Abstract
Aims
To evaluate the cost-effectiveness of bimekizumab, an inhibitor of IL-17F and IL-17A, against biologic and targeted synthetic disease-modifying antirheumatic drugs (DMARD) for psoriatic arthritis (PsA) from the Swedish healthcare system perspective.
Materials and methods
A Markov model was developed to simulate the clinical pathway of biologic [b] DMARD-naïve or tumor necrosis factor inhibitor experienced [TNFi-exp] PsA patients over a lifetime horizon. Treatment response was incorporated as achievement of the American College of Rheumatology 50% (ACR50) and Psoriasis Area and Severity Index 75% (PASI75) response, and changes in the Health Assessment Questionnaire-Disability Index (HAQ-DI) score. The efficacy of bimekizumab was obtained from the BE OPTIMAL (bDMARD-naïve) and BE COMPLETE (TNFi-experienced) trials while a network meta-analysis (NMA) informed the efficacy of the comparators. Resource use and drug costs were obtained from published studies and databases of drug retail prices in Sweden. A willingness-to-pay threshold of €50,000 per quality-adjusted life year (QALY) was applied.
Results
In bDMARD-naïve patients, bimekizumab achieved greater QALYs (14.08) than with all comparators except infliximab (14.22), dominated guselkumab every 4 and 8 weeks, ixekizumab, secukinumab 300 mg, ustekinumab 45 mg and 90 mg, and was cost-effective against risankizumab, tofacitinib, upadacitinib and TNFis, except adalimumab biosimilar. In TNFi-experienced patients, bimekizumab led to greater QALYs (13.56) than all comparators except certolizumab pegol (13.84), and dominated ixekizumab and secukinumab 300 mg while being cost-effective against all other IL-17A-, IL-23- and JAK inhibitors.
Limitations
An NMA informed the comparative effectiveness estimates. Given gaps in evidence of disease management and indirect costs specific to HAQ-DI scores, and sequential clinical trial evidence in PsA, non-PsA cost data from similar joint conditions were used, and one line of active treatment followed by best supportive care was assumed.
Conclusions
Bimekizumab was cost-effective against most available treatments for PsA in Sweden, irrespective of prior TNFi exposure
1. Introduction
Psoriatic arthritis (PsA) is a chronic, inflammatory arthropathy characterized by arthritis, enthesitis, and dactylitisCitation1. The majority of patients with PsA have psoriasis (PSO) at diagnosis, and up to 30% of patients with PSO are expected to progress to concomitant PsACitation2. In Sweden, an estimated 0.34% (24,980 individuals) of the adult population live with PsACitation3,Citation4. The estimated incidence of PsA is 1.69 cases per 100 patient-years among subjects with PSO (approximately 422 patients each year)Citation5.
The complexity and heterogeneous presentation of PsA make it a complicated condition to treat. If not appropriately treated, PsA can lead to long-term deterioration and damage to joints, poor quality of life, and decreased social participation and emotional well-beingCitation6–11. The disease commonly affects working-age patients, and, therefore, PsA also presents a considerable burden on productivityCitation12. Despite the number of available pharmacological treatments such as nonsteroidal anti-inflammatory drugs, corticosteroids, conventional synthetic disease-modifying antirheumatic drugs (csDMARDs) (i.e. methotrexate, sulfasalazine, and leflunomide), biologic DMARDs (bDMARD) (i.e. tumor necrosis factor inhibitors [TNFi], interleukin [IL] 12/23, IL-23, IL-17A inhibitors, and cytotoxic T lymphocyte antigen 4-immunoglobulin [Ig]), or targeted synthetic DMARDs (tsDMARD) (i.e. phosphodiesterase-4 inhibitor, and Janus kinase inhibitors [JAKi]), many patients fail to achieve long-term remission, do not respond, or experience side effects leading to the discontinuation of treatment. Although csDMARDs are typically the first-line DMARD treatment in PsA, they do not always prevent long-term joint damage or provide lasting remissionCitation13.
The clinical burden experienced by subjects often requires specialized care and multiple courses of treatment, translating into a considerable economic burden on healthcare systems. A previous cost-of-illness study in Sweden estimated that PsA was associated with a 97% higher mean annual societal cost per patient compared with patients with PSO without PsA; productivity losses and the cost of biologics were the main drivers of these higher costsCitation14.
The treatment landscape for PsA continues to evolve, and this constitutes a therapeutic advance in the clinical care of patients. However, it also represents a challenge to clinical decision-making due to the need for evidence regarding the comparative effectiveness of these treatments and, in some cases, their economic implicationsCitation15. Current clinical practice guidelines in Sweden recommend initiating treatment with bDMARDs or tsDMARDs for patients with active disease despite three months of treatment with csDMARDs, and considering bDMARDs or tsDMARDs as a first-line treatment for patients with highly active disease at presentationCitation16. In case of insufficient response to csDMARDs, TNFis are recommended as second-line treatment, and for patients where a response is still not achieved, switching to another TNFi, IL-17Ai, or JAKi is recommendedCitation16. EULAR guidelines specifically recommend IL-17 inhibitors in cases where there is peripheral arthritis or axial disease with relevant skin involvement and in cases of unequivocal enthesitisCitation17.
