis and OPKO and they have received research support from OPKO, Sandoz, and Pfizer. The other peer reviewers on this manuscript have no other relevant financial relationships or otherwise to disclose.
Author contributions
All authors contributed equally to the study design, implementation, analysis and interpretation of the data. All authors have contributed to the paper review and agree to be accountable for all aspects of the work.
Previous presentations
A preliminary version of this study has been presented at ISPOR EU 2022 conference – https://doi.org/10.1016/j.jval.2022.09.280.
Supplemental Material
Download MS Word (327.2 KB)Acknowledgements
No assistance in the preparation of this article is to be declared.
References
- National Institute for Health and Care Excellence (NICE). Human growth hormone (somatropin) for the treatment of growth failure in children. Technology appraisal guidance [TA188]. 2010 [cited 2021 May 26]. Available from: https://www.nice.org.uk/guidance/ta188
- Lindsay R, Feldkamp M, Harris D, et al. Utah growth study: growth standards and the prevalence of growth hormone deficiency. J Pediatr. 1994;125(1):29–35. doi: 10.1016/s0022-3476(94)70117-2.
- Thomas M, Massa G, Craen M, et al. Prevalence and demographic features of childhood growth hormone deficiency in Belgium during the period 1986-2001. Eur J Endocrinol. 2004;151(1):67–72. doi: 10.1530/eje.0.1510067.
- Reh CS, Geffner ME. Somatotropin in the treatment of growth hormone deficiency and turner syndrome in pediatric patients: a review. Clin Pharmacol. 2010;2:111–122.
- Stagi S, Scalini P, Farello G, et al. Possible effects of an early diagnosis and treatment in patients with growth hormone deficiency: the state of art. Ital J Pediatr. 2017;43(1):81. doi: 10.1186/s13052-017-0402-8.
- Grimberg A, DiVall SA, Polychronakos C, et al. Guidelines for growth hormone and insulin-like growth factor-I treatment in children and adolescents: growth hormone deficiency, idiopathic short stature, and primary insulin-like growth factor-I deficiency. Horm Res Paediatr. 2016;86(6):361–397. doi: 10.1159/000452150.
- Ranke MB. Short and long-term effects of growth hormone in children and adolescents with GH deficiency. Front Endocrinol. 2021;12:720419. doi: 10.3389/fendo.2021.720419.
- Foo J, Maghnie M, Colao A, et al. Cost-consequence analysis for human recombinant growth hormone (r-hGH) treatment administered via different devices in children with growth hormone deficiency in Italy. Clinicoecon Outcomes Res. 2019;11:525–537. doi: 10.2147/CEOR.S195265.
- Bell J, Parker KL, Swinford RD, et al. Long-term safety of recombinant human growth hormone in children. J Clin Endocrinol Metab. 2010 Jan;95(1):167–177. doi: 10.1210/jc.2009-0178.
- Bell JJ, Lippe B, Romano AA, et al. National cooperative growth study: 25 years of growth hormone data, insights, and lessons for future registries. Pediatr Endocrinol Rev. 2018;16(2):240–255.
- Maghnie M, Ranke MB, Geffner ME, et al. Safety and efficacy of pediatric growth hormone therapy: results from the full KIGS cohort. J Clin Endocrinol Metab. 2022;107(12):3287–3301. doi: 10.1210/clinem/dgac517.
- Sävendahl L, Polak M, Backeljauw P, et al. Treatment of children with GH in the United States and Europe: long-term follow-up from NordiNet® IOS and ANSWER program. J Clin Endocrinol Metab. 2019;104(10):4730–4742. doi: 10.1210/jc.2019-00775.
- Brod M, Alolga SL, Beck JF, et al. Understanding burden of illness for child growth hormone deficiency. Qual Life Res. 2017;26(7):1673–1686. doi: 10.1007/s11136-017-1529-1.
- Brod M, Højbjerre L, Alolga SL, et al. Understanding treatment burden for children treated for growth hormone deficiency. Patient. 2017;10(5):653–666. doi: 10.1007/s40271-017-0237-9.
- Haverkamp F, Johansson L, Dumas H, et al. Observations of nonadherence to recombinant human growth hormone therapy in clinical practice. Clin Ther. 2008;30(2):307–316. doi: 10.1016/j.clinthera.2008.02.017.
- Turner-Bowker DM, Yaworsky A, Palladino A, et al. Development and psychometric evaluation of the life interference questionnaire for growth hormone deficiency (LIQ-GHD) to assess growth hormone injection burden in children and adults. Patient. 2020;13(3):289–306. doi: 10.1007/s40271-019-00405-7.
