Abstract
Aims
The treatment landscape of renal cell carcinoma has changed with the introduction of targeted therapies. While the clinical benefit of cabozantinib is well-established for Japanese patients who have received prior treatment, the economic benefit remains unclear. The objective of this study was to assess the cost-effectiveness of cabozantinib compared with everolimus, axitinib, and nivolumab in patients with advanced renal cell carcinoma who have failed at least one prior therapy in Japan.
Methods
A cost-effectiveness model was developed using a partitioned survival approach and a public healthcare payer’s perspective. Over a lifetime horizon, clinical and economic implications were estimated according to a three-health–state structure: progression-free, post-progression, and death. Key clinical inputs and utilities were derived from the METEOR trial, and a de novo network meta-analysis and cost data were obtained from publicly available Japanese data sources. Costs, quality-adjusted life-years, and incremental cost-effectiveness ratios were estimated. Costs and health benefits were discounted annually at 2%.
Results
Cabozantinib was more costly and effective compared with everolimus and axitinib, with deterministic incremental cost-effectiveness ratios of ¥5,375,559 and ¥2,223,138, respectively. Compared to nivolumab, cabozantinib was predicted to be less costly and more effective. Sensitivity and scenario analyses demonstrated that the key drivers of cost-effectiveness results were the estimation of overall survival and treatment duration, relative efficacy, drug costs, and subsequent treatment costs.
Limitations
METEOR was an international trial but did not enroll any patients from Japan. Efficacy and safety data from METEOR were used as a proxy for the Japanese population following validation by clinical experts, and alternative assumptions specific to clinical practice in Japan were evaluated in scenario analyses.
Conclusions
In Japan, cabozantinib is a cost-effective alternative to everolimus, axitinib, and nivolumab for the treatment of patients with advanced renal cell carcinoma who have received at least one prior line of therapy.
Introduction
Renal cell carcinoma (RCC) is the most common type of kidney cancer, accounting for 80% of all kidney cancer casesCitation1. In Japan, the incidence of RCC is among the highest in Asia, with a reported annual incidence of 11.6/100,000 in men and 5.6/100,000 in womenCitation2. The median overall survival (OS) for an untreated patient with RCC is approximately five months; only 29% survive beyond one yearCitation3.
The treatment landscape of RCC has changed significantly over the past decade with the introduction of targeted therapies. The 2020 Japanese Urological Association treatment guidelines for RCCCitation4 recommend treatment with nivolumab, cabozantinib, and axitinib after failure of tyrosine kinase inhibitor (TKI) therapy; if these treatments are not suitable, everolimus and sorafenib are recommended as alternatives.
Cabozantinib is an oral TKI that has demonstrated significant improvements in clinical outcomes in both treatment-naïve and previously treated patients with advanced RCC (aRCC)Citation5,Citation6. A phase III, randomized, open-label, multicenter trial (METEOR) evaluated the comparative efficacy and safety of 60 mg of cabozantinib once a day vs. 10 mg of everolimus once a day, in adult patients with advanced or metastatic clear-cell RCC previously treated with one or more vascular endothelial growth factor receptor (VEGFR) TKIsCitation7.
Results from METEOR showed cabozantinib was associated with improved progression-free survival (PFS; 0.51 [95% confidence interval (CI): 0.41, 0.62], p < 0.0001), objective response rate (ORR; 17% [95% CI: 13%, 22%] vs. 3% [95% CI: 2%, 6%], p < 0·0001), and OS (hazard ratio [HR]: 0.70 [95% CI: 0.58, 0.85], p = 0.0002) compared to everolimusCitation5,Citation8. A phase II, open-label, single-arm trial in Japanese patients (a bridging study to METEOR with similar patient population) also confirmed the findings from METEOR are generalizable to Japanese patientsCitation9. Based on the results from these trials, cabozantinib received regulatory approval for the treatment of RCC in March 2020 in Japan.
While the clinical benefit of cabozantinib is well established for Japanese patients who have received prior TKIs, the economic benefit compared to other guideline-recommended treatments that are used to treat patients in Japan remains unclear. The demand for the conduct of cost-effectiveness studies to inform the allocation of limited healthcare resources and price adjustments has been burgeoning on a global scale. In addition, the increasing cost of new health technologies, including prescribing drugs, is a pressing matter in Japan, as well as many other industrialized countries.
The necessity of cost-effectiveness data became evident over time in Japan, as the effective and efficient utilization of healthcare resources has become increasingly important. Cost-effectiveness evaluations of health technologies have been implemented in Japan since April 2019, which provides a systematic framework to better inform value-based price adjustments of pharmaceuticals and devices. Unlike in many countries, including the UK, cost-effectiveness data in Japan are used for price adjustment after initial listing, but not to inform reimbursement decisions.
