Abstract
Background
The 15-valent pneumococcal conjugate vaccine (PCV15 or V114) has recently been approved for pediatric vaccination against pneumococcal diseases (PDs) in Japan. The study aims to evaluate the cost-effectiveness of pediatric vaccination with V114 versus 13-valent PCV (PCV13) in Japan.
Methods
The study used a decision analytical Markov model to estimate the cost and effectiveness outcomes for a birth cohort in Japan over a 10-year time horizon. The model tracked the occurrences of acute PD events, including invasive PD (IPD), non-bacteremic pneumococcal pneumonia (NBPP) and pneumococcal acute otitis media (AOM) and the long-term impact of post-meningitis sequalae. Vaccine effectiveness was estimated based on literature and assumptions, and accounted for indirect effects and vaccine waning. The base case took the societal perspective, including both direct and indirect costs, while a healthcare payer perspective was modeled in a scenario analysis. Additional scenario analyses and sensitivity analyses were conducted.
Results
In the base case, V114 was associated with an incremental gain of 24 quality-adjusted life years and a reduction of ¥365,610,955 in total costs compared to PCV13. It was expected to reduce the number of pneumococcal AOM, NBPP, and IPD cases by 1,832, 1,333 and 25, respectively. All scenario analyses and most sensitivity analyses showed that V114 was a dominant strategy compared to PCV13.
Conclusions
Pediatric vaccination with V114 is expected to lead to cost savings and more health benefits compared to PCV13 in Japan from both societal and healthcare payer perspectives. The findings are robust under plausible assumptions and inputs.
Introduction
Pneumococcal disease (PD) is a major cause of morbidity and mortality globally, especially in young children and older adults.Citation1 PD includes a group of diseases caused by Streptococcus pneumoniae (S. pneumoniae, pneumococcus).Citation1–3 The most common types of PD are non-bacteremic pneumococcal pneumonia (NBPP), which can affect individuals of all ages, and pneumococcal acute otitis media (AOM), which primarily affects children.Citation3,Citation4 Invasive pneumococcal disease (IPD), including meningitis, bacteremia without focus, and bacteremic pneumonia, is a less common but more severe form of PD.Citation2–4 Meningitis, in particular, has a high mortality rate and can lead to long-term disabling neurological sequelae, such as hearing loss, seizures, and cognitive impairment.Citation5,Citation6
The introduction of pneumococcal conjugate vaccines (PCVs) was a critical milestone in reducing the burden of PD worldwide.Citation1,Citation7 In Japan, the 7-valent pneumococcal conjugate vaccine (PCV7) was approved in 2009 and included in the National Immunization Program (NIP) in 2013.Citation8 However, it was soon replaced by PCV13, which provides protection against six more serotypes, and has been included in the NIP for the routine pediatric vaccination against PD since 2013.Citation8 Currently, the Japan Pediatric Society recommends 3 + 1 schedule for PCV13, consisting of three doses at 2, 3, and 4 months in the primary series and one booster dose at 12–15 months.Citation9
Routine immunization with PCV7 and PCV13 has drastically reduced incidence of PDs caused by PCV13 serotypes among children in Japan.Citation10–14 Additionally, vaccine-serotype PDs have markedly declined in the adult population in Japan following the introductions of PCV7 and PCV13, which suggests that vaccination with PCVs may reduce the PD incidences in unvaccinated populations—a phenomenon known as the ‘indirect effects’ of pediatric PCV use.Citation13,Citation15–18 For example, the percentage of IPD attributable to PCV13 serotypes decreased by more than half in adults from 2010 to 2016.Citation13 Similar declines were observed in PCV13 serotypes in community acquired pneumonia in Japan adult population.Citation16,Citation17 Combining the direct and indirect effects, pediatric vaccination with PCVs has substantially reduced burden of PD in Japan.Citation10–18
However, despite the success in the pediatric PCV vaccination program in Japan, serotype replacement, which refers to an increase in the incidence rates of PD caused by non-vaccine serotypes (NVTs) after introduction of PCVs, poses new challenges in PD prevention.Citation10,Citation13,Citation14 Between 2014 and 2016, NVTs accounted for nearly half of the pneumococcal serotypes in children, a substantial increase compared to their contributions to the pneumococcal serotypes in the pre-PCV7 era.Citation13 In addition, PCV13 may have limited effectiveness against certain vaccine serotypes, such as serotype 3.Citation13,Citation19–22 Consequently, the epidemiological, economic and humanistic burden of PD in Japan remains high during the post-PCV era.Citation3 Therefore, it is necessary to develop more effective vaccines with broader serotype coverage to further reduce the burden of PD in Japan.
A 15-valent PCV (PCV15 or V114) is approved and recommended for prevention of PDs in children and adults in many countries and regions, including the United States, Canada, and European Unions.Citation23–26 It has recently been approved for pediatric and adult vaccination against PD in Japan.Citation27 V114 provides protection against two additional serotypes, 22 F and 33 F, which contribute to 9.3–10.9% of IPD cases in Japan among children <5 years in the post-PCV13 period.Citation10,Citation28 Pediatric clinical trials have demonstrated that V114 is well-tolerated with a safety profile that is consistent with PCV13, and has a non-inferior immune response for the 13 shared serotypesCitation29–31 and statistically superior immune responses for serotypes 3, 22 F and 33 F as compared to PCV13.Citation30 This study evaluated the cost-effectiveness of routine infant vaccination with V114 versus PCV13 in Japan from a societal perspective.
Method
Model overview
A previously published decision analytical Markov modelCitation32 was utilized to assess the cost-effectiveness of vaccination strategies with V114 versus PCV13 in the pediatric population in Japan (). Both strategies adopted the 3 + 1 dosing schedule as recommended by the guidelines of the Japan Pediatric Society.Citation9 The model assumed that the primary series and the booster dose were administered in the first- and second-year of life, respectively, with all doses from the same vaccine type.
Figure 1. Decision analytic model of pediatric pneumococcal vaccination.
Abbreviations: V114: 15-valent pneumococcal conjugate vaccine; PCV13: 13-valent pneumococcal conjugate vaccine.
