Abstract
Background
The coronavirus disease 2019 (COVID-19) pandemic has imposed significant burden on Brazil’s health system. This study aimed to examine clinical characteristics, overall vaccine uptake, and to assess healthcare resource utilization (HCRU) and costs associated with acute COVID-19 in Brazil during the Omicron predominant period.
Methods
A nationwide retrospective study was conducted using various Brazilian databases including, COVID-19 related databases, public health systems, and other surveillance/demographic datasets. Individuals with positive COVID-19 test results between January 1 2022 and April 30 2022, during Omicron BA.1/BA.2 wave, were identified. Patients’ demographics, vaccine uptake, HCRU and corresponding costs were described by age groups.
Results
A total of 8,160,715 (3.80%) COVID-19 cases were identified in the study cohort, ranging from 2.43% in <5 years to 62.05% in 19-49 years. The uptake of partial (Dose 1) or full immunization (Dose 2) was less than 0.1% in children aged <5 years, whereas in individuals ≥ 19 years, it exceeded 89.78% for Dose 1 and 84.07% for Dose 2. Overall booster vaccine uptake was 38.06%, which was significantly higher among individuals aged ≥ 65 years, surpassing 74.79%. Regardless of vaccination status, 87.2% cases were symptomatic, and 1.48% were hospitalized due to acute COVID-19 (<5 years: 2.33%, 5-11 years: 0.99%, 12-18 years: 0.32%, 19-49 years: 0.40%; 50-64 years: 1.50%, 65-74 years: 5.43%, and ≥ 75 years: 17.89%). Among the hospitalized patients (n = 120,450), 32.57% were admitted to ICU, of whom 31,283 (79.75%) individuals required mechanical ventilation (MV) support. The average cost per day in normal ward and ICU without MV in public/general hospital settings was $104.36 and $302.81, respectively. While average cost per day in normal ward and ICU with MV was $75.91 and $301.22 respectively.
Conclusions
This study quantified the burden of COVID-19 in Brazil, suggesting substantial healthcare resources required to manage the COVID-19 pandemic.
Introduction
Brazil’s health system has been substantially burdened by the coronavirus disease 2019 (COVID-19) pandemic. As of May 2023, over 37.5 million confirmed COVID-19 cases and 702.7 thousand COVID-19-related deaths have been reported in the country.Citation1 The pandemic has also inflicted immense strain on the economy, with total federal government spending related to COVID-19 in 2020 equivalent to 7.0% of the gross domestic product (GDP).Citation2,Citation3
Extensive vaccination campaigns have been implemented across the country to curb the spread of COVID-19. The Brazil National Immunization Program initiated COVID-19 primary vaccination in January 2021, prioritizing elderly individuals and high-risk groups. The program subsequently expanded to recommend primary vaccination for the pediatric population aged 3 years or older in 2022, and eventually extended to include children aged 6 months or older in 2023.Citation4 Consequently, by June 2022, Brazil administered the largest number of COVID-19 vaccine doses in Latin AmericaCitation5 resulting in a notable decline in COVID-19 related hospitalizations and fatalities.Citation6,Citation7
Despite this remarkable progress, the emergence of highly contagious variants such as Alpha, Beta, Gamma, Delta, and Omicron has raised significant concerns within the realm of public health. The Omicron variant, in particular, caused a sharp surge in infections in Brazil in early 2022 due to its increased transmissibility and the potential to partially evade natural or vaccine-induced immunity.Citation7,Citation8
While multiple studies have explored the impact of COVID-19 in Brazil,Citation9–14 there is a notable gap in research specifically focusing on the healthcare resource utilization (HCRU) burden during the predominance of the Omicron variant in the country. Hence, this study aimed to address this critical gap by examining clinical characteristics and overall vaccine uptake among general population, as well as assessing HCRU and corresponding direct healthcare costs among patients with acute COVID-19 in Brazil during January 1, 2022 to April 30, 2022, when Omicron variant was predominant in the country.
Methods
Study design
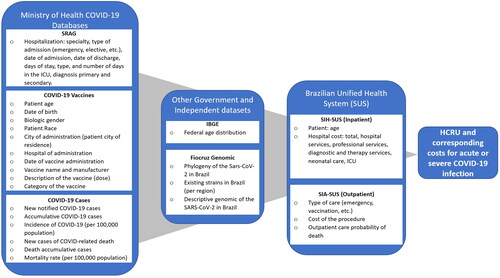
A nationwide retrospective study was conducted using various Brazilian data sources to examine the clinical burden of COVID-19 in terms of confirmed cases and symptomatic infections, and vaccine uptake among the general population in Brazil. Subsequently, the study focused on assessing the HCRU and corresponding direct healthcare costs among individuals hospitalized with acute COVID-19 infection. As illustrated in , a cost estimation model was developed comprising individuals who tested positive for COVID-19 between January 1, 2022, and April 30, 2022, a period when the Omicron variant was predominant in the country.