In order to inform price adjustment in Japan, two sets of three thresholds are established under the Japanese cost-effectiveness evaluation system. One set of thresholds is applicable for general drugs (¥5,000,000, ¥7,500,000, ¥10,000,000 per quality-adjusted life-year [QALY]), and another set is for drugs with special consideration, which applies for oncology drugs (¥7,500,000, ¥11,250,000, ¥15,000,000 per QALY). If the incremental cost-effectiveness ratio (ICER) from the cost-effectiveness evaluation is less than the lowest threshold (i.e. ¥7,500,000 in this case), then a zero-adjustment rate is applied for the drug under consideration (i.e. no price adjustment required). The adjustment rate increases stepwise, and the highest adjustment rate is applied when the ICER is larger than the highest thresholdCitation10.
The aim of this study was to evaluate the cost-effectiveness of cabozantinib vs. everolimus, axitinib, and nivolumab in patients with aRCC previously treated with a TKI, from the perspective of the Japanese healthcare system.
Methods
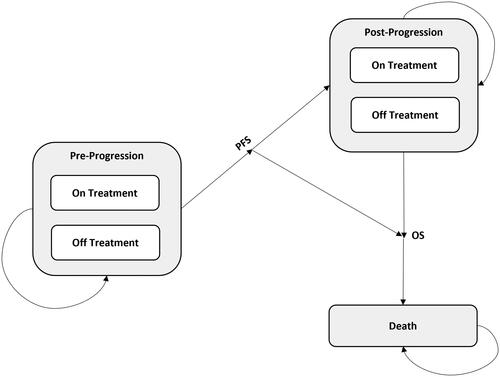
The methods were decided based on the Japanese guideline for cost-effectiveness evaluationCitation11. A cost-effectiveness analysis was the selected method for this economic evaluation, as it is a comparative assessment that considers the impact of treatment on survival as well as health-related quality of life (QoL). In a cost-effectiveness analysis, the natural history of the disease is typically modeled using mutually exclusive, collectively exhaustive health states (). Oncology cost-effectiveness models with three health states (progression-free, post-progression, and death) are commonplace, especially when PFS and OS are key events and are well captured in the trials.
Model framework
A health economic model was developed in Microsoft Excel® to conduct a cost-effectiveness analysis of cabozantinib vs. everolimus, axitinib, and nivolumab for the treatment of aRCC in patients who have failed on at least one prior line.
The model adopts a partitioned survival approach to track a cohort’s costs and health outcomes over a lifetime horizon. All patients are assumed to be progression free at baseline, and each model cycle (i.e. every four weeks), the cohort of patients is redistributed across three health states: progression-free, post-progression, and death. The percentage of patients in a health state at any given time is calculated based on extrapolated treatment-specific survival curves from pivotal clinical trials. Treatment-specific OS curves are directly used to estimate the proportion of patients alive over time. OS is further partitioned to progression-free and post-progression health states to capture specific QoL and cost implications among treatments. The percentage of patients in the progression-free state are estimated using the treatment-specific PFS curves. The post-progression probabilities are inferred as the difference between the OS and PFS survival estimates. Mean PFS and OS survival was estimated by approximating the area under the curve using Riemann sums.
Costs and health benefits were discounted at an annual rate of 2%Citation11 and then accrued for each health state to estimate the expected outcomes. A half-cycle correction was implemented to approximate the area under the curves.
Model parameters and data sources
Patient population
The model population includes Japanese patients with aRCC who have failed at least one prior therapy (i.e. second- or later-line therapies). This population is reflective of the primary data sources used to parameterize the model to conduct the cost-effectiveness analysis: METEOR, AXIS, and CheckMate 025.
Treatment comparators
The primary therapy considered in this study was cabozantinib, as the objective of this study was to evaluate its cost-effectiveness vs. relevant comparators. Comparators were decided based on the Japanese guidelineCitation11 and a discussion between the Center for Outcomes Research and Economic Evaluation for Health (C2H), the Japanese health technology assessment (HTA) body, and the manufacturer. Everolimus was selected because it was compared head-to-head against cabozantinib in the METEOR trial, in which case robust data were available to conduct a rigorous cost-effectiveness analysis. Axitinib was included because, at the time, it was a recommended drug in the Japanese treatment guideline and the most commonly prescribed drug for the second-line treatment of aRCC in Japan. Nivolumab was also included as a comparator for scenario analysis based on the discussion with C2H. Thus, three drugs were included in this analysis as comparators.