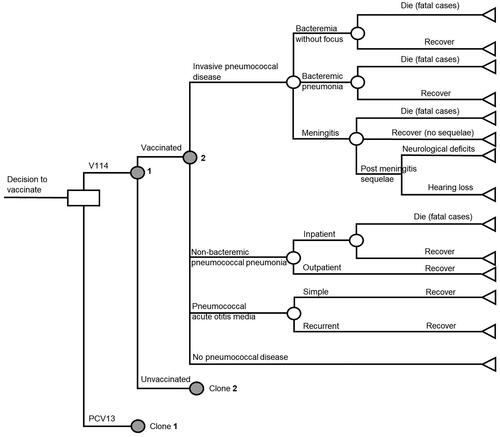
The Markov model consisted of three health states, namely ‘no PD,’ ‘post-meningitis sequelae (PMS),’ and ‘death,’ and was designed to track acute PD events, including IPD, NBPP, and pneumococcal AOM (). Individuals entered in the ‘no PD’ state at cycle 0 and were at risk of developing PD events in each model cycle (i.e. one year). All PD events were modeled as acute events that would resolve within a year. These events would incur short-term resource utilization, costs, and a decrement in quality-adjusted life years (QALYs) during the cycle in which the events occurred.
Individuals with meningitis were at risk of developing PMS, including neurologic deficits and hearing loss,Citation33 and would remain in the ‘PMS’ state until death. They were also at risk of developing non-meningitis IPD, pneumonia, and AOM in later model cycles. Moreover, the general background mortality was applied to all health states, while individuals with IPD or inpatient NBPP would experience additional disease-related death.
In the base case, the model followed a single vaccinated cohort for a 10-year time horizon. The 10-year time horizon was selected considering the duration of a public health program launched by municipalities and the time horizon needed to incorporate the vaccine waning effect, which was assumed to start in Year 6 from the last dose. A societal perspective was applied in the base case, including direct medical costs and indirect costs. The outcomes included incremental cost-effectiveness ratio (ICER), expressed as cost per life year (LY) gained and cost per QALY gained, as well as clinical outcomes, reported as the number of IPD, NBPP, pneumococcal AOM, and PMS cases prevented, and the number of IPD- and pneumonia-related deaths avoided. An annual discount rate of 2% was applied to costs, QALYs and LYs.Citation34 Clinical outcomes such as numbers of PD cases or deaths were not discounted.
Model inputs
Epidemiological inputs
In the base case, the model followed a single birth cohort of 781,093 infants in 2023, the year when V114 was assumed to be available to the pediatric population in Japan. The birth rate and background mortality were obtained from the government data.Citation35,Citation36 While the vaccines were given to the birth cohort, the model considered the direct effects of the vaccines on the birth cohort as well as the indirect effects on other age groups.
Annual PD incidence rates and case fatality rates are presented in . For IPD, incidence rates for children <5 years were estimated using the overall IPD incidence rates and the distribution of different IPD types published by the National Institute of Infectious Disease (NIID) in Japan.Citation37,Citation38 For the age groups between 5 and 65 years, the incidence rates were sourced from Chang et al.Citation39, Fukusumi et al.Citation40 and Tamura et al.Citation15 For the age groups ≥65, the rates were obtained from the NIID as well.Citation38 The proportions developing PMS among patients with meningitis were only considered for the age groups <18 yearsCitation10 due to lack of data in the adult population. Annual incidence rates for NBPP by age group were estimated by multiplying all-cause pneumonia incidence rateCitation11,Citation41 with the percentage of pneumonia incidence attributable to S. pneumoniae in the corresponding age group.Citation11,Citation41,Citation42 The inpatient and outpatient NBPP incidence rates were then estimated by the proportions reported in the government data.Citation35 Annual incidence rates for simple and recurrent pneumococcal AOM were estimated using all-cause AOM rates based on the Sado Otitis Media Study,Citation43 the proportions of simple and recurrent AOMCitation43,Citation44 and the percentage attributable to S. pneumoniae.Citation45 The case fatality ratios for IPD were obtained from the studies by Iwata et al.Citation10 Nakano et al.Citation46 and Chang et al.Citation39 and those for inpatient NBPP were derived from the studies by Tashiro et al.Citation47 and Morimoto et al.Citation41
Table 1. Model inputs on pneumococcal disease incidence and case fatality rates, health utilities and costs.
Vaccine effectiveness
The vaccine coverage rate (VCR) was assumed to be 100% for primary series and the booster dose for both vaccines based on the data published by the Ministry of Health, Labour and Welfare (MHLW) and a previous cost-effectiveness analysis (CEA) study.Citation48,Citation49 Vaccine effectiveness (VE) was estimated based on the literature and assumptions, and was reported as a percentage reduction in baseline PD incidence rates resulting from pneumococcal vaccine administration. Serotype-specific VEs were applied to age-specific serotype distributions for all PDs in the model ().
Table 2. Vaccine effectiveness of PCV13 and V114 in the base case.
The VEs against IPD and pneumococcal AOM were estimated using similar methods. Specifically, the serotype-specific VEs for PCV13 were estimated first. V114 was assumed to have the same VEs as PCV13 for the shared 13 serotypes. For IPD, VEs for PCV13 were estimated based on the VEs for PCV7Citation50 and the study by Moore et al.Citation51 The VEs for serotypes 22 F and 33 F in V114 were assumed to be equal to the average VE among the shared 13 serotypes. The VEs against pneumococcal AOM were obtained from the PCV7 clinical trialCitation52 and the study by Pichichero et al.Citation53 The VEs for serotypes 22 F and 33 F were assumed to the same as the overall VE in the PCV7 clinical trial.Citation52 The same VEs were assumed for simple and recurrent AOM in the base case. The VEs against NBPP were obtained from Lewnard et al.Citation54 and applied to both inpatient and outpatient NBPP.
In addition, the model considered vaccine waning and indirect effects when estimating the VEs. The base case assumed that vaccine waning started in Year 6 after the last dose and decreased linearly to 0% over the next 10 years.Citation55,Citation56 The indirect effects were applied to serotype 22 F and 33 F IPD, assuming an annual incidence rate reduction of 7.8%Citation55 for the first five years. Based on this, a relative reduction in 22 F- and 33 F-related IPD incidence rate of 7.8%, 15.5%, 21.6%, 27.7% and 33.4% compared to the baseline was applied in the first, second, third, fourth, and fifth or more years upon V114 launch, respectively.
Utility weights and QALY decrements
The utilities for ‘no PD’ were assumed to be the same as those in the general population in Japan, as reported by Shiroiwa et al.Citation57 Weighted average utilities of male and female populations were estimated for each age group (). For PMS, the utility values of 0.68 and 0.73 from the study by Rubin et al.Citation58 were applied to neurological deficits and hearing loss, respectively, across all age groups. The QALY decrements associated with PD events were sourced from the study by Rubin et al.Citation58 for children <18 years, and the studies by Mangen et al.Citation59 and Glick et al.Citation60 for adults ().