- Gomez R, Ahmed SF, Maghnie M, et al. Treatment adherence to injectable treatments in pediatric growth hormone deficiency compared with injectable treatments in other chronic pediatric conditions: a systematic literature review. Front Endocrinol. 2022;13:795224. doi: 10.3389/fendo.2022.795224.
- Acerini CL, Segal D, Criseno S, et al. Shared decision-making in growth hormone therapy-implications for patient care. Front Endocrinol. 2018;9:688. doi: 10.3389/fendo.2018.00688.
- Acerini CL, Wac K, Bang P, et al. Optimizing patient management and adherence for children receiving growth hormone. Front Endocrinol. 2017;8:313. doi: 10.3389/fendo.2017.00313.
- Mohseni S, Heydari Z, Qorbani M, et al. Adherence to growth hormone therapy in children and its potential barriers. J Pediatr Endocrinol Metab. 2018;31(1):13–20. doi: 10.1515/jpem-2017-0157.
- Fisher BG, Acerini CL. Understanding the growth hormone therapy adherence paradigm: a systematic review. Horm Res Paediatr. 2013;79(4):189–196. doi: 10.1159/000350251.
- Aydın BK, Aycan Z, Sıklar Z, et al. Adherence to growth hormone therapy: results of a multicenter study. Endocr Pract. 2014;20(1):46–51. doi: 10.4158/EP13194.OR.
- Cutfield WS, Derraik JG, Gunn AJ, et al. Non-compliance with growth hormone treatment in children is common and impairs linear growth. PLoS One. 2011;6(1):e16223. doi: 10.1371/journal.pone.0016223.
- Farfel A, Shalitin S, Morag N, et al. Long-term adherence to growth hormone therapy in a large health maintenance organization cohort. Growth Horm IGF Res. 2019;44:1–5. doi: 10.1016/j.ghir.2018.10.004.
- Maggio MC, Vergara B, Porcelli P, et al. Improvement of treatment adherence with growth hormone by easypod device: experience of an Italian centre. Ital J Pediatr. 2018;44(1):113. doi: 10.1186/s13052-018-0548-z.
- Kapoor RR, Burke SA, Sparrow SE, et al. Monitoring of concordance in growth hormone therapy. Arch Dis Child. 2008;93(2):147–148. doi: 10.1136/adc.2006.114249.
- Loftus J, Miller BS, Parzynski CS, et al. Association of daily growth hormone injection adherence and height among children with growth hormone deficiency. Endocr Pract. 2022;28(6):565–571. doi: 10.1016/j.eprac.2022.02.013.
- Koledova E, Stoyanov G, Ovbude L, et al. Adherence and long-term growth outcomes: results from the easypod™ connect observational study (ECOS) in paediatric patients with growth disorders. Endocr Connect. 2018;7(8):914–923. doi: 10.1530/EC-18-0172.
- de Arriba Munoz A, Muniz VC, Saez JJA, et al. Impact of adherence on growth response during the first 2 years of growth hormone treatment. Endocrine. 2021;72(2):513–523. doi: 10.1007/s12020-020-02560-6.
- Rodríguez Arnao MD, Rodríguez Sánchez A, Díez López I, et al. Adherence and long-term outcomes of growth hormone therapy with easypod™ in pediatric subjects: Spanish ECOS study. Endocr Connect. 2019;8(9):1240–1249. doi: 10.1530/EC-19-0325.
- Canadian Agency for Drugs and Technologies in Health (CADTH). Reimbursement review - somatrogon (Ngenla). Ottawa: CADTH; 2022.
- Deal CL, Steelman J, Vlachopapadopoulou E, et al. Efficacy and safety of weekly somatrogon vs daily somatropin in children with growth hormone deficiency: a phase 3 study. J Clin Endocrinol Metab. 2022;107(7):e2717–e2728. doi: 10.1210/clinem/dgac220.
- Maniatis AK, Carakushansky M, Galcheva S, et al. Treatment burden of weekly somatrogon vs daily somatropin in children with growth hormone deficiency: a randomized study. Journal of the Endocrine Society. 2022;6(10):bvac117. doi: 10.1210/jendso/bvac117.
- Canadian Agency for Drugs and Technologies in Health (CADTH). Commo drug review - pharmaeconomic review report - somatropin (genotropin) for subcutaneous injection. Ottawa: CADTH; 2022.