Clinical inputs
The primary clinical inputs in the model included parameter estimates of OS, PFS, and time-to-treatment discontinuation (TTD) survival functions. Parametric survival analyses were conducted by fitting survival distributions to the patient-level survival data collected in METEOR in order to make long-term projections for cabozantinib and everolimus in the cost-effectiveness model. In particular, six parametric distributions—Weibull, Gompertz, exponential, log-normal, gamma, and log-logistic—were fitted to the time-to-event data using the LIFEREG procedure in SAS version 9.4 and using four-week time intervals. The final survival functions for OS, PFS, and TTD were selected in accordance with the recommendations in the National Institute for Health and Care Excellence (NICE) Decision Support Unit (DSU) reportCitation12. The clinical plausibility of long-term extrapolations was validated by three Japanese clinicians. Distributions with the best fit to the observed data according to Akaike and Bayesian information criterion generally had the most credible long-term predictions and were preferred by the clinicians. The parameter estimates of the final survival functions are specified in , and additional details are provided in the Supplementary Materials.
Table 1. Clinical inputs.
A network meta-analysis (NMA) was conducted via fixed-effects (FE) Bayesian methods to estimate the relative efficacy inputs (i.e. OS and PFS) of the treatment comparators because head-to-head comparisons were not conducted for cabozantinib vs. nivolumab or axitinib. Each NMA provided a central estimate of the relative effect of interest (i.e. hazard ratio [HR]) along with its 95% credible interval (CrI) for all possible comparisons in the network. The HRs estimated via the NMA were applied to the long-term parametric fits for cabozantinib, and the proportional hazards assumption was assumed to hold for the lifetime horizon. Details regarding the methodology and results of the NMA are presented as Supplementary Material. Median time on treatment from AXIS and CheckMate 025 was used to inform the TTD for the comparators not included in METEOR (i.e. axitinib and nivolumab), as HRs or Kaplan-Meier (KM) curves were not available from AXIS and CheckMate 025. Of note, the METEOR trial protocol allowed the continuation of study treatments following progression if the investigator deemed it to be clinically beneficial.
The model includes grade 3+ adverse events (AEs) that were reported in greater than 5% of patients in METEOR, AXIS, or CheckMate 025. According to these criteria, the occurrence of five AEs was modeled: diarrhea, fatigue, palmar-plantar erythrodysesthesia (PPE) syndrome, hypertension, and anemia. The consequences of AEs were modeled in terms of the accrual of associated management costs and disutilities. AEs were only considered for second-line treatments, and AEs associated with subsequent-line treatments were not explicitly modeled.
Utilities
Utility scores represent health-related QoL and are commonly employed to compute QALYs in cost-effectiveness analyses—sometimes referred to more specifically as cost-utility analyses. Linear mixed-effects regression analyses accounting for repeated measurements were performed for the five-level version of the EQ-5D (EQ-5D-5L) data collected in METEOR. EQ-5D-5L utilities were derived by converting EQ-5D-5L responses into utility indexes according to the Japanese version of the EQ-5D-5L value setCitation13, and were predicted as a function of progression status and the occurrence of AEs. Additionally, the utility regression analysis adjusted for differences in mean baseline utility scores between the two treatment arms in METEOR. Treatment was not statistically significant in the univariate or multivariate analysis, and thus was removed from the final multivariate model. Using the linear-mixed effects model, least-squares means (i.e. marginal means) and associated standard errors were estimated for each health state. The cost-effectiveness model applies a one-time disutility due to AEs according to the treatment-specific AE probabilities for a one-cycle duration. The mean health state utilities and AE disutilities are summarized in .
Table 2. Direct Medical cost and utility inputs.
Costs
All costs considered in the analysis were reported in Japanese Yen (¥), and inflation was implemented where requiredCitation14.
Drug acquisition costs reflect prices in Japan derived from the Japanese National Healthcare Insurance (NHI) drug list (). Relative dose intensities (RDIs) for cabozantinib and everolimus were obtained from the METEOR trial, and RDIs for other comparators were derived from previous NICE technology appraisalsCitation15–17 and published trial data ()Citation1,Citation18. The administration-related costs were derived using the data available from the Japanese NHI medical fee table. Patients who received oral drugs were assumed to incur a prescription fee, dispensing fee, and cost of drug instructions. Patients receiving nivolumab were assumed to have an associated intravenous administration cost instead of prescription fees and pharmacy costs. Per the opinion of Japanese clinicians, all drugs were assumed to be administered in an outpatient setting.