Cost inputs
The societal perspective was utilized in the base case, which included both direct medical costs and indirect costs (). All costs were adjusted to 2022 Japanese Yen (JPY) using the National Health Insurance (NHI) conversion rates.Citation61
Direct medical costs
The vaccine acquisition cost for PCV13 was ¥7,200, which was obtained from the MHLW data.Citation62 Price parity was assumed for V114. Vaccine administration costs were estimated at ¥4,400 for both vaccines based on the MHLW guidance report and the NHI treatment fee schedule,Citation63 and included outpatient pediatric visit fee, injection fee, vaccine premium and consumer tax.
Treatment costs per episode for IPD, NBPP, AOM were derived from the literature and varied by age group.Citation49,Citation64–69 Annual medical costs for PMS were estimated separately for the age groups of <2 years and 2–18 years, using the discounted lifetime costs.Citation70
Indirect costs
The model considered productivity loss related to the treatment of PDs, which was incurred by caregivers of pediatric patients and adult patients. The productivity loss was estimated using the human capital approach, which accounted for the wage loss of caregivers or adults patients for each PD episode. The indirect costs for the age groups <18 years were obtained from the study by Shiragami et al.Citation49 For the age groups ≥18 years, productivity loss related to hospitalization due to IPD and inpatient NBPP was considered. Specifically, the length of stay, which was obtained from the Diagnostic Procedure Code (DPC) statistics, were multiplied by the median annual earnings for each age group.Citation65,Citation66,Citation71 In addition, the model accounted for productivity loss due to premature death, which was derived based on the average annual earnings for the age groups ≥16 years, calculated by multiplying the labor force participation rate with the median annual earnings for each age group.Citation35,Citation71
Sensitivity analyses
A series of scenario analyses were carried out to assess the uncertainties associated with specific assumptions and inputs in the model. These analyses involved alternative assumptions regarding the target population, the time horizon, the model perspective, vaccine coverage rate, and the direct and indirect vaccine effects. Specifically, Scenario 1 included the entire Japan population as the target population and used 100-year (i.e. lifetime horizon); Scenario 2 took a healthcare payer perspective instead of the societal perspective; Scenario 3 used VEs against all-cause pneumonia and all-cause AOM to estimate pneumonia- and AOM-related outcomes; Scenario 4 assumed no indirect effects; and lastly, Scenario 5 and Scenario 6 assumed a vaccine coverage rate of 95% and 90%, respectively. Furthermore, one-way sensitivity analysis (SA) was conducted by varying one model input at a time to assess the sensitivity of the model results to various model input values. Additionally, a probabilistic sensitivity analysis (PSA) was conducted using a Monte Carlo simulation with 1,000 iterations generated by simultaneously varying key model parameters based on pre-specified distribution assumptions (Supplementary Table 1). Except for the population inputs and vaccine acquisition costs, the key parameter variations were defined based on the 95% confidence intervals or the standard errors (SEs), as reported in the respective source studies. If the variation of an input parameter was not presented in the source study, an SE of 20% of the base-case value was applied. Lastly, given the uncertainty of the V114 price, a threshold analysis was conducted to estimate the maximum price of V114 for it to be cost saving compared to PCV13.
Table 3. Base-case results.
Results
Base case
The base-case results () showed that by vaccination of a birth cohort of 781,093 infants, V114 was expected to reduce the number of IPD, NBPP and pneumococcal AOM cases by 25, 1,333 and 1,832, respectively, over the 10-year time horizon, compared to PCV13. Furthermore, V114 was projected to prevent two IPD-related deaths and to save seven LYs with an incremental QALY gain of 24, relative to PCV13. V114 was associated with slightly higher vaccine acquisition and administration costs (¥1,919 and ¥1,172, respectively), due to the reduction in the IPD-related deaths associated with V114 during the first year. However, it was predicted to save ¥9,138,561 in IPD treatment costs, ¥126,195,971 in pneumonia treatment costs, ¥98,903,212 in AOM treatment costs and ¥901,144 in PMS treatment costs. Additionally, V114 was predicted to reduce the indirect costs by ¥130,475,159. The total cost savings associated with V114 versus PCV13 amounted to ¥365,610,955. Because V114 was associated with extended LYs and QALYs and lower costs, it was a dominant strategy compared to PCV13 in the base case.
Table 4. Incremental costs and effectiveness outcomes comparing V114 vs. PCV13 in the scenario analyses.
Sensitivity analysis
All scenario analyses conducted were consistent with the base-case results, indicating that V114 was the dominant vaccination strategy in comparison to PCV13 (). In scenario 1, following the entire Japan population over lifetime (i.e. 100 years) resulted in the highest cost savings of ¥13,067,478,826 for V114, with 3,011 extended LYs and 3,221 total QALY gain. When the healthcare payer perspective was applied, the total cost savings associated with V114 relative to PCV13 became ¥235,135,796. In scenario 3, where VEs against all-cause pneumonia and all-cause AOM were applied, the incremental LYs and QALYs were similar to the base case but the total cost savings were greater. Moreover, the scenario analysis assuming no indirect effects resulted in little changes compared to the base case, mainly because the indirect effects were only applied to IPD.
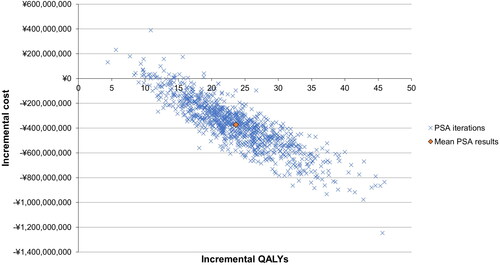
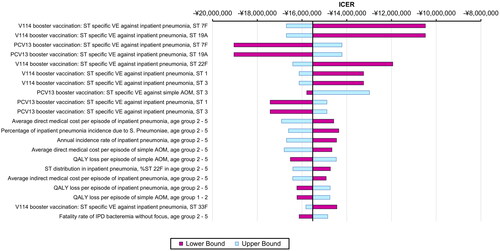
The one-way SA and PSA further supported the robustness of the model results. All ICERs in the one-way SA indicated that V114 was cost saving and yielded higher QALYs (). The ICER was most sensitive to inputs related to inpatient pneumonia, including serotype-specific VE for V114 and PCV13, direct and indirect cost per episode, baseline incidence rate, percentage attributable to S. pneumoniae, serotype distribution, and QALY decrement. Certain inputs related to simple AOM and bacteremia without focus were also among the most impactful ones. In addition, the model inputs for the age group of 2–5 years generally had a greater impact on the results than other age groups (). The threshold analysis showed that the maximum price per dose for V114 was ¥7,318 for it to remain cost saving compared to PCV13, i.e. ¥118 higher than the PCV13 price. Results from the PSA showed that V114 was the dominant strategy in 98.7% of the simulations ().
