Abstract
Background
Apixaban and rivaroxaban are two direct-acting oral anticoagulants (DOACs) recommended for thromboprophylaxis in cancer patients treated with chemotherapy in an ambulatory setting. We aimed to assess the cost-utility of thromboprophylaxis with apixaban and rivaroxaban vs no thromboprophylaxis in ambulatory cancer patients starting chemotherapy with an intermediate-to-high risk of venous thromboembolism (VTE), Khorana score ≥ 2 points.
Methods
A cost-effectiveness analysis was performed from the perspective of Spain’s National Health System (NHS) using an analytical decision model in the short-term (180 days) and a Markov model in the long-term (5 years). Transition probabilities were obtained from randomized, double-blind, placebo-controlled clinical trials of apixaban and rivaroxaban in adult ambulatory patients with cancer at risk for VTE, treated with chemotherapy (AVERT and CASSINI trials). The costs (€2,021) were taken from Spanish sources. The utilities of the model were obtained through the EQ-5D questionnaire. Deterministic (base case) and probabilistic (second-order Monte Carlo simulation) analyses were conducted.
Results
In the probabilistic sensitivity analysis, apixaban generated a cost per patient of €1,082 ± 187, with a 95% confidence interval (CI) of €713–1,442, while no prophylaxis produced a cost per patient of €1,146 ± 218, with a 95% CI of €700–1,491, with a saving of €64 per patient and a gain of 0.008 QALYs. Likewise, rivaroxaban provided a cost per patient of €993 ± 133, with a 95% CI of €748–1,310, while no prophylaxis produced a cost per patient of €872 ± 152, with a 95% CI of €602–1,250, with an additional expense of €121 per patient and a gain of 0.008 QALYs.
Conclusions
In thromboprophylaxis of cancer patients, the use of apixaban and rivaroxaban generated similar costs compared to non-prophylaxis, without the difference found being statistically significant, with a clinically insignificant QALY gain.
Introduction
Venous thromboembolism (VTE) is a frequent complication in cancer patients and has been progressively increasing in the last two decadesCitation1. Cancer-associated thrombosis (CAT) has been associated with a higher mortality, morbidity, healthcare costs, and, more recently, with a significantly negative impact on the quality-of-life of cancer patientsCitation2.
CAT rates vary widely among cancer patientsCitation3. The estimated CAT rate for average-risk patients is 13 per 1,000 person-years, while patients with metastatic disease or receiving thrombogenic therapies have a VTE rate of 68 per 1,000 person-years, with the highest risk reported in lung, pancreatic, and brain cancers (200 per 1,000 person-years). Currently, most venous thromboembolic events occur in the outpatient settingCitation4. Different randomized clinical trialsCitation5,Citation6 and meta-analysesCitation7 have shown that ambulatory thromboprophylaxis is effective with an acceptable bleeding risk profile in this population.
Apixaban (APIX) and rivaroxaban (RIV) are the only direct oral anticoagulants (DOAC) recommended by the American Society of Clinical Oncology (ASCO)Citation8 and the International Initiative on Thrombosis and Cancer (ITAC)Citation9 in ambulatory cancer patients receiving systemic chemotherapy. These recommendations are based on two phase III randomized clinical trials: CASSINICitation10 and AVERTCitation11 trials, that included a wide spectrum of prothrombotic cancers (gastrointestinal, genitourinary, lung, brain tumors and hematological neoplasms such as lymphoma and myeloma) in adult patients. Both studies have a similar design, they compared up to 6 months thromboprophylaxis with RIV (10 mg daily) or APIX (2.5 mg twice daily) to placebo in ambulatory cancer patients initiating systemic anti-cancer therapy with a Khorana score of 2 points or higher. Patient populations share similar clinical features (Supplementary Table S1 and S2). In the AVERT trial, apixaban was associated with a significantly lower incidence of VTE compared to placebo in the primary modified intention-to-treat analysis (mITT), 4.2% versus 10.2%, respectively (hazard ratio = 0.41; 95% confidence interval [CI] = 0.26–0.65; p < 0.001). The rate of major bleeding was significantly higher with apixaban than with placebo in the mITT (3.5% and 1.8%, respectively; HR = 2.00; 95% CI = 1.01–3.95), but not during the treatment period. In the CASSINI trial, VTE incidence was lower with rivaroxaban compared to placebo in the per-protocol analysis, 2.6% versus 6.4% (HR = 0.40; 95% CI = 0.20–0.80), but not in the primary intention-to-treat analysis. Major bleeding occurred in 2% of patients receiving rivaroxaban and 1% receiving placebo (HR = 1.96; 95% CI = 0.59–6.49). This indication is not yet authorized in Spain, but, on the contrary, it is recommended by the Spanish Society of Medical Oncology (SEOM)Citation12. Despite the availability of several validated risk assessment models for VTE in cancer patients and guideline recommendations, rates of VTE prophylaxis in high-risk patients are low in routine clinical practice. A recent survey by the Association of Community Cancer Centers reported that only 9% of oncologists use a risk assessment tool to identify high-risk patients in the outpatient setting and more than 90% of patients with very high-risk do not receive pharmacological thromboprophylaxisCitation13.
