Abstract
Aim
To assess the impact of rifaximin (± lactulose) use following discharge of an initial overt hepatic encephalopathy (OHE) hospitalization on OHE rehospitalizations and healthcare costs in a real-world setting.
Methods
Adults (18–64 years) with an OHE hospitalization were identified from MarketScan® Commercial claims (Q4′15–Q2′20), classified into two mutually exclusive treatment cohorts (i.e. rifaximin and no rifaximin treatment), and further stratified into four subgroups based on decreasing quality of care (QoC; i.e. Type 1 – rifaximin without delay post-discharge; Type 2 – rifaximin with delay post-discharge; Type 3 – lactulose only post-discharge; Type 4 – no rifaximin/lactulose treatment post-discharge). The impact of rifaximin use on 30-day and annualized OHE hospitalizations and healthcare costs were assessed between cohorts and by the QoC subgroup.
Results
Characteristics were similar between the rifaximin (N = 1,452; Type 1: 1,138, Type 2: 314) and no rifaximin (N = 560; Type 3:337, Type 4: 223) treatment cohorts. The 30-day risk of OHE rehospitalization was lower for the rifaximin vs. no rifaximin treatment cohort (odds ratio 0.56, p < .01) and increased with decreasing QoC. The annual rate of OHE hospitalizations was 59% lower for the rifaximin treatment cohort (incidence rate ratio 0.41, p < .01) and increased with decreasing QoC. Compared to the no rifaximin treatment cohort, the rifaximin treatment cohort had higher pharmacy costs, lower medical costs, and no difference in total healthcare costs.
Limitations
This was a claims-based study subject to common data limitations such as billing inaccuracies or omissions in coded claims. Total healthcare costs were reported from a payer’s perspective, which do not capture indirect costs associated with patient burden.
Conclusions
Initiation of rifaximin after an OHE hospitalization was associated with reduced OHE hospitalizations both in the 30-days following and annually. Further, reduced medical costs offset increased pharmacy costs, and no annual cost differences were observed between cohorts.
Introduction
Hepatic encephalopathy (HE) is a serious neurological complication of liver cirrhosis characterized by the reversible loss of normal brain function. Up to 70% of patients with cirrhosis are affected by HE during the course of the diseaseCitation1,Citation2. Overt HE (OHE) is the most severe form of HECitation1. The complex pathogenesis is largely driven by compounds such as inflammatory cytokines and ammonia, some of which can enter the brain via the bloodstream and elicit neurological symptoms if not properly metabolizedCitation1. OHE is associated with a substantial economic burden, with average costs of an OHE hospitalization ranging between $28,063 and $34,810 in the US (2019 USD)Citation3–5. Additionally, patients with OHE often experience hospital readmissionCitation6,Citation7, with several studies reporting HE as the most common cause of readmission among patients hospitalized for complications due to cirrhosisCitation6–9.
Management of OHE is multi-pronged and focuses on addressing underlying contributing factors (e.g. infection, electrolyte disorders, gastrointestinal bleeding) and pharmacologic therapyCitation10. Rifaximin 550 mg twice daily (BID) is the only oral antibiotic treatment currently approved by the US Food and Drug Administration for reducing the risk of OHE recurrence in adultsCitation11. In a clinical trial of patients with recurrent HE, rifaximin treatment resulted in a 58% relative reduction in the risk of a breakthrough HE episode over the 6-month study period when compared with placebo, as well as a 50% reduction in the risk of HE hospitalizationsCitation12. Further, the benefits of rifaximin treatment were in addition to benefits produced by lactulose administration, as over 90% of patients in both treatment arms received lactuloseCitation12. The American Association for the Study of Liver Diseases (AASLD) and European Association for the Study of the Liver (EASL) treatment guidelines recommend the use of rifaximin as an add-on therapy to lactulose in patients who have had at least one episode of OHE while on lactulose to prevent OHE recurrenceCitation10.