Figure 2. One-way sensitivity analysis results (showing the most impactful parameters).
Abbreviations: ICER: incremental cost-effectiveness ratio; PCV13: 13-valent pneumococcal conjugate vaccine; V114: 15-valent pneumococcal conjugate vaccine; QALY: quality-adjusted life year; VE: vaccine effectiveness; IPD: invasive pneumococcal disease; AOM: acute otitis media; ST: serotype.
Notes: Tornado diagram showing the results of the one-way sensitivity analysis. The purple bars show the change in ICER from the base case when the higher value of an input was used whereas the light blue bars show the change in ICER from the base case when the lower value of the selected input was used while all other inputs remain constant.
Discussion
The emergence of PDs caused by NVT and antibiotic-resistant serotypes present new challenges in PD prevention and treatment in Japan,Citation10,Citation13,Citation46 which could be potentially addressed by newer vaccines. V114 includes the 13 serotypes in PCV13 plus two additional serotypes, 22 F and 33 F, which are associated with high mortality, antibiotic resistance, and meningitis.Citation1 Furthermore, V114 provides superior immune response against the shared serotype 3 as compared with PCV13,Citation30 potentially offering better protection against this serotype. Adding to the previously published literature on V114’s immunogenicity and safety, the current study provides economic evidence on the use of V114. The current study shows that V114 is a cost-saving strategy in Japan from both the societal and healthcare payer perspectives compared to PCV13. To our knowledge, this is the first study to evaluate the cost-effectiveness of V114 in Japan. The base case predicts that V114 is associated with total cost savings of ¥365,610,955 and 24 more QALYs. It had the greatest impact in reducing the number of AOM cases, followed by pneumonia cases, while the cost savings were highest for pneumonia treatment costs, followed by treatment costs for AOM and IPD. Indirect cost savings accounted for approximately one-third of the total cost savings in the base case. Despite the variation in the incremental costs and QALYs comparing V114 versus PCV13 in the sensitivity analysis, V114 was consistently the dominant strategy under all plausible alternative assumptions and input values in the scenario analyses and deterministic sensitivity analyses and 98.7% of the simulations in the PSA, demonstrating the robustness of the model results.
The conclusions from the current study align with the recently published cost-effectiveness analyses of pediatric use of V114 versus PCV13 in the United States.Citation32,Citation72 Both US models applied a similar model structure as the one in the current model. Huang et al.Citation32 tracked PD incidence in the entire US population over a lifetime, while Prasad et al.Citation72 tracked PD incidence in a single birth cohort up to 17 years (15 years after the last PCV dose). Despite the differences, both studies showed that that V114 was the dominant strategy compared to PCV13 in the base case and all the sensitivity analyses, including the scenario analysis of no indirect effects. Based on these CEA studies as well as the demonstrated immunogenicity and safety of V114 in the clinical trials, the Centers for Disease Control and Prevention Advisory Committee for Immunization Practices recommends V114 for pediatric immunization in the US.Citation26 The current study adds to the previous CEAs comparing V114 with PCV13 in pediatric populations. Together, they demonstrate that V114 is expected to prevent more PD cases, extend LYs and QALYs, and lead to cost savings in both direct and indirect costs as compared to PCV13. Moreover, consistent across all studies, the conclusions were sustained in almost all scenario analyses and sensitivity analyses, suggesting that the cost saving of V114 vs. PCV13 is robust under different settings, assumptions, and input values.
While the results from the current study cannot be directly compared with other CEAs of pneumococcal vaccines in Japan due to different comparators, the model structure is similar to that of previously published models.Citation49,Citation64,Citation73 Notably, the current study incorporated distinctive improvements upon earlier models. For example, the current study modeled bacteremia without focus and bacteremic pneumonia separately, and treated simple AOM and recurrent AOM as separate PD events with different baseline incidence rates. Additionally, the current study estimated the number of NBPP cases, which is specific to S. pneumoniae vs. all-cause pneumonia. And lastly, the model incorporated indirect effects, which are included in many CEA models in other countriesCitation32,Citation58,Citation74–76 and is supported by the evidence in Japan.Citation13,Citation15–18 The study shows that indirect effects had a negligible effect on the cost savings and QALY gains because the model conservatively applied the indirect effects to IPD caused by 22 F and 33 F only and did not consider the indirect effects on other PD types.
The model should be reviewed in the context of certain limitations. As with all models, limited data availability is a major constraint in the current model. While thorough effort was made to obtain the most relevant model inputs specific to the Japan populations, certain inputs are not available in the literature, such as disutilities associated with PD events in Japan, case fatality ratios related to inpatient pneumonia for children, and VEs for certain serotypes, including 22 F and 33 F. With such limitations, reasonable and conservative assumptions were made with extensive sensitivity analyses to evaluate the uncertainty. One example is indirect effects, which were conservatively assumed to be applicable to IPD in the base case and were removed in the scenario analysis. Another example is the conservative assumption of the same VE between V114 and PCV13 for PDs caused by serotype 3 despite the demonstrated superior immunogenicity of V114 for this serotype.Citation30 Additionally, due to lack of information in Japan, certain inputs were not considered in the current model, e.g. direct non-medical costs and indirect costs associated with PMS. However, based on studies conducted in the US, direct non-medical costs were generally insignificant when compared to direct medical costs and indirect costs due to work loss.Citation77–81 Therefore, the exclusion of direct non-medical costs is unlikely to affect the conclusions of the current study. Similarly, the omission of indirect costs associated with PMS reduces the magnitude of cost saving by V114, resulting in more conservative results, and thus would not impact the study conclusions. Another key model input with uncertainty is the price for V114. V114 price in the pediatric indication was unknown at the time of the analysis. To address this limitation, a threshold analysis was conducted to estimate the maximum price at which V114 remains cost saving compared to PCV13. Furthermore, the current study did not account for certain trends in recent epidemiology of PD. One of them is antibiotic resistance, which may increase the PD treatment costs over time. Notably, serotypes 22 F and 33 F are associated with antibiotic resistance;Citation1 by providing protection against these two serotypes, V114 is expected to further reduce treatment costs compared to the current estimate in the base case. Moreover, the model did not explicitly consider time-varying serotype distribution. However, the study applied vaccine waning to account for reducing VEs over time due to serotype replacement.