Two recently published economic analysesCitation14,Citation15 assessed cost-effectiveness of thromboprophylaxis with DOAC in cancer patients treated with chemotherapy in ambulatory patients with an intermediate to high risk of suffering VTE (Khorana score ≥ 2). In these studies thromboprophylaxis refers to the pharmacological thromboprophylaxis for patients with cancer with high risk of VTE based on Khorana risk score. However, neither of the two studies performed a single drug-based analysis and no formal drug–drug comparison was provided (Supplementary Table S3).
The main hypothesis of this study is that APIX and RIV may have a different cost-effectiveness profile. The aim of this analysis is to know if the use of APIX or RIV is a cost-effective alternative in thromboprophylaxis for ambulatory cancer patients with intermediate-high risk of VTE (Khorana score ≥ 2) in Spain, based on the different results obtained in efficacy and safety with these drugs in placebo-controlled randomized clinical trialsCitation9,Citation10 and on different pharmacokinetic and pharmacodynamic profilesCitation16.
Methods
This cost-effectiveness analysis was performed from the perspective of the Spanish National Health Service, so only healthcare costs (direct medical costs) were included. A discount rate of 3% per year was applied to costs and outcomes. The primary outputs of the model included costs, quality-adjusted life years (QALYs), and incremental cost-effectiveness ratio (ICER). The model was constructed via TreeAge Pro 2018 (TreeAge Software, Inc, Willianston, MA).
Model structure and analysis type
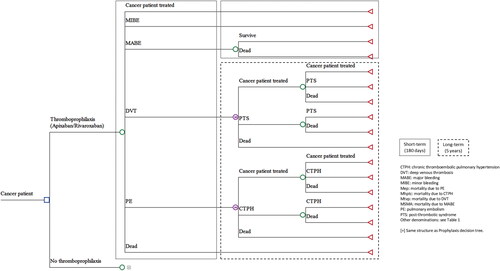
The structure of the model was based on previously published modelsCitation17–20. shows the structure of the economic model. It consists of a short-term (with a 180-day time horizon) decision analysis (DA) and subsequent long-term Markov model (with a 5-year time horizon) and a cycle length of 1 year. The short-term DA includes the following health states: (i) actively treated cancer; (ii) minor bleeding events (MIBE); (iii) major bleeding events (MABE); (iv) deep venous thrombosis (DVT); (v) pulmonary embolism (PE); or (vi) death from any cause. Patients with DVT were expected to evolve in the long-term (Markov model) to the following states: (i) actively treated cancer; (ii) post-thrombotic syndrome (PTS); or (iii) death from any cause. Similarly, the patient with PE was assumed to evolve (Markov) to the following states: (i) actively treated cancer; (ii) chronic thromboembolic pulmonary hypertension (CTPH); or (iii) death from any cause (). Deterministic and probabilistic analysis (through 1.000 second-order Monte Carlo simulations) were performed. Probabilistic analyses allow the assessment of the uncertainty of the variablesCitation21. One-way (tornado) deterministic sensitivity analyses were also performed, modifying probabilities, costs, and utilities. A willingness-to-pay in Spain of EUR 25,000 per QALY gained was considered.