Figure 1. Brazilian real-world data sources used in the cost estimation model.
COVID-19, coronavirus disease 2019; IBGE, Brazilian Institute of Geography and Statistics; ICU, intensive care unit; SAR-CoV-2, severe acute respiratory syndrome coronavirus 2; SUS, Sistema Único de Saúde - Brazilian Unified Health System; SIA-SUS: SUS outpatient Information System; SIH-SUS: SUS inpatient Information System; SRAG, Síndrome Respiratória Aguda Grave
Study population
The reverse transcription-polymerase chain reaction (RT-PCR) or rapid antigen test(s) confirmed COVID-19 cases were included in the cost estimation. Acute COVID-19 cases were defined as individuals with symptomatic infection who may or may not require COVID-19 related hospitalization. Based on their vaccination status, individuals were categorized into three groups of partial or full immunization and booster dose. Partial immunization (Dose 1) was defined for individuals who had received only one dose out of two doses of primary series of vaccine(s), excluding individuals with one dose of Ad26.COV2.S vaccine. Whereas full immunization (Dose 2) was defined if individuals had received both doses of primary series of vaccine(s) or one dose of Ad26.COV2.S vaccine. Any doses received after full immunization were referred to as boosters.
Model input
The model inputs were obtained from different publicly available Brazilian databases including COVID-19 related databases, public health systems, and other surveillance/demographic datasets, which are briefly described below and visually depicted in .
Nationwide public health system databases
Brazil’s national public-founded health system, known as the Unified Health System "Sistema Único de Saúde" (SUS), is designed to achieve universal health coverage across the country. The SUS offers primary care, outpatient care, hospital care, and medications to the population, particularly those who cannot afford private insurance. With federal-level coverage extending to approximately 190 million population, the SUS encompasses both administrative and epidemiological databases.Citation15 At the individual level, the primary administrative databases within the SUS include the SIH-SUS (Inpatient Information System) and SIA-SUS (Outpatient Information System).
The SIH-SUS collects data on hospital interventions that are funded by the SUS, categorizing information into five segments encompassing patient demographics, hospitalization details, hospital costs, sterilization records, and additional information such as high-risk pregnancy, antenatal care, and nosocomial infections.Citation16 On the other hand, the SIA-SUS captures data on outpatient clinic interventions that are also funded by the SUS. This database receives, validates, and consolidates patient-level data, utilizing two sources: the BPA (Outpatient Production Report) and the APAC (High Complexity Procedures Authorization).Citation17 These public health system databases were used to estimate the HCRU and corresponding direct healthcare costs for individuals with acute COVID-19, who required hospitalization.
Nationwide COVID-19 databases from Ministry of health
The Síndrome Respiratória Aguda Grave (SRAG) database, which includes data about all respiratory viruses identified in Brazil since 2009,Citation18 was utilized for the national surveillance of severe acute respiratory syndrome (SARS). Whereas the COVID-19 Vaccines database incorporates the federal vaccination campaign against COVID-19. Both the SRAG and COVID-19 Vaccine database were used in the model. The SRAG database is updated on a weekly basis with COVID-19-related epidemiological and hospitalization data,Citation18 while the COVID-19 Vaccines database is updated daily, compiling anonymized patient-level data on vaccine administration and uptake. The COVID-19 cases database was utilized for COVID-19 related epidemiological data including new and accumulative cases and COVID-19-related deaths.Citation19
Other Brazilian government and independent datasets
The Brazilian Institute of Geography and Statistics (IBGE) dataset, generated by the federal institute responsible for general statistics in the country, was used to define demographic characteristics of the general population in Brazil.Citation20
To describe the SARS-CoV-2 mutations and strain existing in Brazil, an independent dataset of the Fiocruz Genomic Network was used. The Fiocruz Genomic is a project of the Oswaldo Cruz Foundation (Fiocruz) that aims to understand and describe SARS-Cov-2 behavior, and its genetic evolution and mutations to support health decisions in Brazil.Citation21
Analysis
The clinical characteristics, vaccine uptake and rates of symptomatic COVID-19 infections, derived from the COVID-19-specific databases, were stratified by age group. The HCRU and corresponding direct healthcare costs for individuals with acute COVID-19 requiring hospitalization were derived from the SIH-SUS and SIA-SUS databases, and reported for normal ward and intensive care unit (ICU), with and without mechanical ventilation (MV) support for the overall cohort and different age groups.