Despite the limitations of the study, the robust findings suggest that V114 has the potential to address the emerging challenges posed by PD and reduce the burden of communicable diseases in Japan. The study provides timely economic evidence that enables healthcare decision-makers in Japan to make informed decisions about adopting V114 in the NIP. In addition, the study highlights the importance of continuing to monitor the disease and update the model to accurately assess the potential impact of V114 as new information becomes available.
Conclusions
The study demonstrates that using the 3 + 1 dosing schedule of V114 for infant vaccination is a dominant strategy compared to PCV13 in Japan, resulting in both direct and indirect cost savings and improved QALYs. V114 has the potential to prevent more cases of PD, particularly NBPP and AOM. The findings are robust under a wide range of model input values and plausible alternative assumptions, providing valuable economic evidence to healthcare decision-makers in Japan for the adoption of V114.
Transparency
Author contributions
All authors were involved in the model conception, design, and analysis, as well as the interpretation of the results. All authors contributed to the first draft of the paper and subsequent revisions, and the final approval of the version to be published. All authors agree to be accountable for all aspects of the work.
Supplemental Material
Download MS Word (26.4 KB)Acknowledgements
We would like to acknowledge CHEORS for assistance in the model evaluation and Analysis Group, Inc. for assistance in model input collection. We would also like to acknowledge Jipan Xie, MD, PhD, at XL Source, Inc. for medical writing assistance.
Declaration of funding
This work was supported by Merck Sharp & Dohme LLC, a subsidiary of Merck & Co., Inc., Rahway, NJ, USA.
Declaration of financial/other relationships
Atsushi Tajima and Machiko Abe are employees of MSD K.K., Tokyo, Japan. Min Huang and Jessica Weaver are employees of Merck Sharp & Dohme LLC, a subsidiary of Merck & Co., Inc., Rahway, NJ, USA. A reviewer on this manuscript disclosed that they have been part of a health technology assessment project on PCV15. Peer reviewers on this manuscript have received an honorarium from JME for their review work but have no other relevant financial relationships to disclose.
References
- Feldman C, Anderson R. Recent advances in the epidemiology and prevention of Streptococcus pneumoniae infections. F1000Res. 2020;9:338. doi: 10.12688/f1000research.22341.1.
- Bogaert D, De Groot R, Hermans PW. Streptococcus pneumoniae colonisation: the key to pneumococcal disease. Lancet Infect Dis. 2004;4(3):144–154. doi: 10.1016/S1473-3099(04)00938-7.
- Igarashi A, Ueyama M, Idehara K, et al. Burden of illness associated with pneumococcal infections in Japan - a targeted literature review. J Mark Access Health Policy. 2022;10(1):2010956.
- Gierke RW, A, Patricia, Kobayashi M. Pneumococcal disease: centers for disease control and prevention. 2021 [cited 2023 Feb 23]. Available from: https://www.cdc.gov/vaccines/pubs/pinkbook/downloads/pneumo.pdf.
- Yildirim I, Shea KM, Pelton SI. Pneumococcal disease in the era of pneumococcal conjugate vaccine. Infect Dis Clin North Am. 2015;29(4):679–697. doi: 10.1016/j.idc.2015.07.009.
- Schiess N, Groce NE, Dua T. The impact and burden of neurological sequelae following bacterial meningitis: a narrative review. Microorganisms. 2021;9(5):900. doi: 10.3390/microorganisms9050900.
- Myint TT, Madhava H, Balmer P, et al. The impact of 7-valent pneumococcal conjugate vaccine on invasive pneumococcal disease: a literature review. Adv Ther. 2013;30(2):127–151. doi: 10.1007/s12325-013-0007-6.
- Katsuta T, Moser CA, Feemster KA, et al. Comparison of immunization systems in Japan and the United States - What can be learned? Vaccine. 2020;38(46):7401–7408. doi: 10.1016/j.vaccine.2020.09.028.
- Japan Pediatric Society. Immunization schedule recommended by the Japanese pediatric society. 2020. [cited 2023 Jan 19]. Available from: https://www.jpeds.or.jp/modules/activity/index.php?content_id=138.
- Iwata S, Takata M, Morozumi M, et al. Drastic reduction in pneumococcal meningitis in children owing to the introduction of pneumococcal conjugate vaccines: longitudinal analysis from 2002 to 2016 in Japan. J Infect Chemother. 2021;27(4):604–612. doi: 10.1016/j.jiac.2020.11.019.
- Takeuchi N, Naito S, Ohkusu M, et al. Epidemiology of hospitalised paediatric community-acquired pneumonia and bacterial pneumonia following the introduction of 13-valent pneumococcal conjugate vaccine in the national immunisation programme in Japan. Epidemiol Infect. 2020;148:e91. doi: 10.1017/S0950268820000813.
- Ubukata K, Morozumi M, Sakuma M, et al. Etiology of acute otitis media and characterization of pneumococcal isolates after introduction of 13-Valent pneumococcal conjugate vaccine in japanese children. Pediatr Infect Dis J. 2018;37(6):598–604. doi: 10.1097/INF.0000000000001956.
- Ubukata K, Takata M, Morozumi M, et al. Effects of pneumococcal conjugate vaccine on genotypic penicillin resistance and serotype changes, Japan, 2010-2017. Emerg Infect Dis. 2018;24(11):2010–2020. doi: 10.3201/eid2411.180326.
- Suga S, Chang B, Asada K, et al. Nationwide population-based surveillance of invasive pneumococcal disease in japanese children: effects of the seven-valent pneumococcal conjugate vaccine. Vaccine. 2015;33(45):6054–6060. doi: 10.1016/j.vaccine.2015.07.069.
- Tamura K, Chang B, Shimbashi R, et al. Dynamic changes in clinical characteristics and serotype distribution of invasive pneumococcal disease among adults in Japan after introduction of the pediatric 13-valent pneumococcal conjugate vaccine in 2013-2019. Vaccine. 2022;40(24):3338–3344. doi: 10.1016/j.vaccine.2022.04.062.