Probabilities of the model
shows the short- and long-term probabilities considered in the model. The short-term probabilities were mainly obtained from the economic studies of Du et al.Citation14 and Li et al.Citation15, who, in turn, had taken them from phase III, randomized, placebo-controlled, double-blind clinical trials of APIX (AVERT study)Citation10, and RIV (CASSINI study)Citation11 in cancer, as well as from another previously published analysisCitation22. The design and results of the AVERT and CASSINI trials, as well as the baseline demographic and clinical characteristics of the patients, are detailed in Supplementary Tables S1 and S2. The probabilities considered in the long-term Markov model (with 1 year cycles) were obtained from clinical and economic studies published earlierCitation14,Citation17,Citation21–25. Transition probabilities of the model were calculated according to the formula Pt = 1 ‒e‒rt, r being the event rate and t the time in which such rate takes placeCitation26.
Table 1. Probabilities of the economic model in the short- and long-term.
Costs of the model
The costs of the model are presented in . For the calculation of the mean cost of prophylaxis with DOAC, the mean duration of prophylaxis (in days) was calculated using the medians from the AVERT studyCitation10 according to the formula from Pudar et al.Citation27. Thus, a mean duration of prophylaxis of 140 days was considered, between a minimum of 70 and a maximum of 150 days.
Table 2. Economic model costs (2020 €).
The cost of the events (DVP, PE, MABE, MIBE, PTS, and CTPH) in Spain were obtained from the public prices of health services of the Basque Health Service for the year 2020Citation28. The costs of the following diagnostic-related groups (DRG): were considered: DVP (197); PE (134); MABE (44); MIBE (253); PTS (197), and CTPH (207).
Utilities of the model
The utilities considered in the model were the ones used in the recent economic analysis of Du et al.Citation13 (). These utilities derive from a study conducted in the United Kingdom using the EQ-5D instrumentCitation29, excluding the CTPH utility, that was obtained from a study performed in the USACitation30.
Table 3. Economic model utilities.
Clinical validation of the model
The economic model was clinically validated by two Spanish clinical experts with experience in thromboprophylaxis in cancer patients (Dr. Enrique Gallardo, Oncology Department, Hospital Universitario Parc Taulí, Sabadell, Spain; Dr. Andrés J. Muñoz, Medical Oncology Department, Hospital General Universitario Gregorio Marañón, Madrid, Spain).
Sensitivity analysis
A univariable and probabilistic sensitivity analyses were performed to assess the robustness of the model and the uncertainty in parameter estimation. In this study, a probabilistic deterministic sensitivity analysis (PDSA) expressed in incremental net benefit was performed instead of a normal univariant sensitivity analysis, because PDSA offers advantages versus a classic deterministic sensitivity analysis for providing insight on the effect of uncertainty in individual parameters on the estimate cost-effectivenessCitation31.
Results
Base-case analysis
APIX vs no prophylaxis
APIX would be dominant (greater effectiveness with lower costs) compared to no prophylaxis, with a gain of 0.005 quality adjusted life years (QALY) and a cost difference of –€59.49 (). It is estimated that for every 1,000 patients treated with APIX instead of RIV, seven episodes of DVT and 11 episodes of PE would be prevented (Supplementary Table S4).
Table 4. Base-case analysis.
RIV vs no prophylaxis
RIV would not be dominant compared with no prophylaxis. However, it would be cost-effective. The cost of gaining a QALY with RIV would be €18,746.77 (below a willingness to pay of €25,000–60,000 per QALY gained)Citation32. If one considered that the QALY gain was not clinically relevant, with RIV, an additional cost of €116.23 per patient would be generated versus no thromboprophylaxis ().
Probabilistic sensitivity analyses
APIX vs no prophylaxis
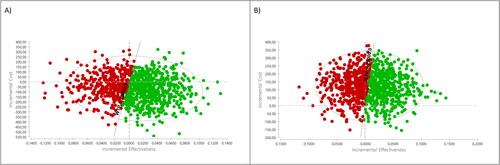
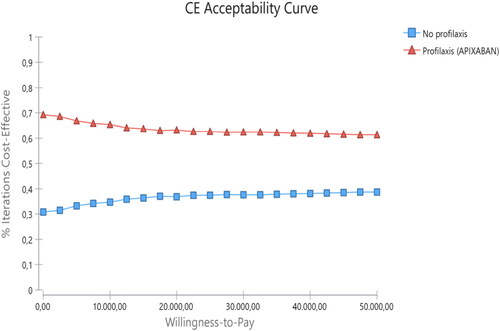
As shown in and , the probabilistic analysis revealed savings of around €64 per patient treated with APIX and a gain of 0.008 QALY per patient treated with APIX compared to no prophylaxis. The probability of APIX being cost-effective, for a willingness-to-pay of €25,000 per QALY gained, was 62.2%. shows the cost-effectiveness acceptability curve (CEAC) of APIX vs no prophylaxis.