For categorical variables, frequency counts and percentages were reported. We employed two statistical methodologies, namely the Chi-Square Test and Generalized Linear Models (GLMs), to investigate the patterns of COVID-19 infection and hospitalization across diverse age groups. The Chi-Square Test was utilized to assess the distribution of COVID-19 cases and analyze symptomatic rates within various age groups. Concurrently, the GLM binomial method was applied to calculate a 95% confidence interval (95% CI) for hospitalization rates. Additionally, the GLM Poisson approach was employed to estimate a 95% CI for the average Length of Stay (LOS) in hospitals across different age groups. Subsequently, we derived 95% CI estimates for the cost of hospitalization in normal wards versus ICUs, stratified by age groups, using the GLM technique. This model predicted the value of the dependent variable (in this case, the cost of hospitalization) based on the values of two or more independent variables (hospitalization type and age group), providing valuable insights into the financial implications of COVID-19-related hospitalizations.
The scope of study was limited to public and/or general hospital settings only, excluding private or other healthcare settings. The analysis was conducted using Microsoft Excel®. All costs were reported in US Dollars (USD) for the year 2022 by applying the exchange rate of USD1 corresponding to the Brazilian Real (BRL) 4.92 as of April 2022.
Results
Clinical characteristics and vaccine uptake of the study population
presents clinical characteristics of the study population, stratified by age group. During the study period of January 1, 2022 to April 30, 2022, a total of 8,160,715 (3.80% of the total study population) COVID-19 confirmed cases were identified, ranging from 2.43% among children aged below 5 years to 62.05% of cases among individuals aged between 19 and 49 years.
Table 1. Clinical characteristics and vaccine uptake of the study population during January 1-April 30 2022.
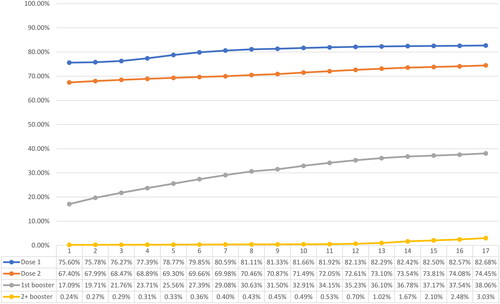
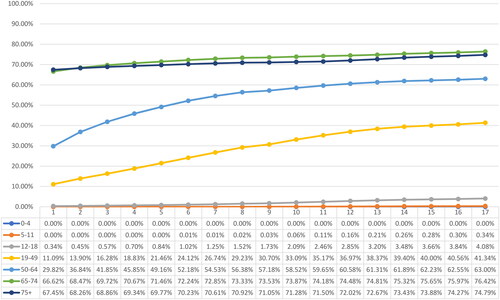
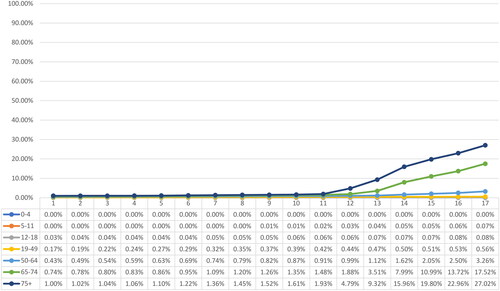
By April 2022, approximately 177.62 million individuals had received partial immunization (Dose 1), 159.94 million were fully immunized (Dose 2), and 81.77 million had received booster dose(s). The overall vaccine uptake for partial and full immunization stood at 82.68% and 74.45%, respectively, while 38.06% of the overall study population had received booster dose(s). A significant disparity was observed in the uptake of vaccinations across age groups. Among children under the age of 5, the uptake of partial or full immunization was less than 0.1%. In contrast, adolescents and individuals aged 19 years and older showed substantially higher uptake rates, surpassing 89.78% for partial immunization and 73.73% for full immunization. Noteworthy, the uptake of booster dose was significantly higher among individuals aged 65 years and above (74.79% and above) compared to the younger population. depict the weekly vaccination uptake for Dose 1, Dose 2 and booster dose(s) by age group.
Figure 3. Weekly vaccination coverage, January 1, 2022 to April 30, 2022 - Dose 1/partial immunization.