- Sando E, Suzuki M, Furumoto A, et al. Impact of the pediatric 13-valent pneumococcal conjugate vaccine on serotype distribution and clinical characteristics of pneumococcal pneumonia in adults: the Japan pneumococcal vaccine effectiveness study (J-PAVE). Vaccine. 2019;37(20):2687–2693. doi: 10.1016/j.vaccine.2019.04.009.
- Akata K, Chang B, Yatera K, et al. Distribution and annual changes in Streptococcus pneumoniae serotypes in adult japanese patients with pneumonia. J Infect Chemother. 2015;21(10):723–728. doi: 10.1016/j.jiac.2015.07.002.
- Ubukata K, Chiba N, Hanada S, et al. Serotype changes and drug resistance in invasive pneumococcal diseases in adults after vaccinations in children, Japan, 2010-2013. Emerg Infect Dis. 2015;21(11):1956–1965. doi: 10.3201/eid2111.142029.
- Balsells E, Guillot L, Nair H, et al. Serotype distribution of Streptococcus pneumoniae causing invasive disease in children in the post-PCV era: a systematic review and meta-analysis. PLoS One. 2017;12(5):e0177113. doi: 10.1371/journal.pone.0177113.
- Pilishvili T. 13-valent pneumococcal conjugate vaccine (PCV13) effects on disease caused by serotype 3. CDC; 2019. [cited 2022 May 31]. Available from: https://www.cdc.gov/vaccines/acip/meetings/downloads/min-archive/min-2019-02-508.pdf.
- Yanagihara K, Kosai K, Mikamo H, et al. Serotype distribution and antimicrobial susceptibility of Streptococcus pneumoniae associated with invasive pneumococcal disease among adults in Japan. Int J Infect Dis. 2021;102:260–268. doi: 10.1016/j.ijid.2020.10.017.
- Furuya Y, Yamagishi Y, Okade H, et al. Impact of the pneumococcal conjugate vaccine on serotype distribution of adult non-invasive Streptococcus pneumoniae isolates in tokai region, Japan, 2008-2016. J Infect Chemother. 2017;23(6):394–399. doi: 10.1016/j.jiac.2017.03.014.
- Food and Drug Administration. Label for pneumococcal 15-valent conjugate vaccine. 2023 [cited 2023 Apr 6]. Available from: https://nctr-crs.fda.gov/fdalabel/services/spl/set-ids/1158fa93-ef41-4a29-8252-9251f94c53c8/spl-doc?hl=Pneumococcal%2015-valent%20Conjugate%20Vaccine.
- European Medicines Agency. Label for pneumococcal 15-valent conjugate vaccine. 2022 [cited 2023 Apr 6]. Available from: https://www.ema.europa.eu/en/documents/product-information/vaxneuvance-epar-product-information_en.pdf.
- An Advisory Committee Statement (ACS) National Advisory Committee on Immunization (NACI). Interim guidance on the use of pneumococcal 15-valent conjugate vaccine (PNEU-C-15) in pediatric populations. 2023 [cited 2023 Jun 29]. Available from: https://www.canada.ca/content/dam/hc-sc/documents/services/publications/vaccines-immunization/national-advisory-committee-immunization-interim-guidance-pneumococcal-15-valent-conjugate-vaccine-pneu-c-15-pediatric-populations/national-advisory-committee-immunization-interim-guidance-pneumococcal-15-valent-conjugate-vaccine-pneu-c-15-pediatric-populations.pdf.
- Kobayashi M, Farrar JL, Gierke R, et al. Use of 15-Valent pneumococcal conjugate vaccine among U.S. Children: updated recommendations of the advisory committee on immunization practices—United States, 2022. MMWR Morb Mortal Wkly Rep. 2022;71(4):109–117. doi: 10.15585/mmwr.mm7104a1.
- Pharmaceuticals and Medical Devices Agency. Label for pneumococcal 15-valent conjugate vaccine. 2023 [cited 2023 Aug 8]. Available from: https://www.pmda.go.jp/PmdaSearch/iyakuDetail/GeneralList/631140K. Japanese.
- Annual Surveillance Data. Epidemiological Status in FY2020. 2023 [cited 2023 Jan 19]. Available from: https://ipd-information.com/?page_id=58.
- Greenberg D, Hoover PA, Vesikari T, et al. Safety and immunogenicity of 15-valent pneumococcal conjugate vaccine (PCV15) in healthy infants. Vaccine. 2018;36(45):6883–6891. doi: 10.1016/j.vaccine.2018.02.113.
- Lupinacci R, Rupp R, Wittawatmongkol O, et al. A phase 3, multicenter, randomized, double-blind, active-comparator-controlled study to evaluate the safety, tolerability, and immunogenicity of a 4-dose regimen of V114, a 15-valent pneumococcal conjugate vaccine, in healthy infants (PNEU-PED). Vaccine. 2023;41(5):1142–1152. doi: 10.1016/j.vaccine.2022.12.054.
- Merck & Co., Inc., Rahway, NJ, USA. U.S. FDA approves merck’s VAXNEUVANCE™ (pneumococcal 15-valent conjugate vaccine) for the prevention of invasive pneumococcal disease in infants and children [Internet]. 2022. [cited 2023 Mar 7]. Available from: https://www.merck.com/news/u-s-fda-approves-mercks-vaxneuvance-pneumococcal-15-valent-conjugate-vaccine-for-the-prevention-of-invasive-pneumococcal-disease-in-infants-and-children/.
- Huang M, Hu T, Weaver J, et al. Cost-Effectiveness analysis of routine use of 15-Valent pneumococcal conjugate vaccine in the US pediatric population. Vaccines (Basel). 2023;11(1):135. doi: 10.3390/vaccines11010135.
- Edmond K, Clark A, Korczak VS, et al. Global and regional risk of disabling sequelae from bacterial meningitis: a systematic review and meta-analysis. Lancet Infect Dis. 2010;10(5):317–328. doi: 10.1016/S1473-3099(10)70048-7.
- Center for Outcomes Research and Economic Evaluation for Health. National institute of public health (C2H). Guideline for preparing cost-effectiveness evaluation to the central social insurance medical council: Core2 health; 2022 [cited 2023 Mar 7]. Available from: https://c2h.niph.go.jp/tools/guideline/guideline_en.pdf.
- Ministry of Health, Labour and Welfare. The patient survey. [cited 2023 Jan 19]. Available from: https://www.e-stat.go.jp/stat-search/files?page=1&toukei=00450022&metadata=1&data=1. Japanese.