Table 5. Probabilistic analysis results.
RIV vs no prophylaxis
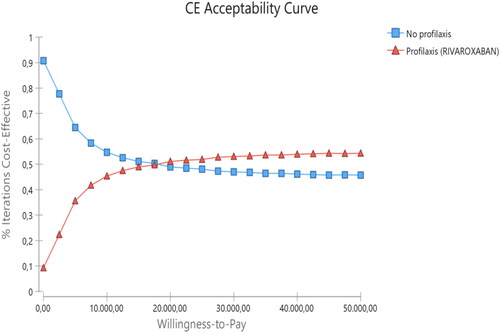
As noted in and , the probabilistic analysis showed an additional cost per patient treated with RIV of around €121 and a gain of 0.008 QALY per patient treated with RIV in comparison with no prophylaxis. The probability of RIV being cost-effective, for a willingness to pay of €25,000 per QALY gained, was 51.9%. shows the CEAC of RIV vs no prophylaxis.
Probabilistic deterministic sensitivity analyses
Incremental net monetary benefit (INMB) was positive (cost-effective) for a willingness to pay of €25,000 per QALY gained for all variables in the case of RIV, with two exceptions: the probability of cancer mortality with or without prophylaxis, in which case the risk of a negative IBNM (not cost-effective) would be 60.9% and 36.2%, respectively. In the case of APIX, the risk of a negative IBNM for the probability of cancer mortality with or without prophylaxis would be 64.0% and 30.8%, respectively. One-way deterministic sensitivity analyses are described in detailed for APIX and RIV in .
Table 6. One-way deterministic sensitivity analyses.
Discussion
In the last three decades the overall incidence of VTE has increased by 3-fold. It’s estimated the risk of VTE in cancer patients is 12-fold higher than in the overall population, and in patients receiving systemic anti-cancer therapy reaches 23-foldCitation1. VTE in cancer patients provokes a significant increase in VTE recurrence and major bleeding during anticoagulant therapy compared to non-cancer patientsCitation33. Furthermore, VTE is associated with temporary or definitive interruption of anticancer therapy with a potential impact on cancer prognosis. These data suggest that prevention of VTE in patients with cancer who receive systemic therapy in an ambulatory setting has a potentially high clinical value. Despite all these facts and available evidence from placebo-controlled randomized clinical trials showing outpatient thromboprophylaxis in cancer patients is effective and safe, it is still seldom used in clinical practice, and the cost-effectiveness relationship has not been well established yet.
In the present economic analysis thromboprophylaxis in cancer patients treated with systemic therapy would be more efficient with APIX than with RIV. APIX is dominant (greater effectiveness with lower costs) compared to no prophylaxis. Nevertheless, the gain of 0.005 QALY per patient might not be clinically relevant, since clinical relevance in utility gain (the smallest difference in quality-of-life that a patient can perceive) is generally considered to be between 0.03 and 0.04 QALY gainedCitation34,Citation35. If this premise is accepted, one would save up to €59.49 per patient with APIX compared to no thromboprophylaxis. This fact shows that in addition to the clinical value derived from the use of APIX and RIV as thromboprophylaxis, the use of these alternatives will generate resource savings for the National Health System in the case of APIX and will be a cost-effective option in the case of RIV. It should be taken into account that there are no studies with this objective from the perspective of the Spanish National Health System.