Hospital readmissions can be costly to healthcare systems and result in poor patient outcomes, including increased morbidity and mortalityCitation6,Citation7,Citation9,Citation13–15. In recent years, the Center for Medicare and Medicaid Services (CMS) has implemented programs, such as the Hospital Readmissions Reduction Program (HRRP), to reduce avoidable hospital readmissions through improved communication and care coordination between patient caregivers and providers post-dischargeCitation16,Citation17. CMS uses 30-day hospital readmission rates as a benchmark for quality of care (QoC) in these programs and publicly reports statistics on 30-day risk-standardized unplanned readmissionsCitation17,Citation18. In addition to its use in quality improvement programs, 30-day hospital readmission is an informative and standardized measure that can be used in real-world studies to evaluate patient outcomesCitation19–21. Accordingly, OHE treatment guidelines emphasize post-discharge care coordination to prevent OHE rehospitalizationCitation10.
In addition to the proven benefits of rifaximin in a clinical trial settingCitation5, real-world studies have demonstrated that treatment with rifaximin can reduce OHE-related hospitalizations and hospitalization costsCitation5,Citation22,Citation23. However, previous studies have assessed OHE-related rehospitalizations (either with OHE as the only outcome or as a composite outcome [e.g. all-cause rehospitalization]), but primarily among those with an initial hospitalization for decompensated cirrhosis (i.e. not OHE-specific; except Kruger et al.)Citation6–9,Citation24,Citation25. Further, these studies did not assess the impact of clinical guidelines concordant use of rifaximin post-discharge. Therefore, this study aimed to describe and compare OHE hospitalizations, including the risk of 30-day rehospitalization and the annual rate of OHE hospitalizations, as well as post-discharge healthcare costs following an initial OHE hospitalization among commercially insured patients with rifaximin treatment versus those without.
Methods
Data source
Data from October 1, 2015 to March 31, 2020 from the Merative MarketScan® Commercial claims were usedCitation26. The database contains employer- and health plan-sourced medical and pharmacy claims data for employees, their spouses, and dependents who are covered by employer-sponsored private health insurance across all US census regions. The data comprise standard demographic variables (except race), records of inpatient services, outpatient services, and prescription-drug claims; information on inpatient medications was not available. The database also includes an employer-paid portion of payments and any out-of-pocket expenses incurred by patients. Data was de-identified and complies with the requirements of the Health Insurance Portability and Accountability Act; therefore, no review by an institutional review board nor informed consent was required per Title 45 of CFR, Part 46.101(b)(4).
Study design
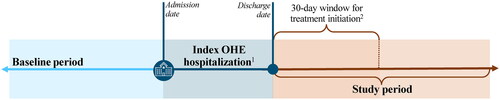
A retrospective cohort study design was used and developed in collaboration with a medical expert (Arun Jesudian [AJ], also a co-author on this study) (). Patients with an OHE hospitalization were defined as those hospitalized with HE as the primary diagnosis and diagnosis-related group 441, 442, or 443 (i.e. liver-related diseases)Citation5 or with a prescription fill for rifaximin 550 mg BID with ≥30 d of supply between the hospital admission and discharge dates. This definition was based on input from the medical expert (AJ) and published literatureCitation5, as an OHE-specific ICD-10-code did not exist at the time of the study period to identify an OHE hospitalization in claims data. The index OHE hospitalization was defined as the first observed OHE hospitalization and spanned from the admission date to the discharge date, inclusively. The index date was defined as the next calendar date after the discharge date of the index OHE hospitalization (i.e. discharge date +1). The baseline period was defined as the 6-month period prior to the admission date of the index OHE hospitalization. The study period was defined as the ≥30-day period following the index date and ended at the earliest of the end of continuous health plan enrollment, the start of Medicare supplemental enrollment, cohort-specific censoring (rifaximin treatment cohort: censoring at rifaximin discontinuation; no rifaximin treatment cohort: censoring at the initiation of rifaximin), liver transplant, or the end of data availability (March 31, 2020). A minimum of 30 d was chosen to align with the CMS 30-day risk-standardized readmission measure within hospitalsCitation18, as well as to allow for a sufficient follow-up to measure readmission rates and healthcare costs.
Figure 1. Study design. Abbreviation. OHE: overt hepatic encephalopathy. 1Due to the lack of an OHE-specific ICD-10 code at the time of the study period to identify an OHE hospitalization in claims data, medical expert input was used to operationalize the definition of OHE hospitalization. 2Medical expert input was used to operationalize the definition of the 30-day window for treatment initiation.