Table 3. Drug and administration costs.
The disease management costs in the model capture routine tests, routine visits, and computed tomography scans of the kidneys. The resource utilization was assumed to be the same for all oral treatments, with increased utilization excepted for those receiving nivolumab (the frequency of outpatient visits and blood tests). Furthermore, it was assumed that disease management costs were equivalent for progression-free and post-progression patients, according to feedback from Japanese clinicians. The costs associated with end-of-life care and the management of AEs were derived from a previously published cost-effectiveness analysis of lenvatinib treatment for patients with unresectable hepatocellular carcinoma in Japan ()Citation19.
Analyses
In the base case analysis, treatments were compared both on a pairwise basis (with cabozantinib) and under the efficiency frontier methodology. The base case analysis was conducted using a deterministic approach, where the mean model outcomes were estimated using point estimates of the mean values for the input parameters. It should be noted that the structure of most cost-effectiveness models, including partitioned survival analyses, typically involve non-linear transformations, in which case the modeled outcome estimated at the mean values of input parameters (deterministic analysis) is not equivalent to the expected value of the outcome evaluated over the probabilistic distributions of input parameters (probabilistic analysis)Citation20,Citation21. Nevertheless, the two approaches generally produce similar results and consistent conclusions in practice, unless the degree of non-linearity is extreme. Approximations based on deterministic analyses have historically been accepted by national HTA bodies, including C2H, although certain HTA bodies like NICE and the Canadian Agency for Drugs and Technologies in Health (CADTH) have recently recommended probabilistic analyses as the base case in their updated guidelinesCitation22,Citation23. For the Japanese cost-effectiveness evaluation, probabilistic analyses were conducted as sensitivity analyses, further detailed below.
A series of four scenario analyses were performed to examine the effect of alternative assumptions and inputs on model-predicted outcomes:
Scenario 1. Axitinib and everolimus are equally effective: Concerns were raised that the OS estimate for axitinib (AXIS) generated by the NMA may underestimate the survival benefit of axitinib. The evidence review group from the associated cabozantinib NICE technology appraisalCitation24 assumed that axitinib and everolimus have equal efficacy (i.e., OS and PFS) in a scenario analysis, which was similarly tested in the following scenario.
Scenario 2. Alternate RDI for a Japanese population: RDIs reflective of data for a Japan-specific population were assumed. The mean RDI of cabozantinib in the 2001 trialCitation25, a phase II study of cabozantinib in a Japanese population with aRCC, was 51%. The real-world prescribed doses estimated using the Japan Medical Data Center (JMDC) claims database were 85.1% for everolimus, 93.8% for axitinib, and 92.3% for nivolumabCitation26.
Scenario 3. Cabozantinib treatment effect on OS wanes: Beyond the end of the METEOR follow-up period (i.e., 3.15 years), cabozantinib survival was assumed to follow the per-cycle risk of mortality of the everolimus arm. A scenario exploring a treatment waning effect for cabozantinib OS was requested by NICE for the technology appraisal of cabozantinib for previously treated aRCC, due to the uncertainty around long-term efficacy assumptionsCitation25.
Scenario 4. Treatment until progression for axitinib: All patients were assumed to remain on axitinib until progression due to limited availability of discontinuation data from AXIS.
Two forms of sensitivity analyses were conducted to study uncertainty: deterministic sensitivity analysis (DSA) and probabilistic sensitivity analysis (PSA). One-way DSAs were conducted by systematically varying parameters from the base case on a univariate basis, and the key drivers of cost-effectiveness were plotted in tornado diagrams. The PSA was performed by running 1,000 simulations, where tests for convergence concluded that 1,000 replicates were sufficient to minimize the Monte Carlo standard errorCitation23.
Results
Base case
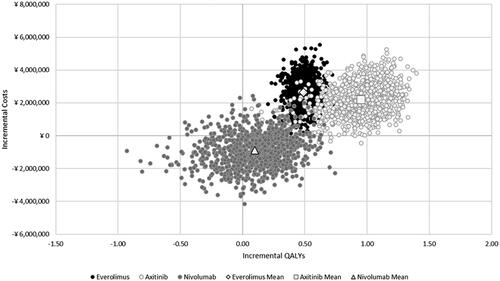
Over a lifetime deterministic analysis, treatment with cabozantinib was predicted to yield a 30% increase in QALYs (+0.49) vs. everolimus, an 87% increase vs. axitinib (+0.98), and a 6% increase vs. nivolumab (+0.11), resulting in ICERs well below the most favorable (i.e. lowest) established threshold of ¥ 7,500,000 (). In particular, cabozantinib was predicted to be less costly and more effective in comparison to nivolumab (i.e. dominant). Results for the deterministic analysis using the efficiency frontier approach are summarized in the Supplementary Material. In the efficiency frontier comparison, axitinib was dominated by everolimus, and nivolumab was dominated by cabozantinib.