Bimekizumab is a novel, humanised monoclonal IgG1 antibody that selectively inhibits IL-17F in addition to IL-17A. The inhibition of both IL-17F and IL-17A has been reported to provide a greater reduction of inflammation compared to the single inhibition of IL-17A based on in vitro studiesCitation18,Citation19. In the BE OPTIMAL (NCT03895203) and BE COMPLETE (NCT03896581) phase III trials, bimekizumab demonstrated significant improvements in efficacy outcomes compared with placebo in adults with PsA who were bDMARD-naïve or had a prior inadequate response or lack of tolerability to TNFi, respectively. Bimekizumab was well tolerated, with a safety profile consistent with prior studies and no unexpected safety findingsCitation20–23. Due to the favourable relationship between the risks and benefits associated with bimekizumab, marketing authorization has been granted for its use as a treatment strategy for active psoriatic arthritis in the European UnionCitation24,Citation25.
A network meta-analysis (NMA) of randomized controlled trials in PsA which evaluated the comparative efficacy and safety of bimekizumab vs. other b/tsDMARDs in biologic-naïve and -experienced patients, found that bimekizumab ranked highly on the achievement of response in joint, skin, and minimal disease activity outcomes against a variety of treatments (comprising 21 studies in biologic-naïve and 16 in TNFi-experienced patients)Citation26. The methods and results were similar to a recent NMA of guselkumab against other comparatorsCitation27.
No published cost-effectiveness analyses have assessed the economic value of bimekizumab or other therapies for PsA in Sweden to date. This study aimed to evaluate the cost-effectiveness of bimekizumab in bDMARD-naïve and -TNFi-experienced patients against previously reimbursed therapies for PsA in Sweden.
2. Methods
2.1. Analytical framework
A cost-utility analysis was conducted using a state-transition cohort model to estimate the expected lifetime costs and health benefits of bimekizumab and a set of comparator treatments from the perspective of the Swedish healthcare system. Health benefits were expressed as quality-adjusted life years (QALY). Costs were estimated in Swedish krona (SEK; cost year 2022 and converted to Euros (€) using a 2022 exchange rate of 10.6 SEK = €1Citation28. Inflation adjustments were conducted when relevant using customer price index data based on recommended methodsCitation29. Costs and QALYs were discounted at 3.0% per year in line with guidelines for conducting economic evaluations in Sweden from The Dental and Pharmaceutical Benefits AgencyCitation30. A willingness-to-pay threshold (WTP) of 500,000 SEK (approximately €50,000) per incremental QALY was applied to determine the cost-effectiveness of bimekizumab, such that for any comparison yielding an ICER of less than 500,000 SEK it would be considered cost-effective, whereas comparisons with ICERs greater than 500,000 SEK it would be considered not cost-effectiveCitation30,Citation31. This threshold is commonly referred to as the reference threshold for conditions of moderate severity level in Sweden, and a similar threshold has been used in previous cost-effectiveness analyses of therapies in inflammatory diseasesCitation30,Citation32. Scenario-, deterministic- and probabilistic sensitivity analyses were conducted to estimate the uncertainty around the incremental cost-effectiveness ratio (ICER) and its sensitivity to key parameters or structural model assumptions. The analyses were conducted and reported according to best-practice guidelines for reporting economic evaluations from The Professional Society for Health Economics and Outcomes Research and the Consolidated Health Economic Evaluation Reporting StandardsCitation33,Citation34. The clinical studies that informed the efficacy of bimekizumab were conducted in accordance with the Declaration of Helsinki and the International Conference on Harmonisation Guidance for Good Clinical Practice, with ethical approval from the institutional review boards of the included participating sitesCitation20,Citation21.
2.2. Target population
The economic evaluation modelled adults with symptomatic PsA with or without concomitant PSO who had not responded adequately to prior treatment with csDMARDs. This population was divided into two subpopulations based on previous exposure to biologic treatments: (1) b/tsDMARD-naïve and (2) TNFi-experienced. The baseline characteristics of the modelled populations were obtained from the observed characteristics of patients in the BE OPTIMAL and BE COMPLETE clinical trialsCitation20,Citation21. The severity of concomitant PSO was incorporated for each population based on observed baseline Psoriasis Area Severity Index (PASI) scores from the same trials ().
Table 1. Baseline characteristics of the modelled PsA patient populations.
2.3. Intervention and comparators
Bimekizumab was compared with previously reimbursed TNFis (adalimumab original and biosimilar, certolizumab pegol, etanercept original and biosimilar, infliximab original and biosimilar, and golimumab), IL-17Ais (ixekizumab, secukinumab 150 mg and 300 mg), IL-23is (guselkumab 100 mg Q8W and Q4W, risankizumab), IL-12/23i (ustekinumab 45 mg and 90 mg), JAKis (upadacitinib and tofacitinib), abatacept, and csDMARDs. Only treatments with available relative efficacy estimates in each subpopulation could be included as comparators in the analyses. For the purpose of the analyses and to avoid assumptions about later-line therapy use and effectiveness under data scarcity, patients were assumed to receive one line of active therapy followed by best supportive care. Patients were assumed to receive concomitant csDMARDs alongside the active treatments and to stop active treatment (go back to csDMARDs only) after discontinuation or lack of responseCitation35 (Supplementary material 1). The dosing regimens for the treatments were obtained from their respective marketing authorization in SwedenCitation36 (Supplementary Material Citation2).