Sample selection
Patients with HE were included in the study if they met the following eligibility criteria: ≥1 of four HE criteria as previously defined by Volk et al.Citation5; ≥1 OHE hospitalization; no diagnosis for secondary malignant neoplasm of liver (ICD-10-CM: C78.7x) at any time in the data; aged 18-64 years old as of the index date; no liver transplant on or before the discharge date of the index OHE hospitalization; ≥6 months and ≥30 d of continuous enrollment prior to and following the index OHE hospitalization, respectively.
Cohort classification
Following the index OHE hospitalization, all patients were classified into two mutually exclusive cohorts, based on treatment initiation within 30 days post-discharge: (1) rifaximin treatment (550 mg BID with ≥30 days of supply ± lactulose) and (2) no rifaximin treatment.
The rationale behind a 30-day window for treatment initiation was based on input from the medical expert (AJ), who recognized that clinicians in real-world settings may refer patients to a hepatologist for treatment after their hospitalization (15 d) and/or patients may take time to fill their prescription following that visit (15 d).
Based on AASLD treatment guideline recommendationsCitation10,Citation27 and input from the medical expert (AJ) according to clinical practice experience, the two cohorts were further stratified into four subgroups representing decreasing QoC: (1) Type 1: Received rifaximin without any time gap following the index OHE hospitalization; (2) Type 2: Received rifaximin within 30 d post-discharge; (3) Type 3: Received lactulose within 30 days post-discharge; and (4) Type 4: Received no rifaximin/lactulose within 30 d post-discharge.
To account for real-world treatment patterns typically observed in clinical practice, two adjustments were made to the cohort classification. First, patients who qualified in the rifaximin treatment cohort but experienced a second OHE hospitalization prior to initiating rifaximin (i.e. the admission date of the hospitalization occurred before the prescription fill date) were classified in the no rifaximin treatment cohort, as the event occurred prior to treatment and would not be associated with rifaximin. Second, leftover treatment was considered for patients who received treatment prior to their index OHE hospitalization and had remaining days of supply as of the admission date, as they may have been discharged with the intention of continuing their prior treatment. The window for treatment initiation among these patients was extended by the number of remaining days of supply as of the OHE hospitalization admission date.
Characteristics and study outcomes
Patient characteristics were evaluated and included age, sex, region, gastroenterology consults, indicators of portal hypertension (e.g. ascites, paracentesis, varices), OHE-related treatments and other treatments, cirrhosis-specific comorbidity scoring system (CirCom) score, Charlson comorbidity index (CCI), and selected comorbidities. During the index OHE hospitalization, the duration and cost of hospitalization were reported.
During the study period, annual rates of OHE hospitalizations were reported. The number of OHE hospitalizations were reported per-patient-per-month (PPPM). During the 30-day post-discharge period, the proportion of patients with ≥1 OHE rehospitalization was also evaluated.
During the study period, all-cause total healthcare costs (medical and pharmacy) were reported PPPM. Costs were measured from a payer’s perspective (i.e. health plan payment + coordination of benefits, excluding patients’ payment) and adjusted for inflation using the US Medical Care consumer price index from the Bureau of Labor Statistics from the US Department of Labor and reported in 2020 US dollarsCitation28.
Statistical analysis
Descriptive characteristics were summarized using means, standard deviations (SD), and medians for continuous variables and frequency counts and percentages for categorical variables.
OHE hospitalizations and healthcare costs were compared between the rifaximin treatment and no rifaximin treatment cohorts. For OHE hospitalizations, the number of events during follow-up was assessed using negative binomial regressions to estimate incidence rate ratios (IRR) with 95% confidence intervals (Cis) and p-values. The proportion of patients with ≥1 OHE hospitalization in the 30-day post-discharge period was assessed using logistic regression to estimate odds ratios (OR) with 95% Cis and p-values. For healthcare costs, adjusted cost differences with 95% Cis and p-values were estimated using multivariate generalized linear regression models with a gamma distribution and a log link. All analyses were adjusted for a priori selected potential confounding factors associated with OHE hospitalizations and costs (i.e. age, gender, health plan, region, CirCom score, prior gastroenterology consult, duration of index OHE hospitalization, treatment during baseline [e.g. rifaximin, HE prophylaxis with antibiotics], indicators of portal hypertension [e.g. varices], and factors associated with HE [e.g. frailty index]).