- Ministry of Health, Labour and Welfare. Overview of the 23rd life table (complete life table). 2023 [cited 2023 Mar 7]. Available from: https://www.mhlw.go.jp/toukei/saikin/hw/life/23th/index.html. Japanese.
- National Institute of Infectious Disease. Notification Status of Invasive Pneumococcal Infection, 1st week 2014 ∼ 35th week. 2021 [cited 2023 Jan 19]. Available from: https://www.niid.go.jp/niid/ja/pneumococcal-m/pneumococcal-idwrs/10779-ipd-211126.html.
- National Institute of Infectious Disease. IASR 2023. 2023 [cited 2023 Jan 19]. Available from: https://www.niid.go.jp/niid/ja/pneumococcal-m/1372-idsc/iasr-topic/11763-515t.html.
- Chang B, Tamura K, Fujikura H, et al. Pneumococcal meningitis in adults in 2014-2018 after introduction of pediatric 13-valent pneumococcal conjugate vaccine in Japan. Sci Rep. 2022;12(1):3066. doi: 10.1038/s41598-022-06950-w.
- Fukusumi M, Chang B, Tanabe Y, et al. Invasive pneumococcal disease among adults in Japan, april 2013 to march 2015: disease characteristics and serotype distribution. BMC Infect Dis. 2017;17(1):2. doi: 10.1186/s12879-016-2113-y.
- Morimoto K, Suzuki M, Ishifuji T, et al. The burden and etiology of community-onset pneumonia in the aging japanese population: a multicenter prospective study. PLoS One. 2015;10(3):e0122247. doi: 10.1371/journal.pone.0122247.
- Okada T, Morozumi M, Sakata H, et al. A practical approach estimating etiologic agents using real-time PCR in pediatric inpatients with community-acquired pneumonia. J Infect Chemother. 2012;18(6):832–840. doi: 10.1007/s10156-012-0422-7.
- Otsuka T, Kitami O, Kondo K, et al. Incidence survey of acute otitis media in children in sado island, Japan–sado otitis media study (SADOMS). PLoS One. 2013;8(7):e68711. doi: 10.1371/journal.pone.0068711.
- Yamanaka N, Hotomi M, Sugita R. Disease-burden of acute otitis media on children and cost-effectiveness of pneumococcal conjugate vaccine in Japan. Jpn J Pediat. 2009;21(1):37–48.
- Suzuki K, Kurono Y, Ikeda K, et al. The seventh nationwide surveillance of six otorhinolaryngological infectious diseases and the antimicrobial susceptibility patterns of the isolated pathogens in Japan. J Infect Chemother. 2020;26(9):890–899. doi: 10.1016/j.jiac.2020.05.020.
- Nakano S, Fujisawa T, Ito Y, et al. Nationwide surveillance of paediatric invasive and non-invasive pneumococcal disease in Japan after the introduction of the 13-valent conjugated vaccine, 2015-2017. Vaccine. 2020;38(7):1818–1824. doi: 10.1016/j.vaccine.2019.12.022.
- Tashiro M, Fushimi K, Takazono T, et al. A mortality prediction rule for non-elderly patients with community-acquired pneumonia. BMC Pulm Med. 2016;16:39. doi: 10.1186/s12890-016-0199-z.
- Ministry of Health, Labour and Welfare. Number of routine vaccinations. 2023 [cited 2023 Mar 7]. Available from: https://www.mhlw.go.jp/topics/bcg/other/5.html. Japanese.
- Shiragami M, Mizukami A, Leeuwenkamp O, et al. Cost-Effectiveness evaluation of the 10-Valent pneumococcal non-typeable Haemophilus influenzae protein D conjugate vaccine and 13-Valent pneumococcal vaccine in japanese children. Infect Dis Ther. 2014;4(1):93–112. doi: 10.1007/s40121-014-0053-7.
- Whitney CG, Pilishvili T, Farley MM, et al. Effectiveness of seven-valent pneumococcal conjugate vaccine against invasive pneumococcal disease: a matched case-control study. Lancet. 2006;368(9546):1495–1502. doi: 10.1016/S0140-6736(06)69637-2.
- Moore MR, Link-Gelles R, Schaffner W, et al. Effectiveness of 13-valent pneumococcal conjugate vaccine for prevention of invasive pneumococcal disease in children in the USA: a matched case-control study. Lancet Respir Med. 2016;4(5):399–406. doi: 10.1016/S2213-2600(16)00052-7.
- Eskola J, Kilpi T, Palmu A, et al. Efficacy of a pneumococcal conjugate vaccine against acute otitis media. N Engl J Med. 2001;344(6):403–409. doi: 10.1056/NEJM200102083440602.
- Pichichero M, Kaur R, Scott DA, et al. Effectiveness of 13-valent pneumococcal conjugate vaccination for protection against acute otitis media caused by Streptococcus pneumoniae in healthy young children: a prospective observational study. Lancet Child Adolesc Health. 2018;2(8):561–568. doi: 10.1016/S2352-4642(18)30168-8.
- Lewnard JA, Givon-Lavi N, Dagan R. Effectiveness of pneumococcal conjugate vaccines against community-acquired alveolar pneumonia attributable to vaccine-serotype Streptococcus pneumoniae among children. Clin Infect Dis. 2021;73(7):e1423–e1433. doi: 10.1093/cid/ciaa1860.
- Stoecker C, Hampton LM, Link-Gelles R, et al. Cost-effectiveness of using 2 vs 3 primary doses of 13-valent pneumococcal conjugate vaccine. Pediatrics. 2013;132(2):e324-32–e332. doi: 10.1542/peds.2012-3350.
- Treskova M, Scholz SM, Kuhlmann A. Cost effectiveness of elderly pneumococcal vaccination in presence of Higher-Valent pneumococcal conjugate childhood vaccination: systematic literature review with focus on methods and assumptions. Pharmacoeconomics. 2019;37(9):1093–1127. doi: 10.1007/s40273-019-00805-5.
- Shiroiwa T, Noto S, Fukuda T. Japanese population norms of EQ-5D-5L and health utilities index mark 3: disutility catalog by disease and symptom in community settings. Value Health. 2021;24(8):1193–1202. doi: 10.1016/j.jval.2021.03.010.