Table 4. Base case (deterministic) results.
Scenario analyses
The cost-effectiveness results for the four pre-specified scenarios are summarized in . Cabozantinib was cost-effective in all head-to-head comparisons at a threshold of ¥ 7,500,000 across the four pre-specified scenarios. The most favorable ICERs for cabozantinib were estimated in the second scenario, where alternative RDIs representative of a Japanese population were assumed. Cabozantinib was still cost-effective vs. everolimus and vs. axitinib when conservatively assuming treatment effect waning.
Table 5. Scenario analysis results (cabozantinib vs. comparators).
Sensitivity analyses
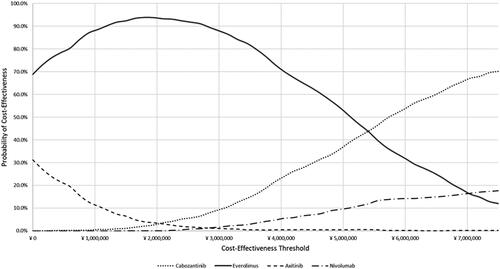
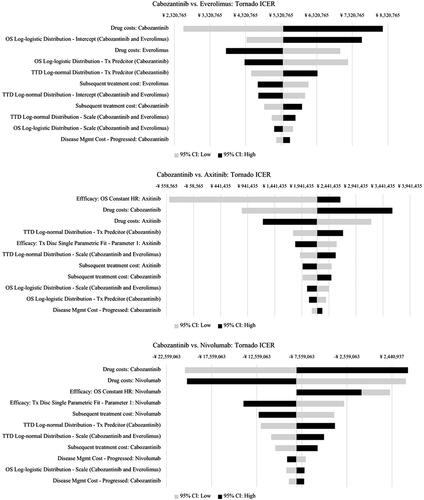
The DSA found that the ICERs were most sensitive to changes in the OS parameters, TTD parameters, and drug costs in all three head-to-head comparisons (). In the PSA, the average cost-effectiveness results were comparable to the base-case (deterministic) analysis ( and Supplement), with mean probabilistic ICERs of ¥ 5,390,419 and ¥ 2,327,798 for cabozantinib vs. everolimus and vs. axitinib, respectively. As in the base-case (deterministic) analysis, cabozantinib was, on average, less costly and more effective than nivolumab (i.e. dominant) in the PSA. When considering all four treatment options in tandem in probabilistic simulations, the PSA predicted that cabozantinib was the most cost-effective option in 70.1% of the simulations at the most favorable threshold of ¥ 7,500,000, while everolimus, axitinib, and nivolumab were the most cost-effective in 12%, 0.2%, and 17.7% of the simulations, respectively (). Tornado diagrams for incremental costs and QALYs, and additional results for the PSA are available in the Supplementary Material.
Figure 2. Tornado ICER Results, DSA. Abbreviations. CI, Confidence interval; HR, Hazard ratio; ICER: Incremental cost-effectiveness ratio; OS, Overall survival; TTD: Time-to-treatment discontinuation; Tx, Treatment.
Discussion
To our knowledge, this is the first study to assess the cost-effectiveness of cabozantinib as a second or later-line treatment for patients with aRCC from the perspective of the Japanese healthcare system. Findings demonstrate the clinical and economic benefits of cabozantinib compared to everolimus, axitinib, and nivolumab. Compared to nivolumab, cabozantinib was found to be a dominant treatment option (i.e. less costly and more effective). Comparisons to everolimus and axitinib highlight the clinical benefit of cabozantinib (i.e. prolonged PFS and OS) at acceptable additional costs, with deterministic and probabilistic ICERs falling below the most favorable established threshold of ¥ 7,500,000: ¥ 5,375,559 vs. everolimus and ¥ 2,223,138 vs. axitinib in the base case (deterministic) results. The results of the deterministic and probabilistic analyses were comparable, leading to consistent conclusions.