Statistical comparisons were replicated among the 4 subgroups of QoC. Specifically, comparisons of OHE hospitalizations and healthcare costs for each subgroup (Type 2–4) versus Type 1, separately. Additionally, OHE hospitalizations were descriptively reported among the 4 QoC subgroups, stratified by OHE-related treatment received pre-index OHE hospitalization (i.e. rifaximin, lactulose, or neither treatment).
Results
Patient characteristics
After patient selection, 1,452 patients were included in the rifaximin treatment cohort (Type 1: n = 1,138, Type 2: n = 314) and 560 patients were included in the no rifaximin treatment cohort (Type 3: n = 337, Type 4: n = 223; ). Baseline demographic and clinical characteristics were similar between the two cohorts (). At index, the mean age was 54.2 years and 39.3% were female in the rifaximin treatment cohort, while the mean age was 55.2 years and 42.7% were female in the no rifaximin treatment cohort. During baseline, more than half of patients had been seen by a gastroenterologist (rifaximin treatment cohort: 64.4%; no rifaximin treatment cohort: 58.9%) and mean CCI scores were 4.3 in both cohorts. Ascites (rifaximin treatment cohort: 53.5%; no rifaximin treatment cohort: 47.9%), paracentesis (rifaximin treatment cohort: 30.6%; no rifaximin treatment cohort: 28.6%), and varices (rifaximin treatment cohort: 37.1%; no rifaximin treatment cohort: 34.5%) were the most common indicators of portal hypertension in both cohorts.
Figure 2. Sample selection. Abbreviations. HE, hepatic encephalopathy; N, number; OHE, overt HE. 1Patients were selected using four criteria based on diagnoses (HE or liver-related complications) and treatment (i.e. rifaximin and/or lactulose) that were developed based on medical expert input on real-world clinical practice for coding for HE (described in Volk et al.Citation5) 2Due to the lack of an OHE-specific ICD-10 code at the time of the study period to identify an OHE hospitalization in claims data, medical expert input was used to operationalize the definition of OHE hospitalization.
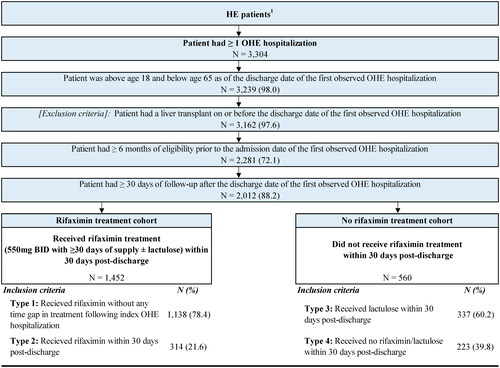
Table 1. Patient characteristics.
The mean length of the index OHE hospitalization was 10.8 d with an average cost of $48,225 in the rifaximin treatment cohort, compared to 8.0 d and $29,108 in the no rifaximin treatment cohort ().
Table 2. Comparison of OHE hospitalizations and healthcare costs.
OHE hospitalization
The mean duration of follow-up was 5.7 months in the rifaximin treatment cohort and 6.9 months in the no rifaximin treatment cohort (). Following the index OHE hospitalization, the odds of 30-day OHE rehospitalization was lower for the rifaximin treatment cohort (6.8%) compared to the no rifaximin treatment cohort (10.5%; adjusted OR [aOR]: 0.56; p < .01; ). Decreasing QoC (from Type 1 to Type 4) was associated with a higher 30-day odds of OHE rehospitalization; compared to Type 1, the aORs were 1.31 for Type 2 (p = .28), 1.74 for Type 3 (p = .01), and 1.90 for Type 4 (p = .01; ).
Figure 3. OHE hospitalizations. *Significant at the 5% level. Abbreviations. CI: confidence interval; OHE: overt hepatic encephalopathy.