Overall, data coming from cost-effectiveness analysis of ambulatory thromboprophylaxis of cancer patients (including DOAC, low-molecular-weight heparins, or vitamin K antagonists) from western European countries are scarce. These results are quite reassuring in order to expand outpatient thromboprophylaxis with low dose DOAC. In addition, it is expected to achieve a better cost-effectiveness result if other risk assessment models of VTE recently published, that have shown to significantly improve the capability of VTE risk prediction compared to Khorana score, are implemented in clinical practiceCitation36,Citation37. Another significant finding of this analysis, and to our knowledge not described before in this setting, is the different cost-effectiveness profile of APIX and RIV. These results are in line with other clinical findings in cancer patients, mainly in safety, with a better bleeding risk profile for APIX compared to RIV (gastrointestinal tract bleeding and gastrointestinal cancers)Citation38,Citation39, that suggest these drugs are not directly interchangeable. Lastly, these results have a direct local applicability from Spanish National Health System perspective and are in line with the recommendation of the Spanish National Guideline of Thrombosis and Cancer (Spanish Society of Medical Oncology, SEOM)Citation12.
This result should be assessed considering the strengths and weaknesses of the study. One of the main strengths would be the clinical validation of the model by two expert physicians with experience in thromboprophylaxis in cancer patients. Secondly, a second-order Monte Carlo simulation allowed us to analyze the uncertainty of the data (probabilities, costs, utilities) included in the analysis. This mathematical method allows the replication of simultaneous and random changes in the model parameters (probabilities, costs, utilities) in an attempt to simulate clinical evolution of patients in real lifeCitation40,Citation41. The confidence of the results was analyzed by the confidence intervals of 95%, with probabilities of cost-effectiveness of 62.2% and 51.9% in patients treated with APIX or RIV compared to no prophylaxis, respectively. In both cases, the probability of cost-effectiveness versus no thromboprophylaxis would be low (under 70%) for the indicated willingness to pay.
As far as the limitations of the study are concerned, first of all, it has to be taken into account that the theoretical model developed is, by definition, a simplified simulation of reality. The fact that there was no direct comparison between thromboprophylaxis with APIX and RIV can be considered a study limitation. Nonetheless, this was not possible for several reasons. Firstly, there are no clinical trials that compare these two drugs directly. The available clinical trials concerning DVT prophylaxis in cancer patients compared APIX (AVERT study)Citation10 and RIV (CASSINI study)Citation11 with a placebo control (no prophylaxis). Secondly, the results obtained in the placebo group of patients differed considerably between both studies, in particular, the probability to suffer symptomatic VTE, minor bleedings and VTE-related mortality, although this point did not invalidate the results of this study. However, the patients from both studies (AVERT and CASSINI) had similar baseline characteristics regarding age, gender, Khorana score, and previous thromboembolism rateCitation8,Citation11. The fact that only two clinical trials are available should be considered a major limitation of the study. Despite there not being any indications or signals that there would be subgroups of patients in which RIV was more cost-effective, we can’t exclude that in certain subgroups RIV may be a better option. This type of subgroup analysis of cancer patients would require dedicated studies. Finally, quality-of-life studies in this setting are needed and should be performed to complement cost-effectiveness analysis.
Conclusions
From the present study the following conclusions can be drawn in ambulatory thromboprophylaxis of cancer patients receiving systemic anti-cancer therapy: (i) APIX is dominant (generating savings) compared to no prophylaxis; (ii) RIV is cost-effective (generating an additional cost) compared to no prophylaxis; (iii) QALY gains with both drugs are small and would probably not be clinically relevant (less than 0.03 QALY); (iv) the probability of prophylaxis with APIX being cost-effective (for a willingness to pay of 25,000€/QALY) is 62.6% and 51.9% with RIV (10.7% higher with APIX). However, the probability that both treatments are cost-effective vs. no prophylaxis was low (<70%).
Transparency
Declaration of funding
No funding was received to produce this article.
Author contributions
Conception and design: AJM, JMS
Data analysis: DRR, CRT, EG, AJM.
Interpretation of data: AJM, EG, DRR, CRT, JMS.
Paper drafting: AG, DRR, CRT, AJM, JMS
Revising it critically for intellectual content: AG, BM, PGA
Final approval: all authors.
No assistance in the preparation of this article is to be declared.
Supplemental Material
Download MS Word (23.5 KB)Supplemental Material
Download MS Word (27.1 KB)Acknowledgements
None stated.