Precedence is a relevant consideration when determining a suitable model structure for cost-effectiveness evaluations. Partitioned survival analyses are regularly used in oncology modeling to inform reimbursement decision-making for new anti-cancer therapies and have been implemented in other technology appraisals for previously treated aRCC; in particular, five of six technology appraisals identified in a targeted literature review of NICE technology appraisals from 2014-2019 used a partitioned survival approach to evaluate cost-effectivenessCitation16,Citation17,Citation27–30. Overall, NICE evidence review groups from these appraisals deemed the partitioned survival approach suitable for this indication. The partitioned survival modeling approach is limited by the assumption of independence between OS and PFS, as well as challenges in modeling the causal relationship between event probabilities (e.g. mortality) and all relevant time-varying parameters (e.g. current treatment, health state)Citation31. Future research could test the robustness of our findings using alternative model structures, such as a state-transition approach.
The cost-effectiveness evaluation presented herein was based on the best-available evidence for all treatments. The METEOR study showed the statistically significant clinical benefit of cabozantinib compared to everolimus in terms of OS and PFS, which provided strong evidence to evaluate its cost-effectiveness. Although this de novo model was developed following the best modeling practices, structural and parameter uncertainties still exist due to data limitations. As determined when conducting the sensitivity analyses, varying the OS and TTD parameters and drug costs had the largest impact on cost-effectiveness results. AEs in subsequent lines were not explicitly modeled, but this was expected to have a negligible impact on the results, as AE management costs were not a key driver of cost-effectiveness.
The clinical inputs for cabozantinib and everolimus were derived based on patient-level data from the METEOR study, whereas an NMA was required to estimate the relative efficacy vs. axitinib and nivolumab. The NMA results suggested relatively low OS HRs of cabozantinib vs. axitinib (HR = 0.56 [95%CrI: 0.20, 1.60]), which is not reflective of real-world clinical practices. The NMA was associated with a higher degree of uncertainty for comparisons to axitinib, which were mainly driven by the fact that two additional trials, TARGET (sorafenib vs. placebo) and RECORD-1 (everolimus vs. placebo), were required to create a connected network between cabozantinib and axitinib. The addition of these studies introduced heterogeneity to the network with regards to the cross-over trial designs, the number and type of prior therapies, and baseline prognostic scores. Given this limitation, one of the model scenarios assumed axitinib to be equally effective as everolimus, with findings consistent to the base case (with an ICER of ¥ 3,736,376 vs. axitinib) in that both were well below the threshold of ¥ 7,500,000.
The METEOR study included patients from North America, Europe, Asia Pacific (including Australia), and Latin America—but no patients from Japan. For the purposes of the cost-effectiveness analysis, data from the METEOR study were used as a proxy for the Japanese population, and alternative assumptions specific to clinical practice in Japan were evaluated in scenario analyses using Japanese patients’ RDI derived from the cabozantinib phase II study and a commercially available Japanese claims database. The METEOR-based, long-term OS and PFS projections were presented to three Japanese clinicians to determine the most clinically plausible long-term projections to assume for a Japanese population.
Cabozantinib was designated for cost-effectiveness evaluation at the time of NHI price listing and reimbursement approval in May 2020 in Japan. The evaluation by the C2H and the appraisal by the Cost-effectiveness Evaluation Expert Committee was completed, and cabozantinib was appraised as a cost-effective treatment. Based on these processes, the price of cabozantinib was determined to remain at its original level at Central Social Insurance Medical Council (Cyuikyo) in August 2022. Cabozantinib was recommended by the NICE committee in 2019 as a subsequent line of treatment in the United Kingdom. The associated technology appraisal included an economic evaluation in which cabozantinib was found to be dominant compared with nivolumab, consistent with the findings of this study, and cost-effective compared with axitinib at a £50,000 threshold.
Conclusions
Given the associated clinical benefits and acceptable health economics profiles, cabozantinib treatment represents a cost-effective option for the treatment of patients in Japan with aRCC who have failed at least one prior therapy.
Transparency
Declaration of financial/other interests
CC, HB, and SC are employed by Evidera, an independent research company that provides consulting and other research services to life science companies; in their salaried positions, they work with a variety of companies and are precluded from receiving payment or honoraria directly from these organizations for these services rendered. Evidera received payment from Takeda Pharmaceutical Company Limited for the conduct of this study. YM has received research grants from MSD K.K. and Ono Pharmaceutical Co. Ltd.; and has received honoraria from Takeda Pharmaceutical Company Limited, Bristol-Myers Squibb K.K., and Eisai Co. Ltd. TO has received honoraria from Takeda Pharmaceutical Company Limited. HU has received research grants from Takeda Pharmaceutical Company Limited, Bayer Yakuhin Ltd., Sanofi K.K., and Daiichi Sankyo Co. Ltd.; and has received honoraria from Janssen Pharmaceutical K.K., Bayer Yakuhin Ltd., Sanofi K.K., Astellas Pharma Inc., AstraZeneca K.K., and Takeda Pharmaceutical Company Limited. HK and MK are employees of Takeda Pharmaceutical Company Limited and have stock in Takeda Pharmaceutical Company Limited.