- Rubin JL, McGarry LJ, Strutton DR, et al. Public health and economic impact of the 13-valent pneumococcal conjugate vaccine (PCV13) in the United States. Vaccine. 2010;28(48):7634–7643. doi: 10.1016/j.vaccine.2010.09.049.
- Mangen M-JJ, Rozenbaum MH, Huijts SM, et al. Cost-effectiveness of adult pneumococcal conjugate vaccination in The Netherlands. Eur Respir J. 2015;46(5):1407–1416. doi: 10.1183/13993003.00325-2015.
- Glick HA, Miyazaki T, Hirano K, et al. One-Year quality of life Post-Pneumonia diagnosis in japanese adults. Clin Infect Dis. 2021;73(2):283–290. doi: 10.1093/cid/ciaa595.
- Deloitte. Summary of medical reimbursement revisions, 2022. 2023 [cited 2023 Jan 19]. Available from: https://www2.deloitte.com/jp/ja/pages/life-sciences-and-healthcare/articles/hc/hc-hoshu-kaitei.html. Japanese.
- Ministry of Health, Labour and Welfare. Pneumococcal 13-valent conjugate vaccine [diphtheria CRM197 protein] suspension for intramuscular injection. 2023 [cited 2023 Mar 3]. Available from: https://www.mhlw.go.jp/stf/shingi/2r985200000371fc-att/2r985200000371td.pdf. Japanese.
- Ministry of Health, Labour and Welfare. Guidelines for evaluating the cost-effectiveness of vaccination. 2023 [cited 2023 Mar 3]. Available from: https://www.mhlw.go.jp/stf/shingi/2r98520000014ryv-att/2r98520000014sdi.pdf. Japanese.
- Hoshi SL, Kondo M, Okubo I. Economic evaluation of vaccination programme of 13-valent pneumococcal conjugate vaccine to the birth cohort in Japan. Vaccine. 2013;31(25):2762–2771. doi: 10.1016/j.vaccine.2013.03.052.
- Ministry of Health, Labour and Welfare. DPC NHI score list. 2023 [cited 2023 Jan 19]. Available from: https://www.mhlw.go.jp/stf/seisakunitsuite/bunya/0000198757_00004.html. Japanese.
- Ministry of Health, Labour and Welfare. DPC statistics (In Japanese). 2023 [cited 2023 Jan 19]. Available from: https://www.mhlw.go.jp/stf/shingi2/0000196043_00005.html.
- Jiang Y, Yang X, Taniguchi K, et al. A cost-effectiveness analysis of revaccination and catch-up strategies with the 23-valent pneumococcal polysaccharide vaccine (PPV23) in older adults in Japan. J Med Econ. 2018;21(7):687–697. doi: 10.1080/13696998.2018.1465272.
- Konomura K, Nagai H, Akazawa M. Economic burden of community-acquired pneumonia among elderly patients: a japanese perspective. Pneumonia (Nathan). 2017;9:19. doi: 10.1186/s41479-017-0042-1.
- Ishiwada N, Iwata S, Sakata H, et al. Burden of illness of pneumonia caused by Streptococcus pneumoniae in children. Jpn J Pediat. 2008;61(11):2194–2204. Japanese.
- Iwata S, Ishiwada N, Sakata H, et al. Burden of illness of bacterial meningitis and bacteremia caused by Streptococcus pneumoniae in children. Jpn J Pediat. 2008;61(11):2206–2220. Japanese.
- Ministry of Health. Labour and welfare. Outline of basic survey on wage structure, 2020. 2021 [cited 2023 Mar 7]. Available from: https://www.mhlw.go.jp/english/database/db-l/schedule_releases.html. Japanese.
- Prasad N, Stoecker C, Xing W, et al. Public health impact and cost-effectiveness of 15-valent pneumococcal conjugate vaccine use among the pediatric population of the United States. Vaccine. 2023;41(18):2914–2921. doi: 10.1016/j.vaccine.2023.03.045.
- Hoshi SL, Kondo M, Okubo I. Economic evaluation of vaccination programme of 7-valent pneumococcal conjugate vaccine to the birth cohort in Japan. Vaccine. 2012;30(22):3320–3328. doi: 10.1016/j.vaccine.2012.02.033.
- Rozenbaum MH, Sanders EA, van Hoek AJ, et al. Cost effectiveness of pneumococcal vaccination among dutch infants: economic analysis of the seven valent pneumococcal conjugated vaccine and forecast for the 10 valent and 13 valent vaccines. BMJ. 2010;340:c2509. doi: 10.1136/bmj.c2509.
- Ray GT, Whitney CG, Fireman BH, et al. Cost-effectiveness of pneumococcal conjugate vaccine: evidence from the first 5 years of use in the United States incorporating herd effects. Pediatr Infect Dis J. 2006;25(6):494–501. doi: 10.1097/01.inf.0000222403.42974.8b.
- McIntosh ED, Conway P, Willingham J, et al. Pneumococcal pneumonia in the UK–how herd immunity affects the cost-effectiveness of 7-valent pneumococcal conjugate vaccine (PCV). Vaccine. 2005;23(14):1739–1745. doi: 10.1016/j.vaccine.2004.08.051.
- Capra AM, Lieu TA, Black SB, et al. Costs of otitis media in a managed care population. Pediatr Infect Dis J. 2000;19(4):354–355. doi: 10.1097/00006454-200004000-00019.
- Cho BH, Stoecker C, Link-Gelles R, et al. Cost-effectiveness of administering 13-valent pneumococcal conjugate vaccine in addition to 23-valent pneumococcal polysaccharide vaccine to adults with immunocompromising conditions. Vaccine. 2013;31(50):6011–6021. doi: 10.1016/j.vaccine.2013.10.024.
- Kobayashi M, Stoecker C, Xing W, et al. Cost-effectiveness of implementing 13-valent pneumococcal conjugate vaccine for U.S. adults aged 19 years and older with underlying conditions. Hum Vaccin Immunother. 2021;17(7):2232–2240. doi: 10.1080/21645515.2020.1861876.
- Lieu TA, Ray GT, Black SB, et al. Projected cost-effectiveness of pneumococcal conjugate vaccination of healthy infants and young children. Jama. 2000;283(11):1460–1468. doi: 10.1001/jama.283.11.1460.
- Stoecker C, Kobayashi M, Matanock A, et al. Cost-effectiveness of continuing pneumococcal conjugate vaccination at age 65 in the context of indirect effects from the childhood immunization program. Vaccine. 2020;38(7):1770–1777. doi: 10.1016/j.vaccine.2019.12.029.