Declaration of financial/other relationships
AM:
Consultant or advisory role:
Pfizer-BMS alliance, Sanofi, Celgene, Leo Pharma, Incyte, Astra-Zeneca, MSD, Lilly, Servier, Roche.
Research funding:
Leo Pharma, Sanofi, Rovi, Celgene.
Speakers’ bureau:
Rovi, Bayer, Menarini, Stada, Daichii Sankyo.
Patents, Royalties, Other Intellectual Property:
Risk assessment model in venous thromboembolism in cancer patients.
LO:
Speakers’ bureau:
Sanofi, Leo Pharma.
AG:
No conflict of interests.
EG:
Consultant or advisory role:
Sanofi, Janssen, Astellas, Bayer, Ipsen, Pfizer, Roche, Novartis, Eisai, EUSA Pharma, BMS, AstraZeneca, Merck, Rovi, Daiichi Sankyo, Techdow.
Speakers’ bureau:
Astellas, Janssen, Sanofi, Bayer, Ipsen, Pfizer, Roche, BMS, Rovi, Daiichi Sankyo, Leo Pharma, Menarini, Eisai, MSD, Boehringer Ingelheim, Merck, EUSA Pharma, Novartis.
Grant support (personal/institutional):
Astellas, Janssen, Sanofi, Bayer, Ipsen, Ferrer, Pfizer, Roche, GSK, BMS, Novartis, Eisai, Pierre Fabre.
DRR:
Consultant for pharmaceutical companies Pfizer-BMS Alliance.
CRT:
Consultant for pharmaceutical companies Pfizer-BMS Alliance.
BM:
No conflict of interests.
PGA:
Consultant or advisory role:
Roche, Lilly, Sanofi, Merck, Amgen, Servier, Piere Fabre.
Speakers’ bureau:
Roche, Lilly, Sanofi, Merck, Amgen, Servier, Piere Fabre.
JMS:
No conflict of interests.
Reviewer disclosures
Peer reviewers on this manuscript have no relevant financial or other relationships to disclose.
References
- Mulder FI, Horváth-Puhó E, van Es N, et al. Venous thromboembolism in cancer patients: a population-based cohort study. Blood. 2021;137(14):1959–1969. doi: 10.1182/blood.2020007338.
- Marin-Barrera L, Muñoz-Martin AJ, Rios-Herranz E, et al. A case-control analysis of the impact of venous thromboembolic disease on quality of life of patients with cancer: quality of life in cancer (qca) study. Cancers (Basel). 2019;12(1):75. doi: 10.3390/cancers12010075.
- Horsted F, West J, Grainge MJ. Risk of venous thromboembolism in patients with cancer: a systematic review and meta-analysis. PLoS Med. 2012;9(7):e1001275. doi: 10.1371/journal.pmed.1001275.
- Khorana AA. Cancer-associated thrombosis: updates and controversies. Hematol Am Soc Hematol Educ Program. 2012;2012(1):626–630. doi: 10.1182/asheducation.V2012.1.626.3798655.
- Agnelli G, Gussoni G, Bianchini C, et al. Nadroparin for the prevention of thromboembolic events in ambulatory patients with metastatic or locally advanced solid cancer receiving chemotherapy: a randomised, placebo-controlled, double-blind study. Lancet Oncol. 2009;10(10):943–949. doi: 10.1016/S1470-2045(09)70232-3.
- Agnelli G, George DJ, Kakkar AK, et al. Semuloparin for thromboprophylaxis in patients receiving chemotherapy for cancer. N Engl J Med. 2012;366(7):601–609. doi: 10.1056/NEJMoa1108898.
- Rutjes AW, Porreca E, Candeloro M, et al. M. Primary prophylaxis for venous thromboembolism in ambulatory cancer patients receiving chemotherapy. Cochrane Database Syst Rev. 2020;12(12):CD008500.
- Key NS, Khorana AA, Kuderer NM, et al. Venous thromboembolism prophylaxis and treatment in patients with cancer: ASCO clinical practice guideline update. J Clin Oncol. 2020;38(5):496–520. doi: 10.1200/JCO.19.01461.