2.4. Model structure
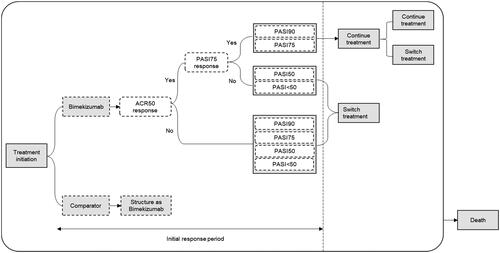
A state-transition cohort model was developed to simulate the clinical pathway of PsA based on the York modelling framework, a widely used model structure in economic evaluations of treatments in PsA ()Citation37. This model structure was considered appropriate due to its ability to recreate the chronic nature of PsA and incorporate the effect of therapies on both skin and joint symptomsCitation37. A hypothetical cohort of patients was modelled over a lifetime horizon (i.e. 50 years) in two periods. The short-term induction phase spanned the initiation of treatment with any of the evaluated therapies until the first assessment of improvement in both joint and skin symptoms. In the long-term maintenance phase, patients achieving response continued their initial treatment until subsequent discontinuation due to loss of efficacy and/or adverse events. Patients were assumed to stay on treatment during the induction period. Patients who did not respond after three months of induction treatment with any of the evaluated therapies or discontinuing (e.g. due to a safety event or loss of efficacy) switched back to csDMARDs. No initial treatment response assessment was made for this last line of treatment. The model used different cycle lengths for the initial response period and long-term follow-up. The first cycle is from induction to the end of maximum response on physical function (See Efficacy section for further details) in the initial response period (four weeks); the second cycle to the end of the initial response period (13 weeks); the third cycle from the end of the initial response period to the end of week 39 and the fourth cycle from the end of week 39 to week 52. After the end of the first year, a one-year cycle length was used. At any time in the model, patients could die. Mortality due to PsA was incorporated into the model by adjusting general population mortality estimates in Sweden with a PsA-specific mortality rate ratio of 1.05Citation38.
2.5. Model inputs
2.5.1. Efficacy
The effect of treatment was incorporated in the model as improvement in joint or skin symptoms and physical function. Response was assessed at the end of induction against the American College of Rheumatology 50% (ACR50) and Psoriasis Area and Severity Index 75% (PASI75) response criteriaCitation39,Citation40. For responders, physical function and skin symptoms were modelled as a change in the Health Assessment Questionnaire-Disability Index (HAQ-DI) score and PASI score, respectively (). A systematic literature review and NMA of relevant clinical trials were used to obtain relative effect estimates of clinical response for bimekizumab and comparatorsCitation26. A lack of efficacy data in TNFi-experienced patients for adalimumab, etanercept, golimumab, infliximab, and guselkumab every four weeks hindered inclusion in the analyses of this population. Furthermore, apremilast could not be included in either the bDMARD-naïve or the TNFi-experienced populations for the same reason (). Treatment discontinuation probability was derived from a previous drug-survival study by Egeberg et al. and was assumed equal for all treatments at 26% per annumCitation41. The overall estimate of the median time to treatment discontinuation from the study was converted to an annual probability of discontinuation. An exponential distribution was assumed, and a weighted average using the frequency of patients in each treatment strategy was obtained. This discontinuation parameter was obtained from the overall sample of patients with PsA in the study that included both bDMARD-naïve and b/tsDMARD-experienced patients.
Table 2. Clinical parameters used in the model.
2.5.2. HAQ-DI and PASI trajectories
Patients achieving both an ACR50 and PASI75 response at the end of the induction period were assumed to stay on treatment and to experience a reduction in their baseline HAQ-DI and PASI scores at that point (). The magnitude of HAQ-DI change for ACR50 responders was estimated from patient-level data in the BE OPTIMAL and BE COMPLETE trials for the two patient populations, based on changes observed in patients on bimekizumab (assumed to apply to all comparators), adalimumab (applied to adalimumab only) and placebo (applied to csDMARD-treatment only)Citation20,Citation21. HAQ-DI change was divided into three phases: induction, end of induction to week 36, and change after week 36Citation35,Citation42. The induction HAQ-DI change was assumed to be achieved by week 4 of treatment, as observed in the BE OPTIMAL and BE COMPLETE trials of bimekizumab and the DISCOVER I and II of guselkumabCitation20,Citation21,Citation43,Citation44. For b/tsDMARD-naïve patients, induction to week 36 HAQ-DI change was applied as the change over the same period in these clinical trials, and HAQ-DI change after week 36 was retrieved from trial data up to week 52 and assumed to continue at the same level for patients continuing on treatment. For TNFi-experienced patients, HAQ-DI was assumed to remain stable after induction while on treatment due to the absence of treatment-specific data after week 16 in BE COMPLETE. PASI scores were estimated from baseline PASI scores for patients in BE COMPLETE and BE OPTIMAL and adjusted for responders based on the proportion of patients achieving a given response level taken from the NMA (PASI50, PASI75, and PASI90). After induction, PASI scores were assumed to remain stable while on treatment at their new level; after stopping active treatment, HAQ-DI and PASI scores returned to their initial levels specific to each subpopulation to align with previous cost-effectiveness analyses in PsACitation45–47.
2.5.3. Utilities
Utility values were estimated as a function of HAQ-DI and PASI scores. To estimate this relationship, EQ-5D-5L data from the BE OPTIMAL and BE COMPLETE trials were mapped to utilities using the Swedish value setCitation20,Citation21,Citation48. Repeated-measures general estimating equation regression models were fitted to assess the relationship between the HAQ-DI, PASI, sex of patients, and their utility estimates. One model was fitted for each subpopulation (See Supplementary material 3 for further details).