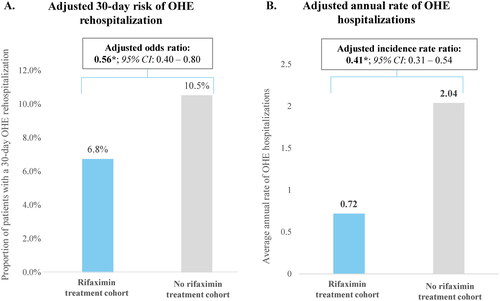
Figure 4. OHE hospitalizations by QoC. *Significant at the 5% level. Abbreviations. CI: confidence interval; OHE: overt hepatic encephalopathy; QoC: quality of care
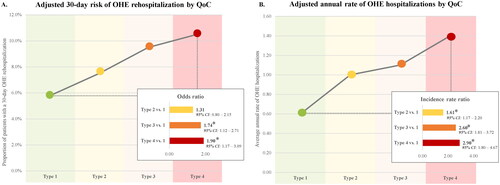
The adjusted annual rate of OHE hospitalizations following the index OHE hospitalization was 59% lower for the rifaximin treatment cohort compared to the no rifaximin treatment cohort (0.72 vs. 2.04; adjusted IRR: 0.41; p < .01; ). Decreasing QoC (from Type 1 to Type 4) was associated with higher annual rates of OHE hospitalization; specifically, the annual rate for Type 1 was 0.6 whereas it ranged to as high as 1.4 for Type 4. Compared to Type 1, the adjusted IRRs were 1.61 for Type 2, 2.60 for Type 3, and 2.90 for Type 4 (all p < .01; ). Regardless of whether patients received treatment with rifaximin, lactulose, or neither prior to the index OHE hospitalization, annual rates of OHE hospitalization were numerically lower in Type 1 (0.6–0.7) compared to Type 2 (1.1–1.2), Type 3 (1.3–3.7), and Type 4 (1.2–2.6).
Healthcare costs
During follow-up, mean total healthcare costs PPPM were $20,030 in the rifaximin treatment cohort and $18,457 in the no rifaximin treatment cohort (). In the rifaximin treatment cohort, adjusted pharmacy costs were $2,033 greater (p < .01) while adjusted medical costs were −$2,991 lower (p = .13); no significant annual cost differences were observed between the two cohorts (adjusted cost difference: −$959; p = .63).
Discussion
In this retrospective, real-world cohort study, higher QoC after an OHE hospitalization (i.e. rifaximin treatment within 30 d post-discharge) was associated with a reduced risk of 30-day OHE rehospitalizations and fewer OHE hospitalizations in the year following discharge. These findings demonstrate that there are both short- and longer-term benefits associated with treatment with rifaximin, which remained consistent regardless of patient exposure to rifaximin or lactulose prior to admission. Importantly, receiving rifaximin immediately from the day of discharge after the initial OHE hospitalization was associated with the lowest 30-day risk and annual rates of OHE hospitalizations. Additionally, the reduction in medical costs associated with fewer hospitalizations offset the increased pharmacy costs in the rifaximin treatment cohort, and no significant cost differences were observed between the cohorts.
The findings of this study are consistent with prior real-world and clinical trial studies that showed both reduced risk and reduced rates of OHE rehospitalizations with rifaximin treatmentCitation30–32. A retrospective study of hospitalized patients with OHE found that 25% of patients who received rifaximin at discharge experienced an OHE hospitalization or office visit in the following year, compared to 50% of patients who did not receive rifaximin at dischargeCitation32. A chart review study of cirrhotic patients who were admitted and treated for HE found that the rate of 180-day hospital readmission was lower for patients who received lactulose and rifaximin combination therapy compared to those who received lactulose monotherapy (2.5% vs. 16.2%; p = .02)Citation30. In this study, the 59% reduction in the adjusted annual rate of OHE hospitalizations is consistent with results from a clinical trial, which found that treatment with rifaximin reduced the risk of OHE hospitalization by 50% compared to placeboCitation12.
This study also demonstrated that adhering to the best care practices (i.e. initiation of rifaximin immediately after an initial OHE hospitalization) is an economically viable treatment strategy for preventing rehospitalizations within this vulnerable population. The benefits of this approach outweigh the associated costs, indicating that it is a viable option for healthcare providers and payers. This finding is corroborated by a systematic literature review of economic studies of OHE and rifaximin and/or lactulose, which found that rifaximin combined with lactulose has a favorable cost-effectiveness profile compared to lactulose aloneCitation3. Additionally, a previous retrospective chart review study of patients with OHE found that among those who switched from lactulose to rifaximin treatment, hospitalization charges decreased by $42,413 per patient (2005 USD) during a 6-month treatment periodCitation22. Preventing hospitalizations may also bring economic benefits at the societal level, for example, by keeping adults in the workforce. Furthermore, given the challenges associated with allocating sometimes scarce healthcare resources, savings in the rate of hospitalization as a result of prompt treatment with rifaximin can be re-allocated to improve other healthcare services that may benefit society at large.