- Farge D, Frere C, Connors JM, et al. 2022 International clinical practice guidelines for the treatment and prophylaxis of venous thromboembolism in patients with cancer, including patients with COVID-19. Lancet Oncol. 2022;23(7):e334–e347. doi: 10.1016/S1470-2045(22)00160-7.
- Carrier M, Abou-Nassar K, Mallick R, AVERT Investigators, et al. Apixaban to prevent venous thromboembolism in patients with cancer. N Engl J Med. 2019;380(8):711–719. doi: 10.1056/NEJMoa1814468.
- Khorana AA, Soff GA, Kakkar AK, et al. Rivaroxaban for thromboprophylaxis in high-risk ambulatory patients with cancer. N Engl J Med. 2019;380(8):720–728. doi: 10.1056/NEJMoa1814630.
- Muñoz Martín AJ, Gallardo Díaz E, García Escobar I, et al. SEOM clinical guideline of venous thromboembolism (VTE) and cancer (2019). Clin Transl Oncol. 2020;22(2):171–186. doi: 10.1007/s12094-019-02263-z.
- Holmes CE, Ades S, Gilchrist S, et al. Successful model for guideline implementation to prevent cancer-associated thrombosis: venous thromboembolism prevention in the ambulatory cancer clinic. J Clin Oncol Oncol Pract. 2020;16(9):e868–e874. pdoi: 10.1200/JOP.19.00697.
- Du J, Wu B. New oral anticoagulants for thromboprophylaxis in patients with cancer receiving chemotherapy: an economic evaluation in a Chinese setting. Clin Drug Investig. 2020;40(7):653–663. doi: 10.1007/s40261-020-00926-2.
- Li A, Carlson JJ, Kuderer NM, et al. Cost-effectiveness analysis of low-dose direct oral anticoagulant (DOAC) for the prevention of cancer-associated thrombosis in the United States. Cancer. 2020;126(8):1736–1748. doi: 10.1002/cncr.32724.
- Frost C, Song Y, Barrett YC, et al. A randomized direct comparison of the pharmacokinetics and pharmacodynamics of apixaban and rivaroxaban. Clin Pharmacol. 2014;6:179–187. doi: 10.2147/CPAA.S61131.
- Gómez-Cerezo JF, Gómez-Arrayás I, Suárez-Fernández C, et al. Análisis coste-efectividad de apixaban frente a dabigatrán en la prevención de la tromboembolia venosa en pacientes intervenidos de artroplastia total de rodilla o de cadera. Rev Esp Cir Ortop Traumatol. 2012;56(6):459–470. doi: 10.1016/j.recot.2012.07.009.
- Kimpton M, Kumar S, Wells PS, et al. Cost-utility analysis of apixaban compared with usual care for primary thromboprophylaxis in ambulatory patients with cancer. CMAJ. 2021;193(40):E1551–E1560. doi: 10.1503/cmaj.210523.
- Ryan E, Salinaro J, Havrilesky LJ, et al. Venous thromboembolism prophylaxis in ambulatory cancer patients initiating chemotherapy: a cost-effectiveness analysis. J Clin Oncol. 2020;38(15_suppl):7074–7074. Available at. doi: 10.1200/JCO.2020.38.15_suppl.7074.
- Glickman A, Brennecke A, Tayebnejad A, et al. Cost-effectiveness of apixaban for prevention of venous thromboembolic events in patients after gynecologic cancer surgery. Gynecol Oncol. 2020;159(2):476–482. doi: 10.1016/j.ygyno.2020.07.096.
- Briggs A, Claxton K, Sculpher M. Decision modelling for health economic evaluation. Oxford (UK): Oxford University Press; 2006.
- Agnelli G. Direct oral anticoagulants for thromboprophylaxis in ambulatory patients with cancer. N Engl J Med. 2019;380(8):781–783. doi: 10.1056/NEJMe1816060.
- Martinez C, Wallenhorst C, Teal S, et al. Incidence and risk factors of chronic thromboembolic pulmonary hypertension following venous thromboembolism, a population-based cohort study in England. Pulm Circ. 2018;8(3):2045894018791358–2045894018791310. doi: 10.1177/2045894018791358.
- Pengo V, Lensing AW, Prins MH, et al. Incidence of chronic thromboembolic pulmonary hypertension after pulmonary embolism. N Engl J Med. 2004;350(22):2257–2264. doi: 10.1056/NEJMoa032274.