2.5.4. Costs and healthcare resource use
The following cost categories were considered in the base-case analyses: medications, management, and drug administration (i.e. intravenous or subcutaneous injection by a nurse)Citation49,Citation50. Indirect costs and monitoring costs are included in separate scenario analyses. Disease management costs were incorporated as a function of HAQ-DI and PASI scores. The average annual costs of treating PsA (i.e. visits to primary, specialty, and inpatient care) in a sample of patients in Sweden reported by Löfvendahl et al. were usedCitation14. In a scenario analysis, indirect costs due to losses in productivity (i.e. sick leave and disability pension valued based on the human capital approach) from the same study were includedCitation14. The frequency distribution of costs due to medical resource utilization for different HAQ-DI categories reported in a Swedish cost-of-illness study in patients with rheumatoid arthritis was used to obtain estimates of disease management and indirect costs by HAQ-DI severityCitation14,Citation51. The distribution of costs in rheumatoid arthritis was considered an acceptable proxy for the distribution of costs in PsA in the absence of PsA-specific data. Costs related to the management of skin symptoms in patients with PSO were included based on a previous retrospective cost-of-illness study in Sweden by Ekelund et al. for different categories of PASI scoresCitation52. In this study, approximately 25% of the study sample had concomitant PsA with PSO. Total costs per HAQ-DI and PASI categories were incorporated in each cycle in the model based on the HAQ-DI and PASI score trajectories over time. In another scenario, the costs of medical resource use related to disease monitoring were incorporated by multiplying the individual cost of these services by their expected administration frequency from: (1) treatment initiation to three months, (2) four to six months, and (3) after six monthsCitation53.
Drug prices were based on pharmacy retail or procured prices in Sweden in December of 2022, and acquisition costs per cycle were calculated by multiplying pack prices by the doses and frequency of administrations according to the manufacturers’ labelsCitation49,Citation50. Two dosing schedules of secukinumab (150 mg and 300 mg), guselkumab (100 mg Q4W and Q8W), and ustekinumab (45 mg and 90 mg) were considered as separate comparators in the model when required efficacy data was reported. The frequency of concomitant methotrexate administration was incorporated into the model based on input from clinicians in Sweden and to reflect marketing authorizations. The costs of subcutaneous drug administration included a one-time visit to a health care center for an initial injection administration by a nurse. It was assumed that patients would self-administer the treatments at home after this initial visit (See Supplementary material Citation2) ().
Table 3. Cost inputs of the included treatment strategies.
2.6. Model validation
Methodological experts from the York Health Economics Consortium evaluated the model’s internal validity (comparability with other models), face validity (representativeness of clinical practice), and cross-validity (accuracy and consistency according to specification) (see Supplementary material 7). The model was considered robust and consistent with previous health technology appraisals of therapies for PsA. Extensive quality controls (i.e. extreme values and formula auditing) were also conducted to ensure the consistency of the obtained estimates.
2.7. Sensitivity analyses
Probabilistic sensitivity analyses were performed by running 400 Monte Carlo simulations in each pairwise comparison to account for parameter uncertainty. Scenario- and deterministic sensitivity analyses for the base case analysis of bimekizumab vs. ixekizumab were conducted to evaluate the potential effect of modifying structural assumptions in the model in the ICER estimates. These separate scenarios included assuming a 10-year time horizon; standardized mortality ratio of 1.20; requiring only ACR50 continuation (without PASI); 20% or 30% annual discontinuation probability; HAQ-DI scores returning to natural history levels post discontinuation; utility estimated based on data from BE OPTIMAL and BE COMPLETE (i.e. unadjusted utility estimates, summarized by health state and timepoint); disease management costs without variation by HAQ-DI scores; monitoring costs included; indirect costs included; different frequencies of PSO severity; frequency of patients per sex of the simulated populations modified; and treatments compared as part of a longer treatment sequence (see Supplementary material Citation4).
3. Results
3.1. Base-case analyses
The base-case results are shown in . In bDMARD-naïve PsA, infliximab treatment provided the greatest lifetime QALYs (14.22) followed by bimekizumab (14.08). Bimekizumab dominated (i.e. was more effective and produced lower costs against) guselkumab Q4W and Q8W, secukinumab 300 mg, ixekizumab, and ustekinumab 45 mg and 90 mg while being cost-effective (i.e. more effective, with each additional QALY gained incurring fewer than €50,000 additional costs) against risankizumab, tofacitinib, upadacitinib, and TNFis, except adalimumab biosimilar. For TNFi-experienced patients with PsA, certolizumab pegol provided the greatest lifetime QALYs (13.84), followed by bimekizumab QALYs (13.56). Bimekizumab dominated ixekizumab and secukinumab 300 mg while being cost-effective against all other comparators (including IL-17A-, IL-23- and JAK inhibitors). Disaggregated base-case lifetime cost estimates are reported in Supplementary material 5 and illustrate that although drug costs varied across compared treatments, the majority of the costs in the model were associated with disease management and varied less between treatments. Patients gained between 13.5 (csDMARDs) and 14.22 (infliximab) QALYs across the lifetime horizon of the model. The majority of QALYs were experienced in later treatment lines, since between induction efficacy and long-term discontinuation, patients spent between 0.71 (tofacitinib) and 1.5 (infliximab) years on first line treatment before transitioning to best supportive care.
Table 4. Base-case results over a lifetime horizon.
3.2. Sensitivity analyses
The results of the scenario analyses in a pairwise comparison of bimekizumab vs. ixekizumab are shown in and Supplementary material Citation6. Bimekizumab continued to dominate ixekizumab in all scenarios in bDMARD naïve patients indicating the robustness of the conclusions to uncertainty around structural and evidence assumptions. In TNFi-experienced patients, bimekizumab remained cost-effective or dominant in all scenarios. The inclusion of indirect costs and assuming only ACR50 as the response criterion at the end of the induction period were associated with increases in cost savings compared to the base-case estimates. The highest increase in ICER was observed in a scenario assuming disease management costs not varying by HAQ-DI scores, where bimekizumab remained cost-effective against ixekizumab with an ICER of €10,456. Incremental net benefit estimates, including confidence intervals based on probabilistic sensitivity analysis, are shown in Supplementary material 6.