- Saz-Parkinson Z, del Sol Granados Alonso M, Co JM. Study of adherence to recombinant growth hormone treatment of children with a GH deficiency: contributions to treatment control and economic impact. Public Health Technology Assessment Report IPEAmate Blan2013/70. Spanish HTA Agency; 2013.
- Scottish Medicines Consortium (SMC). somatropin for injection, 5mg/mL vial of powder and solvent for solution for subcutaneous injection and 3.3mg/mL and 6.7mg/mL penfill cartridge of solution for subcutaneous injection (OmnitropeÒ) No. (598/10). Scottish Medicines Consortium; 2010.
- Vorontsovaa M, Nagaevaa E, Naigovzinab N. Cost-effectiveness of growth hormone therapy in children in Russia. SPE Abstracts. 2018;89: P-P3-208.
- Howard SR, Butler GE. An analysis of the clinical and cost effectiveness of growth hormone replacement therapy before and during puberty: should we increase the dose? Horm Res Paediatr. 2013;79(2):75–82. doi: 10.1159/000346687.
- Lee JM, Davis MM, Clark SJ, et al. Estimated cost-effectiveness of growth hormone therapy for idiopathic short stature. Arch Pediatr Adolesc Med. 2006;160(3):263–269. doi: 10.1001/archpedi.160.3.263.
- Christensen T, Fidler C, Bentley A, et al. The cost-effectiveness of somatropin treatment for short children born small for gestational age (SGA) and children with growth hormone deficiency (GHD) in Sweden. J Med Econ. 2010;13(1):168–178. doi: 10.3111/13696991003652248.
- Joshi AV, Munro V, Russell MW. Cost-utility of somatropin (rDNA origin) in the treatment of growth hormone deficiency in children. Curr Med Res Opin. 2006;22(2):351–357. doi: 10.1185/030079906X80503.
- Solem C, Smith C, Wiegand P, et al. PDB29 budget impact analysis of norditropin versus three leading market growth hormone therapies. Value Health. 2012;15(4):A175. doi: 10.1016/j.jval.2012.03.950.
- Briggs AH, O’Brien BJ. The death of cost-minimization analysis? Health Econ. 2001;10(2):179–184. doi: 10.1002/hec.584.
- Dakin H, Wordsworth S. Cost-minimisation analysis versus cost-effectiveness analysis, revisited. Health Econ. 2013;22(1):22–34. doi: 10.1002/hec.1812.
- Drummond M, Sculpher M, Claxton K, et al. Methods for the economic evaluation of health care programmes. 4th ed. Oxford: Oxford University Press; 2015.
- Ranke MB, Lindberg A, Board KI. Observed and predicted growth responses in prepubertal children with growth disorders: guidance of growth hormone treatment by empirical variables. J Clin Endocrinol Metab. 2010;95(3):1229–1237. doi: 10.1210/jc.2009-1471.
- Quigley CA, Child CJ, Zimmermann AG, et al. Mortality in children receiving growth hormone treatment of growth disorders: data from the genetics and neuroendocrinology of short stature international study. J Clin Endocrinol Metab. 2017;102(9):3195–3205. doi: 10.1210/jc.2017-00214.
- Spandonaro F, Mancusi L. Valutazione di efficienza nella somministrazione dell’ormone della crescita (GH). Farmeconomia. Health economics and therapeutic pathways, 2013;14(1):7–17.
- Takeda A, Cooper K, Bird A, et al. Recombinant human growth hormone for the treatment of growth disorders in children: a systematic review and economic evaluation. Health Technol Assess. 2010;14(42):1–209. iii–iv. doi: 10.3310/hta14420.
- Husereau D, Drummond M, Augustovski F, et al. Correction to: consolidated health economic evaluation reporting standards 2022 (CHEERS 2022) statement: updated reporting guidance for health economic evaluations. Appl Health Econ Health Policy. 2022;20(5):781–782. doi: 10.1007/s40258-022-00743-y.
- Cole TJ, Singhal A, Fewtrell MS, et al. Weight centile crossing in infancy: correlations between successive months show evidence of growth feedback and an infant-child growth transition. Am J Clin Nutr. 2016;104(4):1101–1109. doi: 10.3945/ajcn.116.139774.
- Cole TJ, Freeman JV, Preece MA. British 1990 growth reference centiles for weight, height, body mass index and head circumference fitted by maximum penalized likelihood. Statist Med. 1998;17(4):407–429. doi: 10.1002/(SICI)1097-0258(19980228)17:4 < 407::AID-SIM742 > 3.0.CO;2-L.