From a QoC perspective, reducing hospital readmissions benefits both patients and healthcare systems. In a longitudinal observational study of hospitalized Medicare patients, the 1-year mortality rate among patients with early readmission was 38.7% compared to 12.1% for patients who were not readmitted early (p < .001)Citation33. In a separate retrospective study of patients with cirrhosis, patients readmitted ≤90 d after an initial admission had a significantly higher risk of death than patients readmitted >90 d or patients who were not readmitted (HR: 1.56; 95% CI: 1.53–1.59; p < .001)Citation34. Patients with HE, as well as their informal caregivers, have also reported experiencing major psychological impacts as a result of HE, including fear and anxietyCitation35. Thus, reducing the risk of OHE rehospitalization is likely to translate to improvements in health-related quality of life, as well as survival, for patients with OHE. Although the HRRP was developed for programs to reduce avoidable hospital readmissionsCitation18, studies in other therapeutic areas have used 30-day hospital readmissions as a benchmark for QoCCitation19,Citation20, similar to the present study which demonstrated that providing the highest guideline recommended QoC is an effective strategy for reducing the risk of 30-day hospital readmissions in a cost neutral way for hospitalized patients with OHE.
Findings from this study are subject to limitations. This claims-based study focused on a commercially insured population and may not be generalizable to all cirrhosis patients in the US. As with all claims-based studies, it is subject to common data limitations including billing inaccuracies or omissions in coded procedures, diagnoses, and pharmacy claims. Further, claims data contain limited clinical and laboratory information; as such, it was not possible to observe inpatient medications (including albumin infusions) in claims data nor obtain patients’ cirrhosis severity (e.g. MELD score, Child-Pugh grades). Information on mortality was also not available and survival could not be assessed. Despite adjustments for baseline characteristics in OHE hospitalization and cost comparisons, residual selection bias and confounding may have remained. Due to the lack of a specific ICD-10-CM diagnosis code for HE at the time the analyses were conducted, patients with HE were identified using an algorithm developed based on medical expert input, as described by Volk et al.Citation5 Total healthcare costs were reported from a payer’s perspective, which do not capture indirect costs associated with the patient burden (e.g. caregiving costs, impacts on quality of life). Challenges associated with approximating medication adherence are known limitation of using claims dataCitation36. For the purposes of this study, adherence to prescribed medication was assumed to have been complete. Finally, due to the retrospective design of the study, no causality can be established.
Conclusions
This real-world observational study demonstrated that initiation of rifaximin immediately after an initial OHE hospitalization provides the best QoC and was associated with reduced OHE hospitalizations both in the 30-d following hospitalization and annually. Additionally, reduced medical costs associated with fewer hospitalizations offset increased pharmacy costs of rifaximin, such that treatment with rifaximin is cost-neutral from a payer’s perspective when the best QoC standards are followed.
Transparency
Author contributions
All authors participated in the conceptualization and methodology of the study. PGS, RB, JC, and AG participated in the formal analysis, investigation, software, visualization, and validation. All authors participated in the writing (review & editing). All authors agree to be accountable for all aspects of the work and approved the final version to be published.
Reviewer disclosures
Peer reviewers on this manuscript have no relevant financial or other relationships to disclose.
Acknowledgement
Medical writing assistance was provided by Cody Patton, an independent consultant working on behalf of Analysis Group, Inc., and funded by Bausch Health US, LLC.
Declaration of funding
This study was funded by Bausch Health US, LLC. The study sponsor was involved in several aspects of the research, including the study design, the interpretation of data, the writing of the manuscript, and the decision to submit the manuscript for publication.
Declaration of financial/other relationship
AJ served as a consultant to Bausch Health US, LLC and received speaking fees from Salix Pharmaceuticals at the time this work was performed. ZH, AAD, and BB employees of Bausch Health US, LLC. GJ was an employee of Bausch Heath US, LLC at the time of the development of the study and is currently employed by BioNTech US Inc. DB was completing a Rutgers Institute for Pharmaceutical Industry Fellowship at Bausch Health US, LLC at the time of the development of the study. PGS, RB, JC, and AG are employees of Analysis Group, Inc., a consulting company that has provided paid consulting services to Bausch Health US, LLC, which funded the development and conduct of this study and manuscript.