Table 5. Results of scenario analysis (pairwise comparison of bimekizumab vs. ixekizumab).
4. Discussion
This study was the first to evaluate the cost-effectiveness of bimekizumab against previously reimbursed treatments for patients with active PsA in Sweden. The results suggested that bimekizumab may be cost-effective against most IL-17Ais, IL-23is, JAKis and TNFis at the established €50,000 per QALY threshold. For TNFi-experienced patients, bimekizumab was cost-effective against all included comparators, and dominant over ixekizumab and secukinumab 300 mg.
The cost-effectiveness of ILis has previously been investigated in the literature. The majority of previous cost-effectiveness analyses of ILs in PsA have been conducted from the United Kingdom (UK) perspective, and differences in the clinical management of patients and treatment costs across settings make it difficult to compare these estimates with the present studyCitation54. Purmonen et al. adopted the PsA Response Criteria (PsARC) as the main efficacy criterion without PASI response in the base case, and therefore, the current results are not completely comparableCitation55. A study by Schweikert et al. in the UK reported that sequential treatment with ixekizumab dominated secukinumab in both bDMARD-naive and -experienced patients with greater estimated QALYs but only modest cost-savingsCitation56. The results of the current analyses align with these two previous studies on ILis as a relevant treatment strategy for patients with PsA and provide additional evidence regarding the economic value of bimekizumab with a unique mechanism of action, which inhibits IL-17F in addition to IL-17A. Scenario analyses confirmed the robustness of the base-case estimates to assumptions around structure and parameters in the comparison of bimekizumab to ixekizumab in both b/tsDMARD-naïve and TNFi-experienced patients. An exploratory treatment sequencing analysis also yielded similar results to that of the base case without treatment sequence in both populations. Indirect costs contributed significantly to the modelled lifetime costs and the results were sensitive to their inclusion.
This study fills an important gap in the literature regarding the economic value of biological and targeted synthetic treatments for PsA in Sweden. The strengths of the analyses include the analytical approach, which was based on the York modelling framework, a widely accepted and validated method for assessing the economic value of therapies in PsA by health technology assessment agencies worldwide. It allowed for the evaluation of the health and cost benefits associated with the different treatments on joint and skin symptoms of patients with PsA. The model was previously validated by methodological experts to ensure validity, and extensive quality controls were conducted to ensure consistent estimates of costs and health benefits. The analyses were based on pharmacy retail or procured prices for most treatments which reflected the relevant costs for the Swedish healthcare system. Comprehensive comparisons against a large group of interventions that approximate the current basket of treatments available for PsA were provided. Last, efficacy and utilities for bimekizumab were informed by robust trial data and the efficacy for the comparators was based on a comprehensive recently published systematic literature review and NMA in PsACitation26.
Nevertheless, this study should be considered with limitations. Economic modelling necessarily represents a simplification of expected real-world experiences and outcomes. The disease natural history and treatment pathway presented cannot capture all the potential experiences of PsA patients in Sweden, but efforts were made to capture the most important and meaningful elements in terms of both patient health outcomes and costs. Using a recently published NMA to obtain efficacy data of the evaluated treatments might have introduced some uncertainties in this economic evaluation, but was unavoidable due to the lack of head-to-head trials between the comparators in PsACitation26. The absence of sequential clinical trial evidence in PsA required the base case to only consider one line of active treatment followed by best supportive care. This might not reflect the real-world management of patients with PsA in Sweden, where a sequential approach of treatments is recommendedCitation16. Including treatment sequencing in a scenario to address this limitation was also associated with uncertainty as the sequential treatment efficacy, discontinuation, or relapse rates of treatments used in sequential settings could not be substantiated with clinical- or real-world evidence. Utilities were assessed based on individual patients within a clinical trial, and these may vary from those observed in a clinical setting due to differences in patient characteristics, treatment adherence, and so on. Furthermore, there was no PsA-specific evidence of disease management and indirect costs specific to HAQ-DI scores in Sweden; these estimates were distributed according to HAQ-DI score distributions observed in rheumatoid arthritis following clinical consensusCitation14,Citation57. Confidential price discounts and regional pricing differences for JAKis, risankizumab, ustekinumab and infliximab in Sweden could not be incorporated but could have impacted their cost-effectiveness. Last, the long-term treatment discontinuation rates were assumed to be equal between therapies in the base-case analyses due to limited or no evidence for long-term discontinuation for newer therapies, although it is expected that discontinuation would not be equal between therapies with different mechanisms of action. However, this was however tested in the scenario analyses yielding similar results. Finally, the costs and health effects of adverse events associated with treatment were excluded from the analysis on the assumption of similarity between treatments, and this represents a possible gap in the analysis, which may be informed with longer-term monitoring, especially of newer treatments.
5. Conclusion
Bimekizumab can be considered a cost-effective intervention relative to previously reimbursed treatments in Sweden in both b/tsDMARD-naïve and TNFi-experienced patients from the perspective of the Swedish healthcare system.
Transparency
Author contributions
All authors contributed to the conception or design of the work or the acquisition, analysis, or interpretation of data for the work. All authors supported drafting the work or revising it critically for important intellectual content. All authors provided final approval of the version to be published. All authors agree to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part are appropriately investigated and resolved.
Supplemental Material
Download MS Word (85.3 KB)Acknowledgements
The manuscript was drafted by Devian Parra at Cytel with input from all co-authors.