- Prandoni P, Villalta S, Bagatella P, et al. The clinical course of deep-vein thrombosis: prospective long-term follow-up of 528 symptomatic patients. Haematologica. 1997;82(4):423–428.
- Petitti DB. Meta-analysis, decision analysis and cost-effectiveness analysis. Methods for quantitative synthesis in medicine. New York: Oxford University Press, 1994.
- Pudar S, Djulbegovic B, Hozo I. Estimating the mean and variance from the median, range, and the size of a sample. BMC Med Res Method. 2005;5:13.
- Tarifas para facturación de servicios sanitarios y docentes del servicio vasco de salud para el año. 2020. Diciembre 2019. Available at https://www.osakidetza.euskadi.eus/contenidos/informacion/osk_servic_para_empresas/es_def/adjuntos/LIBRO-DE-TARIFAS_2020_osakidetza.pdf. (accessed 17 September 2020).
- Lloyd AJ, Dewilde S, Noble S, et al. What impact does venous thromboembolism and bleeding have on cancer patients’ quality of life? Value Health. 2018;21(4):449–455. doi: 10.1016/j.jval.2017.09.015.
- Hu B, Fu AZ. Predicting utility for joint health states: a general framework and a new nonparametric estimator. Med Decis Making. 2010;30(5):E29–E39. doi: 10.1177/0272989X10374508.
- Vreman RA, Geenen JW, Knies S, et al. The application and implications of novel deterministic sensitivity analysis methods. Pharmacoeconomics. 2021;39(1):1–17. doi: 10.1007/s40273-020-00979-3.
- Sacristán JA, Oliva J, Campillo-Artero C, et al. ¿Qué es una intervención sanitaria eficiente en España en 2020? [What is an efficient health intervention in Spain in 2020?]. Gac Sanit. 2020;34(2):189–193. doi: 10.1016/j.gaceta.2019.06.007.
- Prandoni P, Lensing AW, Piccioli A, et al. Recurrent venous thromboembolism and bleeding complications during anticoagulant treatment in patients with cancer and venous thrombosis. Blood. 2002;100(10):3484–3488. doi: 10.1182/blood-2002-01-0108.
- Kaplan RM. The minimally clinically important difference in generic utility-based measures. COPD. 2005;2(1):91–97. doi: 10.1081/copd-200052090.
- Wee HL, Machin D, Loke WC, et al. Assessing differences in utility scores: a comparison of four widely used preference-based instruments. Value Health. 2007;10(4):256–265. doi: 10.1111/j.1524-4733.2007.00174.x.
- Muñoz A, Ay C, Grilz E, et al. A clinical-genetic risk score for predicting cancer-associated venous thromboembolism: a development and validation study involving two independent prospective cohorts. J Clin Oncol. 2023;41(16):2911–2925. doi: 10.1200/JCO.22.00255.
- Li A, La J, May SB, et al. Derivation and validation of a clinical risk assessment model for cancer-associated thrombosis in two unique US health care systems. J Clin Oncol. 2023;41(16):2926–2938. doi: 10.1200/JCO.22.01542.
- Agnelli G, Becattini C, Meyer G, et al. Apixaban for the treatment of venous thromboembolism associated with cancer. N Engl J Med. 2020;382(17):1599–1607. doi: 10.1056/NEJMoa1915103.
- Young AM, Marshall A, Thirlwall J, et al. Comparison of an oral factor Xa inhibitor with low molecular weight heparin in patients with cancer with venous thromboembolism: results of a randomized trial (SELECT-D). J Clin Oncol. 2018;36(20):2017–2023. doi: 10.1200/JCO.2018.78.8034.
- Isla D, De Castro J, Juan O, et al. Costs of adverse events associated with erlotinib or afatinib in first-line treatment of advanced EGFR-positive non-small cell lung cancer. Clinicoecon Outcomes Res. 2017;9:31–38. doi: 10.2147/CEOR.S121093.
- Anguita P, González C, Cañete M, et al. Coste de los efectos adversos asociados a enzalutamida o apalutamida en el tratamiento del cáncer de próstata resistente a la castración no metastásico en España. Rev Esp Econ Salud. 2019;14(4):794–805.