Author contributions
All authors satisfied ICMJE authorship criteria.
Previous presentations
None
Supplemental Material
Download MS Word (2.5 MB)Acknowledgements
The authors thank Michael Grossi, Senior Editor at Evidera, for his assistance in the editing, formatting, and submission of the manuscript.
Data availability statement
The authors declare that all input data to parameterize the model are available within the article and the Supplementary Material. The model can be re-built entirely based on the detailed description of the model structure and information provided.
Additional information
Funding
References
- Escudier B, Porta C, Schmidinger M, et al. Renal cell carcinoma: ESMO clinical practice guidelines for diagnosis, treatment and follow-updagger. Ann Oncol. 2019;30(5):706–720. doi: 10.1093/annonc/mdz056.
- Marugame T, Matsuda T. Comparison of time trends in kidney cancer incidence (1973-97) in East Asia, Europe and USA, from cancer incidence in five continents, vols IV-VIII. Jpn J Clin Oncol. 2008;38(7):508–509. doi: 10.1093/jjco/hyn060.
- Gangadaran SGD. Current management options in metastatic renal cell cancer. Oncol Rev. 2017;11(2):339. doi: 10.4081/oncol.2017.339.
- Japanese Urological Association. Clinical practice guideline for renal cancer (revision of 2017 guidelines) 2017 [September 2020]. Available from: https://www.urol.or.jp/lib/files/other/guideline/33_renal_cancer_2017_rev2019.pdf.
- Choueiri TK, Escudier B, Powles T, et al. Cabozantinib versus everolimus in advanced renal cell carcinoma (METEOR): final results from a randomised, open-label, phase 3 trial. Lancet Oncol. 2016;17(7):917–927. doi: 10.1016/S1470-2045(16)30107-3.
- Chen RC, Choueiri TK, Feuilly M, et al. Cabozantinib versus sunitinib as initial therapy for metastatic renal cell carcinoma of intermediate or poor risk (alliance A031203 CABOSUN randomised trial): progression-free survival by independent review and overall survival update. Eur J Cancer. 2020;126(24):5311–5318. doi: 10.1016/j.ejca.2018.02.012.
- Choueiri TK, Escudier B, Powles T, et al. Cabozantinib versus everolimus in advanced renal-cell carcinoma. N Engl J Med. 2015;373(19):1814–1823. doi: 10.1056/NEJMoa1510016.
- Motzer RJ, Escudier B, Powles T, et al. Long-term follow-up of overall survival for cabozantinib versus everolimus in advanced renal cell carcinoma. Br J Cancer. 2018;118(9):1176–1178. doi: 10.1038/s41416-018-0061-6.
- Tomita Y, Tatsugami K, Nakaigawa N, et al. Cabozantinib in advanced renal cell carcinoma: a phase II, open-label, single-arm study of Japanese patients. Int J Urol. 2020;27(11):952–959. doi: 10.1111/iju.14329.
- Hasegawa M, Komoto S, Shiroiwa T, et al. Formal implementation of cost-effectiveness evaluations in Japan: a unique health technology assessment system. Value Health. 2020;23(1):43–51. doi: 10.1016/j.jval.2019.10.005.
- CORE2 HEALTH (C2H). Guideline for preparing cost-effectiveness evaluation to the Central Social Insurance Medical Council. Version 2.0 approved by CSIMC on 20th February, 2019.
- Latimer NR. Survival analysis for economic evaluations alongside clinical trials–extrapolation with patient-level data: inconsistencies, limitations, and a practical guide. Med Decis Making. 2013;33(6):743–754. doi: 10.1177/0272989X12472398.
- Ikeda S. Developing a Japanese version of the EQ-5D-5L value set. J Public Health. 2015;64(1):47–55.
- e-Stat portal site of official statistics of Japan. Statistics of Japan. 2020;8. Available from: https://www.e-stat.go.jp/en/
- National Institute for Health and Care Excellence. Pazopanib for the first-line treatment of advanced renal cell carcinoma: Technology appraisal guidance. [TA215]. 2013. Available from: https://www.nice.org.uk/guidance/ta215.
- National Institute for Health and Care Excellence. Axitinib for treating advanced renal cell carcinoma after failure of prior systemic treatment: technology appraisal guidance [TA333] 2015. Available from: https://www.nice.org.uk/guidance/ta333.