Declaration of financial and other relationships
V Sigurdardottir has received consulting fees from Novartis, AstraZeneca, Abbvie, Janssen, Galapagos, Sanofi, and UCB. A Engstrom, P Berling, L Melis and D Willems are employees and stockholders of UCB Pharma. T Olofsson has performed consulting tasks for Merck Sharp & Dohme. L Ooldsberg is an employee of Quantify Research, providing consultancy services to pharmaceutical companies and other private and public organizations and institutions; receiving consulting fees from UCB for the current work. S Sadler and D Parra are employees of Cytel, which was contracted as a consultant on a research project by UCB Pharma to carry out modelling work and to adapt the model to the Swedish setting and draft the manuscript. The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed. Peer reviewers on this manuscript have no relevant financial or other relationships to disclose.
Data availability statement
The authors declare that all input data to parameterize the model are available within the article and the Supplementary Material. Data from non-clinical studies are outside of UCB's data-sharing policy and are unavailable for sharing.
Additional information
Funding
References
- McArdle A, Pennington S, FitzGerald O. Clinical features of psoriatic arthritis: a comprehensive review of unmet clinical needs. Clin Rev Allergy Immunol. 2018; Dec55(3):271–294. doi: 10.1007/s12016-017-8630-7.
- Ritchlin CT, Colbert RA, Gladman DD. Psoriatic arthritis. N Engl J Med. 2017; Mar 9376(10):957–970. doi: 10.1056/NEJMra1505557.
- Exarchou S, Wallman J, Di Giuseppe D, et al. The national prevalence of clinically diagnosed psoriatic arthritis in Sweden 2017. J Rheumatol. 2023;50(6):781–788. doi: 10.3899/jrheum.221139.
- Statistics Sweden. Official Statistics of Sweden: population: statistics Sweden; 2022 [cited 2023 28/07/2023]. Available from: https://www.statistikdatabasen.scb.se/pxweb/en/ssd/START__BE/.
- Lindberg I, Lilja M, Geale K, et al. Incidence of psoriatic arthritis in patients with skin psoriasis and associated risk factors: a retrospective population-based cohort study in swedish routine clinical care. Acta Derm Venereol. 2020;100(18):adv00324. doi: 10.2340/00015555-3682.
- Gladman DD, Stafford-Brady F, Chang CH, et al. Longitudinal study of clinical and radiological progression in psoriatic arthritis. J Rheumatol. 1990;17(6):809–812.
- McHugh NJ, Balachrishnan C, Jones SM. Progression of peripheral joint disease in psoriatic arthritis: a 5-yr prospective study. Rheumatology (Oxford). 2003; 42(6):778–783. doi: 10.1093/rheumatology/keg217.
- Torre Alonso JC, Rodriguez Perez A, Arribas Castrillo J, et al. Psoriatic arthritis (PA): a clinical, immunological and radiological study of 180 patients. Br J Rheumatol. 1991;30(4):245–250. doi: 10.1093/rheumatology/30.4.245.
- Coates LC, Orbai AM, Azevedo VF, et al. Results of a global, patient-based survey assessing the impact of psoriatic arthritis discussed in the context of the psoriatic arthritis impact of disease (PsAID) questionnaire. Health Qual Life Outcomes. 2020;18(1):173. doi: 10.1186/s12955-020-01422-z.
- Gudu T, Gossec L. Quality of life in psoriatic arthritis. Expert Rev Clin Immunol. 2018; 14(5):405–417. doi: 10.1080/1744666X.2018.1468252.
- Husni ME, Merola JF, Davin S. The psychosocial burden of psoriatic arthritis. Semin Arthritis Rheum. 2017;47(3):351–360.
- Kennedy M, Papneja A, Thavaneswaran A, et al. Prevalence and predictors of reduced work productivity in patients with psoriatic arthritis. Clin Exp Rheumatol. 2014;32(3):342–348.
- Kane D, Stafford L, Bresnihan B, et al. A prospective, clinical and radiological study of early psoriatic arthritis: an early synovitis clinic experience. Rheumatology (Oxford). 2003;42(12):1460–1468. Decdoi: 10.1093/rheumatology/keg384.
- Lofvendahl S, Petersson IF, Theander E, et al. Incremental costs for psoriasis and psoriatic arthritis in a population-based cohort in Southern Sweden: is it all psoriasis-attributable morbidity? J Rheumatol. 2016;43(3):640–647. doi: 10.3899/jrheum.150406.
- Van den Bosch F, Coates L. Clinical management of psoriatic arthritis. Lancet. 2018; 391(10136):2285–2294. doi: 10.1016/S0140-6736(18)30949-8.
- Swedish Rheumatology Association. Guidelines and recommendations: psoriatic arthritis – guidelines for drug treatment: Swedish Rheumatology Association; 2022 [cited 2023 28/07/2023]. Available from: https://riktlinjer.svenskreumatologi.se/riktlinjer-och-rekommendationer/psa/.
- Gossec L, Baraliakos X, Kerschbaumer A, et al. EULAR recommendations for the management of psoriatic arthritis with pharmacological therapies: 2019 update. Ann Rheum Dis. 2020;79(6):778–786. Jundoi: 10.1136/annrheumdis-2020-217163.
- Glatt S, Baeten D, Baker T, et al. Dual IL-17A and IL-17F neutralisation by bimekizumab in psoriatic arthritis: evidence from preclinical experiments and a randomised placebo-controlled clinical trial that IL-17F contributes to human chronic tissue inflammation. Ann Rheum Dis. 2018;77(4):523–532. doi: 10.1136/annrheumdis-2017-212127.
- Glatt S, Helmer E, Haier B, et al. First-in-human randomized study of bimekizumab, a humanized monoclonal antibody and selective dual inhibitor of IL-17A and IL-17F, in mild psoriasis. Br J Clin Pharmacol. 2017;83(5):991–1001. doi: 10.1111/bcp.13185.