Correction Statement
This article was originally published with errors, which have now been corrected in the online version. Please see Correction (http://dx.doi.org/10.1080/13696998.2023.2275924)
References
- Elwir S, Rahimi RS. Hepatic encephalopathy: an update on the pathophysiology and therapeutic options. J Clin Transl Hepatol. 2017;5(2):142–151.
- Patidar KR, Bajaj JS. Covert and overt hepatic encephalopathy: diagnosis and management. Clin Gastroenterol Hepatol. 2015;13(12):2048–2061. doi: 10.1016/j.cgh.2015.06.039.
- Neff G, Zachry W. III. Systematic review of the economic burden of overt hepatic encephalopathy and pharmacoeconomic impact of rifaximin. Pharmacoeconomics. 2018;36(7):809–822. doi: 10.1007/s40273-018-0641-6.
- Stepanova M, Mishra A, Venkatesan C, et al. In-hospital mortality and economic burden associated with hepatic encephalopathy in the United States from 2005 to 2009. Clin Gastroenterol Hepatol. 2012;10(9):1034–1041 e1. doi: 10.1016/j.cgh.2012.05.016.
- Volk ML, Burne R, Guérin A, et al. Hospitalizations and healthcare costs associated with rifaximin versus lactulose treatment among commercially insured patients with hepatic encephalopathy in the United States. J Med Econ. 2021;24(1):202–211. doi: 10.1080/13696998.2021.1877148.
- Seraj SM, Campbell EJ, Argyropoulos SK, et al. Hospital readmissions in decompensated cirrhotics: factors pointing toward a prevention strategy. World J Gastroenterol. 2017;23(37):6868–6876. doi: 10.3748/wjg.v23.i37.6868.
- Sood KT, Wong RJ. Hepatic encephalopathy is a strong predictor of early hospital readmission among cirrhosis patients. J Clin Exp Hepatol. 2019;9(4):484–490. doi: 10.1016/j.jceh.2019.01.005.
- Tapper EB, Halbert B, Mellinger J. Rates of and reasons for hospital readmissions in patients with cirrhosis: a multistate population-based cohort study. Clin Gastroenterol Hepatol. 2016;14(8):1181–1188.e2. doi: 10.1016/j.cgh.2016.04.009.
- Bajaj JS, Reddy KR, Tandon P, et al. The 3-month readmission rate remains unacceptably high in a large North American cohort of patients with cirrhosis. Hepatology. 2016;64(1):200–208. doi: 10.1002/hep.28414.
- Vilstrup H, Amodio P, Bajaj J, et al. Hepatic encephalopathy in chronic liver disease: 2014 practice guideline by the American association for the study of liver diseases and the European association for the study of the liver. Hepatology. 2014;60(2):715–735. doi: 10.1002/hep.27210.
- Food and Drug Administration. XIFAXAN® (rifaximin) label. 2022.
- Bass NM, Mullen KD, Sanyal A, et al. Rifaximin treatment in hepatic encephalopathy. N Engl J Med. 2010;362(12):1071–1081. doi: 10.1056/NEJMoa0907893.
- Beauvais B, Whitaker Z, Kim F, et al. Is the hospital Value-Based purchasing program associated with reduced hospital readmissions? J Multidiscip Healthc. 2022;15:1089–1099. doi: 10.2147/JMDH.S358733.
- Jencks SF, Williams MV, Coleman EA. Rehospitalizations among patients in the medicare fee-for-service program. N Engl J Med. 2009;360(14):1418–1428. doi: 10.1056/NEJMsa0803563.
- Tapper EB. Building effective quality improvement programs for liver disease: a systematic review of quality improvement initiatives. Clin Gastroenterol Hepatol. 2016;14(9):1256–1265.e3. doi: 10.1016/j.cgh.2016.04.020.
- McIlvennan CK, Eapen ZJ, Allen LA. Hospital readmissions reduction program. Circulation. 2015;131(20):1796–1803. doi: 10.1161/CIRCULATIONAHA.114.010270.
- Centers for Medicare & Medicaid Services. Hospital readmissions reduction program (HRRP) 2023 [cited 2023 March 16]. https://www.cms.gov/medicare/medicare-fee-for-service-payment/acuteinpatientpps/readmissions-reduction-program
- Centers for Medicare & Medicaid Services. Readmissions measures 2023 [cited 2023 March 13]. https://qualitynet.cms.gov/inpatient/measures/readmission.