- National Institute for Health and Care Excellence. Nivolumab for previously treated advanced renal cell carcinoma: technology appraisal guidance [TA417]. 2016. Available from: https://www.nice.org.uk/guidance/ta417.
- Motzer RJ, Barrios CH, Kim TM, et al. Phase II randomized trial comparing sequential first-line everolimus and second-line sunitinib versus first-line sunitinib and second-line everolimus in patients with metastatic renal cell carcinoma. J Clin Oncol. 2014;32(25):2765–2772. doi: 10.1200/JCO.2013.54.6911.
- Kobayashi M, Kudo M, Izumi N, et al. Cost-effectiveness analysis of lenvatinib treatment for patients with unresectable hepatocellular carcinoma (uHCC) compared with sorafenib in Japan. J Gastroenterol. 2019;54(6):558–570. doi: 10.1007/s00535-019-01554-0.
- Thom H. Deterministic and probabilistic analysis of a simple Markov model: how different could they be? Appl Health Econ Health Policy. 2022;20(3):447–449. doi: 10.1007/s40258-021-00700-1.
- Xie X, Yeung MW, Wang Z, et al. Comparison of the expected rewards between probabilistic and deterministic analyses in a Markov model. Expert Rev Pharmacoecon Outcomes Res. 2020;20(2):169–175. doi: 10.1080/14737167.2019.1615886.
- Canadian Agency for Drugs and Technologies in Health. Guidelines for the Economic Evaluation of Health Technologies: Canada. 4th Edition. [updated March 2017]. Available from: https://www.cadth.ca/guidelines-economic-evaluation-health-technologies-canada-4th-edition
- National Institute for Health and Care Excellence. NICE health technology evaluations: the manual [updated 31 January 2022]. Available from: https://www.nice.org.uk/process/pmg36/chapter/introduction-to-health-technology-evaluation
- National Institute for Health and Care Excellence. Cabozantinib for previously treated advanced renal cell carcinoma: single Technology Appraisal [ID931] 2017 cited 2020 October 8]. Available from: https://www.nice.org.uk/guidance/ta463/documents/committee-papers.
- ClinicalTrials.gov. NCT03339219: a phase 2 study of cabozantinib in Japanese patients with advanced renal cell carcinoma 2017 [cited 2020 October 8]. Available from: https://clinicaltrials.gov/ct2/show/NCT03339219.
- JMDC. JMDC claims database. [cited 2020 October 8]. Available from: https://www.jmdc.co.jp/en/jmdc-claims-database.
- National Institute for Health and Care Excellence. Cabozantinib for previously treated advanced renal cell carcinoma Technology appraisal guidance [TA463 ]/. 2017. Available from: https://www.nice.org.uk/guidance/ta463.
- National Institute for Health and Care Excellence. Lenvatinib with everolimus for previously treated advanced renal cell carcinoma: technology appraisal guidance [TA498] 2018. Available from: https://www.nice.org.uk/guidance/ta498.
- National Institute for Health and Care Excellence. Everolimus for advanced renal cell carcinoma after previous treatment: technology appraisal guidance [TA432] 2017. Available from: https://www.nice.org.uk/guidance/ta432.
- National Institute for Health and Care Excellence. Bevacizumab (first-line), sorafenib (first- and second-line), sunitinib (second-line) and temsirolimus (first-line) for the treatment of advanced and/or metastatic renal cell carcinoma: technology appraisal guidance [TA178]. 2009. Available from: https://www.nice.org.uk/guidance/ta178.
- Coyle D, Haines A, Lee K. Extrapolating clinical evidence within economic evaluations. CJHT. 2023;3(5):1–21. doi: 10.51731/cjht.2023.649.
- Rini BI, Escudier B, Tomczak P, et al. Comparative effectiveness of axitinib versus sorafenib in advanced renal cell carcinoma (AXIS): a randomised phase 3 trial. Lancet. 2011;378(9807):1931–1939. doi: 10.1016/S0140-6736(11)61613-9.
- Motzer RJ, Escudier B, McDermott DF, et al. Nivolumab versus everolimus in advanced renal-cell carcinoma. N Engl J Med. 2015;373(19):1803–1813. doi: 10.1056/NEJMoa1510665.
- Sternberg CN, Hawkins RE, Wagstaff J, et al. A randomised, double-blind phase III study of pazopanib in patients with advanced and/or metastatic renal cell carcinoma: final overall survival results and safety update. Eur J Cancer. 2013;49(6):1287–1296. doi: 10.1016/j.ejca.2012.12.010.