- McInnes IB, Asahina A, Coates LC, et al. Bimekizumab in patients with psoriatic arthritis, naive to biologic treatment: a randomised, double-blind, placebo-controlled, phase 3 trial (BE OPTIMAL). Lancet. 2023;401(10370):25–37. doi: 10.1016/S0140-6736(22)02302-9.
- Merola JF, Landewe R, McInnes IB, et al. Bimekizumab in patients with active psoriatic arthritis and previous inadequate response or intolerance to tumour necrosis factor-alpha inhibitors: a randomised, double-blind, placebo-controlled, phase 3 trial (BE COMPLETE). Lancet. 2023;401(10370):38–48. doi: 10.1016/S0140-6736(22)02303-0.
- Coates LC, McInnes IB, Merola JF, et al. Safety and efficacy of bimekizumab in patients with active psoriatic arthritis: three-Year results from a phase IIb randomized controlled trial and its Open-Label extension study. Arthritis Rheumatol. 2022;74(12):1959–1970. Decdoi: 10.1002/art.42280.
- Ritchlin CT, Kavanaugh A, Merola JF, et al. Bimekizumab in patients with active psoriatic arthritis: results from a 48-week, randomised, double-blind, placebo-controlled, dose-ranging phase 2b trial. Lancet. 2020;395(10222):427–440. doi: 10.1016/S0140-6736(19)33161-7.
- Committee for Medicinal Products for Human Use (CHMP). European public assessment reports: bimzelx. Procedure No. EMEA/H/C/005316/II/0011 European Medicines Agency 2023 [cited 2023 28/07/2023]. Available from: https://www.ema.europa.eu/en/documents/variation-report/bimzelx-h-c-5316-ii-0011-epar-assessment-report-variation_en.pdf.
- European Medicines Agency. Summary of product characteristics: bimzelx: european Medicines Agency; 2023 cited 2023 28/07/2023]. Available from: https://www.ema.europa.eu/en/documents/product-information/bimzelx-epar-product-information_en.pdf.
- Mease PJ, Gladman D, Merola JF, et al. SA64 Comparative Effectiveness of Bimekizumab in Patients with Psoriatic Arthritis: Results from a Systematic Literature Review and Network Meta-Analysis. Value Health. 2023;26(6).
- Mease PJ, McInnes I, Tam L, et al. Comparative effectiveness of guselkumab in psoriatic arthritis: results from systematic literature review and network meta-analysis. Rheumatology (Oxford). 2021;60(5):2109–2121. doi: 10.1093/rheumatology/keab119.
- Central European Bank. Average exchange rates - SEK to Euro 2022 [cited 2023 28/07/2023]. Available from: https://www.ecb.europa.eu/stats/policy_and_exchange_rates/euro_reference_exchange_rates/html/eurofxref-graph-sek.en.html.
- Turner HC, Lauer JA, Tran BX, et al. Adjusting for inflation and currency changes within health economic studies. Value Health. 2019;22(9):1026–1032. Sepdoi: 10.1016/j.jval.2019.03.021.
- Dental and Pharmaceutical Benefits Agency (TLV). Amendment to the Dental and Pharmaceutical Benefits Agency’s General Advice (TLVAR 2003:2) on Economic Evaluations; 2017 [cited 2023 Sep 20]. Available from: https://www.tlv.se/download/18.467926b615d084471ac3230c/1510316374332/TLVAR_2017_1.pdf
- Viollet J, O'Leary E, Camacho-Gonzalez C, et al. Willingness to pay for different severity levels in Sweden: an analysis of TLV decisions (2014-2022). Value in Health. 2022;25(12):S341. doi: 10.1016/j.jval.2022.09.1686.
- Hansson-Hedblom A, Almond C, Borgstrom F, et al. Cost-effectiveness of ustekinumab in moderate to severe crohn’s disease in Sweden. Cost Eff Resour Alloc. 2018;16(1):28. doi: 10.1186/s12962-018-0114-y.
- Husereau D, Drummond M, Augustovski F, et al. Consolidated health economic evaluation reporting standards 2022 (CHEERS 2022) statement: updated reporting guidance for health economic evaluations. Value Health. 2022;25(1):3–9. doi: 10.1016/j.jval.2021.11.1351.
- Ramsey SD, Willke RJ, Glick H, et al. Cost-effectiveness analysis alongside clinical trials II-an ISPOR good research practices task force report. Value Health. 2015;18(2):161–172. doi: 10.1016/j.jval.2015.02.001.
- National Institute for Health and Care Excellence (NICE). Guselkumab for treating active psoriatic arthritis after inadequate response to DMARDs. Technology appraisal guidance [TA711]. London (UK); 2021.
- Swedish Electronic Medicines Compendium (FASS). Drugs database: swedish Electronic Medicines Compendium (FASS); 2022 [cited 2023 28/07/2023]. Available from: https://www.fass.se/LIF/startpage.
- Rodgers M, Epstein D, Bojke L, et al. Etanercept, infliximab and adalimumab for the treatment of psoriatic arthritis: a systematic review and economic evaluation. Health Technol Assess. 2011;15(10):i–xxi. 1–329. doi: 10.3310/hta15100.
- Ali Y, Tom BD, Schentag CT, et al. Improved survival in psoriatic arthritis with calendar time. Arthritis Rheum. 2007;56(8):2708–2714. doi: 10.1002/art.22800.