- Chan L, Chauhan K, Poojary P, et al. National estimates of 30-Day unplanned readmissions of patients on maintenance hemodialysis. Clin J Am Soc Nephrol. 2017;12(10):1652–1662. doi: 10.2215/CJN.02600317.
- Lin E, Bhattacharya J, Chertow GM. Prior hospitalization burden and the relatedness of 30-Day readmissions in patients receiving hemodialysis. J Am Soc Nephrol. 2019;30(2):323–335. doi: 10.1681/ASN.2018080858.
- Shaw JA, Stiliannoudakis S, Qaiser R, et al. Thirty-day hospital readmissions: a predictor of higher all-cause mortality for up to two years. Cureus. 2020;12(7):e9308. doi: 10.7759/cureus.9308.
- Leevy CB, Phillips JA. Hospitalizations during the use of rifaximin versus lactulose for the treatment of hepatic encephalopathy. Dig Dis Sci. 2007;52(3):737–741. doi: 10.1007/s10620-006-9442-4.
- Neff GW, Kemmer N, Zacharias VC, et al. Analysis of hospitalizations comparing rifaximin versus lactulose in the management of hepatic encephalopathy. Transplant Proc. 2006;38(10):3552–3555. doi: 10.1016/j.transproceed.2006.10.107.
- Kruger AJ, Aberra F, Black SM, et al. A validated risk model for prediction of early readmission in patients with hepatic encephalopathy. Ann Hepatol. 2019;18(2):310–317. doi: 10.1016/j.aohep.2018.08.001.
- Volk ML, Tocco RS, Bazick J, et al. Hospital readmissions among patients with decompensated cirrhosis. Am J Gastroenterol. 2012;107(2):247–252. doi: 10.1038/ajg.2011.314.
- Merative. MarketScan 2023 [cited 2023 March 16]. https://www.merative.com/real-world-evidence.
- Kanwal F, Tapper EB, Ho C, et al. Development of quality measures in cirrhosis by the practice metrics committee of the American association for the study of liver diseases. Hepatology. 2019;69(4):1787–1797. doi: 10.1002/hep.30489.
- Bureau of Labor Statistics. Consumer price index. 2019. https://www.bls.gov/cpi/tables/supplemental-files/home.htm.
- Kim DH, Schneeweiss S, Glynn RJ, Lipsitz LA, Rockwood K, Avorn J. Measuring frailty in medicare data: development and validation of a claims-based frailty index. J Gerontol Med Sci. 2017;73(6):980–987.
- Courson A, Jones GM, Twilla JD. Treatment of acute hepatic encephalopathy: comparing the effects of adding rifaximin to lactulose on patient outcomes. J Pharm Pract. 2016;29(3):212–217. doi: 10.1177/0897190014566312.
- Tapper EB, Aberasturi D, Zhao Z, et al. Outcomes after hepatic encephalopathy in population-based cohorts of patients with cirrhosis. Aliment Pharmacol Ther. 2020;51(12):1397–1405. doi: 10.1111/apt.15749.
- Stoll AM, Guido M, Pence A, et al. Lack of access to rifaximin upon hospital discharge is frequent and results in increased hospitalizations for hepatic encephalopathy. Ann Pharmacother. 2023;57(2):133–140. doi: 10.1177/10600280221100537.
- Lum HD, Studenski SA, Degenholtz HB, et al. Early hospital readmission is a predictor of one-year mortality in community-dwelling older medicare beneficiaries. J Gen Intern Med. 2012;27(11):1467–1474. doi: 10.1007/s11606-012-2116-3.
- Mah JM, Dewit Y, Groome P, et al. Early hospital readmission and survival in patients with cirrhosis: a population-based study. Can Liver J. 2019;2(3):109–120. Summerdoi: 10.3138/canlivj.2018-0025.
- Fabrellas N, Moreira R, Carol M, et al. Psychological burden of hepatic encephalopathy on patients and caregivers. Clin Transl Gastroenterol. 2020;11(4):e00159. doi: 10.14309/ctg.0000000000000159.
- Galozy A, Nowaczyk S, Sant’Anna A, et al. Pitfalls of medication adherence approximation through EHR and pharmacy records: definitions, data and computation. Int J Med Inform. 2020;136:104092. doi: 10.1016/j.ijmedinf.2020.104092.