- Boye KS, Matza LS, Walter KN, et al. Utilities and disutilities for attributes of injectable treatments for type 2 diabetes. Eur J Health Econ. 2011;12(3):219–230. doi: 10.1007/s10198-010-0224-8.
- Evans M, Jensen HH, Bøgelund M, et al. Flexible insulin dosing improves health-related quality-of-life (HRQoL): a time trade-off survey. J Med Econ. 2013;16(11):1357–1365. doi: 10.3111/13696998.2013.846262.
- Henry N, Jovanović J, Schlueter M, et al. Cost-utility analysis of life-long prophylaxis with recombinant factor VIIIFc vs recombinant factor VIII for the management of severe hemophilia a in Sweden. J Med Econ. 2018;21(4):318–325. doi: 10.1080/13696998.2017.1405816.
- Polster M, Zanutto E, McDonald S, et al. A comparison of preferences for two GLP-1 products–liraglutide and exenatide–for the treatment of type 2 diabetes. J Med Econ. 2010;13(4):655–661. doi: 10.3111/13696998.2010.529377.
- Rajan N, Boye KS, Gibbs M, et al. Utilities for type 2 diabetes treatment-related attributes in a South Korean and Taiwanese population. Value Health Reg Issues. 2016;9:67–71. doi: 10.1016/j.vhri.2015.11.006.
- Ridderstråle M, Evans LM, Jensen HH, et al. Estimating the impact of changes in HbA1c, body weight and insulin injection regimen on health related quality-of-life: a time trade off study. Health Qual Life Outcomes. 2016;14:13. doi: 10.1186/s12955-016-0411-0.
- IMS Institute for Healthcare Informatics. IMS Health Ireland 2022. Market shares by SKU BrandUnit. Dublin, Ireland; 2022.
- HPRA. HPRA - Health Products Regulatory Authority; [cited 2023 Jan 16]. Available from: https://www.hpra.ie/homepage/medicines/medicines-information/find-a-medicine/results?query=norditropin&field=
- Health Information and Quality Authority (HIQA). Guidelines for the economic evaluation of health technologies in Ireland; 2020 [cited 2022 Feb 12]. Available from: https://www.hiqa.ie/reports-and-publications/health-technology-assessment/guidelines-economic-evaluation-health
- Briggs A, Sculpher M, Claxton K. Decision modelling for health economic evaluation. Oxford: Oxford University Press; 2006.
- Mehl A, Goh A, Gupta S. EE589 cost-effectiveness of once-daily somatropin from Sandoz versus once-weekly somatrogon for the treatment of growth hormone deficiency in children and adolescents. Value Health. 2022;25(12):S171. doi: 10.1016/j.jval.2022.09.828.
- Bouillon R, Koledova E, Bezlepkina O, et al. Bone status and fracture prevalence in Russian adults with childhood-onset growth hormone deficiency. J Clin Endocrinol Metab. 2004;89(10):4993–4998. doi: 10.1210/jc.2004-0054.
- Health Service Executive - Primary Care Reimbursement Service. List of reimbursable items; 2022 Dec. Available from: https://www.hse.ie/eng/staff/pcrs/online-services/
- Human growth hormone (Somatropin) for treatment of adult growth hormone deficiency prescribing support information; [cited 2022 Feb 12]. Available from: https://www.cambridgeshireandpeterboroughccg.nhs.uk/easysiteweb/getresource.axd?assetid=18581&type=0&servicetype=1
- Ghia P, Pluta A, Wach M, et al. Acalabrutinib versus investigator’s choice in relapsed/refractory chronic lymphocytic leukemia: final ASCEND trial results. Hemasphere. 2022;6(12):e801. doi: 10.1097/HS9.0000000000000801.
- Healthcare Pricing Office (HPO). Activity based funding (ABF) 2022 admitted patient price list. Indicative patient day case. K64B endocrine disorders, MINC; 2022 [cited 2022 Feb 15]. Available from: https://www.hpo.ie/abf/ABF2022AdmittedPatientPriceList.pdf
- Fola Care Prices. [cited 2022 Feb 12]. Available from: https://fola.care/prices/
- Affidea Prices. 2022; [cited 2022 Feb 12]. Available from: https://www.affidea.ie/prices/
- The GP Surgery Prices. [cited 2022 Feb 12]. Available from: https://www.thegpsurgery.co.uk/blood-test/endocrinology/