- Chung CP, Thompson JL, Koch GG, et al. Are American college of rheumatology 50% response criteria superior to 20% criteria in distinguishing active aggressive treatment in rheumatoid arthritis clinical trials reported since 1997? A meta-analysis of discriminant capacities. Ann Rheum Dis. 2006;65(12):1602–1607. doi: 10.1136/ard.2005.048975.
- Feldman SR, Krueger GG. Psoriasis assessment tools in clinical trials. Ann Rheum Dis. 2005;64 (Suppl 2):ii65–ii68. doi: 10.1136/ard.2004.031237.
- Egeberg A, Roseno NAL, Aagaard D, et al. Drug survival of biologics and novel immunomodulators for rheumatoid arthritis, axial spondyloarthritis, psoriatic arthritis, and psoriasis - A nationwide cohort study from the DANBIO and DERMBIO registries. Semin Arthritis Rheum. 2022;53:151979.
- National Institute for Health and Care Excellence (NICE). Upadacitinib for treating active psoriatic arthritis after inadequate response to DMARDs. Technology appraisal guidance (TA768). 2021. London, United Kingdom.
- Deodhar A, Helliwell PS, Boehncke WH, et al. Guselkumab in patients with active psoriatic arthritis who were biologic-naive or had previously received TNFalpha inhibitor treatment (DISCOVER-1): a double-blind, randomised, placebo-controlled phase 3 trial. Lancet. 2020;395(10230):1115–1125. doi: 10.1016/S0140-6736(20)30265-8.
- Mease PJ, Rahman P, Gottlieb AB, et al. Guselkumab in biologic-naive patients with active psoriatic arthritis (DISCOVER-2): a double-blind, randomised, placebo-controlled phase 3 trial. Lancet. 2020;395(10230):1126–1136. doi: 10.1016/S0140-6736(20)30263-4.
- Bravo Vergel Y, Hawkins NS, Claxton K, et al. The cost-effectiveness of etanercept and infliximab for the treatment of patients with psoriatic arthritis. Rheumatology (Oxford). 2007;46(11):1729–1735. doi: 10.1093/rheumatology/kem221.
- Bojke L, Epstein D, Craig D, et al. Modelling the cost-effectiveness of biologic treatments for psoriatic arthritis. Rheumatology 2011;50 Suppl 4:iv39–iv47. Sepdoi: 10.1093/rheumatology/ker245.
- Cummins E, Asseburg C, Punekar YS, et al. Cost-effectiveness of infliximab for the treatment of active and progressive psoriatic arthritis. Value Health. 2011;14(1):15–23. Jandoi: 10.1016/j.jval.2010.10.016.
- Burstrom K, Sun S, Gerdtham UG, et al. Swedish experience-based value sets for EQ-5D health states. Qual Life Res. 2014;23(2):431–442. doi: 10.1007/s11136-013-0496-4.
- Dental and Pharmaceutical Benefits Agency. (Tandvårds- och läkemedelsförmånsverket T. Price and decision database: dental and Pharmaceutical Benefits Agency (Tandvårds- och läkemedelsförmånsverket, TLV); 2022 [cited 2023 28/07/2023]. Available from: https://www.tlv.se/beslut/sok-priser-och-beslut-i-databasen.html.
- Region Stockolm and Gotland. Price list Contract drugs Region Stockholm and Region Gotland Region Stockolm and Gotland; 2022 [cited 2023 28/07/2023]. Available from: https://contracts.tendsign.com/ContractArea/Details/1579312?eId=2XwXmS0zykSquwLRsSricwA%3d.
- Wallman JK, Eriksson JK, Nilsson JA, et al. Costs in relation to disability, disease activity, and health-related quality of life in rheumatoid arthritis: observational data from Southern Sweden. J Rheumatol. 2016;43(7):1292–1299. doi: 10.3899/jrheum.150617.
- Ekelund M, Mallbris L, Qvitzau S, et al. A higher score on the dermatology life quality index, being on systemic treatment and having a diagnosis of psoriatic arthritis is associated with increased costs in patients with plaque psoriasis. Acta Derm Venereol. 2013;93(6):684–688. Novdoi: 10.2340/00015555-1591.
- Södra sjukvårdsregionen. Regionala priser och ersättningar för södra sjukvårdsregionen: södra sjukvårdsregionen; 2022 [cited 2023 28/07/2023]. Available from: https://sodrasjukvardsregionen.se/download/regionala-priser-och-ersattningar-for-sodra-sjukvardsregionen-2022/?wpdmdl=24791&masterkey=61decc148fc78.
- D'Angiolella LS, Cortesi PA, Lafranconi A, et al. Cost and cost effectiveness of treatments for psoriatic arthritis: a systematic literature review. Pharmacoeconomics. 2018;36(5):567–589. Maydoi: 10.1007/s40273-018-0618-5.
- Purmonen T, Puolakka K, Bhattacharyya D, et al. Cost-effectiveness analysis of secukinumab versus other biologics and apremilast in the treatment of active psoriatic arthritis: a finnish perspective. Cost Eff Resour Alloc. 2018;16(1):56. doi: 10.1186/s12962-018-0162-3.
- Schweikert B, Malmberg C, Akerborg O, et al. Cost-effectiveness analysis of sequential biologic therapy with ixekizumab versus secukinumab in the treatment of active psoriatic arthritis with concomitant moderate-to-Severe psoriasis in the UK. Pharmacoecon Open. 2020;4(4):635–648. Decdoi: 10.1007/s41669-020-00202-1.
- Burton W, Morrison A, Maclean R, et al. Systematic review of studies of productivity loss due to rheumatoid arthritis. Occup Med. 2006;56(1):18–27. doi: 10.1093/occmed/kqi171.