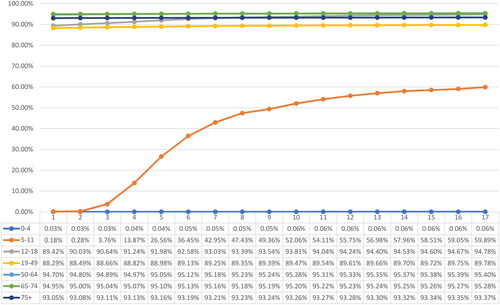
Figure 4. Weekly vaccination coverage, January 1, 2022 to April 30, 2022 - Dose 2/full immunization.
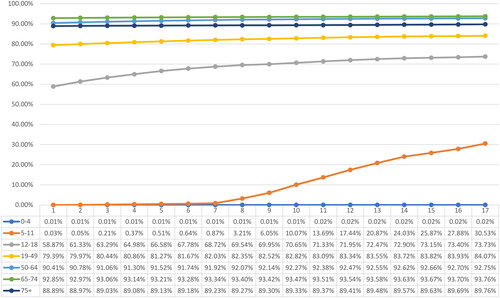
Among the total confirmed COVID-19 cases, regardless of vaccination status, a total of 7,113,685 individuals (87.17%) experienced symptomatic manifestations. Notably, similar rates of symptomatic infections were observed across different age groups.
Healthcare resource utilization (HCRU) among hospitalized individuals with acute COVID-19 infection during January 1, 2022 – April 30, 2022
The HCRU among hospitalized individuals with acute COVID-19 infection is illustrated in . Over the course of the four-month study period, a total of 120,450 (95% CI: 119,123-121,682) cases of acute COVID-19 resulted in hospitalization. The overall hospitalization rate was 1.48%, which varied across age groups, ranging from 0.32% (95% CI: 0.31-0.33%) in 12-18 years, 5.43% (95% CI: 5.38-5.49%) in 65-74 years to 17.89% (95% CI: 17.76-18.01%) among individuals aged 75 years and above. Young children had relatively lower hospitalization rates of 2.33% (95% CI: 2.27-2.38%) among under the age of 5 and 0.99% (95% CI: 0.96-1.02%) among 5-11 years old than adults.
Table 2. Healthcare resource utilization (HCRU) among hospitalized patients with acute COVID-19 infection during January 1-April 30 2022.
Among the hospitalized individuals, a total of 39,226 (32.57%) were admitted to the ICU, of which 31,283 (79.75%) individuals required MV support. This need for MV was consistently observed across the various age groups. On the other hand, 81,224 (67.43%) individuals were admitted to normal wards. The utilization of MV was also significantly high in normal ward, with an overall rate of 50.85%, reaching up to 55.52% (95% CI: 54.83-56.20%) and 61.33% (95% CI: 60.87-61.78%) among the age groups of 65-74 years and ≥ 75 yrs, respectively.
The duration of hospitalization, indicated by length of stay (LOS), varied significantly among different patient groups. Individuals in the ICU receiving MV support experienced significantly longer hospital stays ranging from 14.28 days (95% CI: 14.21-14.35) among those aged ≥ 75 yrs to 17.36 (95% CI: 16.77-17.98) among adolescents aged 12-18 years in contrast to relatively shorter LOS of 13.08 days (ranging from 9.57 days in those aged 12-18 years old to 14.91 days in those aged 50-64 years) among ICU patients not requiring MV. Whereas patients in normal wards, regardless of MV support, had a relatively shorter LOS ranging from 6.07 to 10.48 days.
Healthcare costs associated with acute COVID-19 infection
and present the direct healthcare costs per day associated with acute COVID-19 infection for both normal ward and ICU, with/without MV support, in the Brazilian public/general hospital settings. The average cost per day in a normal ward and ICU without MV support, was $104.36 and $302.81, respectively. The cost per day in ICU without MV support was significantly higher for the adult population (ranging from $298.20 in 19-49 years to $326.50 in ≥75 years) compared to young children ($199.09-201.74). Whereas relatively similar daily costs were observed across different age groups in normal wards without MV support.
Figure 7. Average hospitalization cost per day (USD 2022), normal ward without mechanical ventilation support.
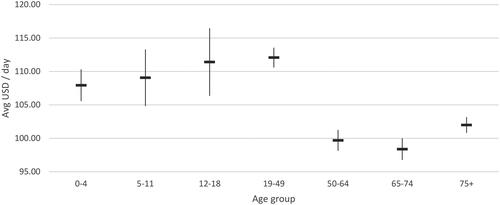
Figure 8. Average hospitalization cost per day (USD 2022), normal ward with mechanical ventilation support.
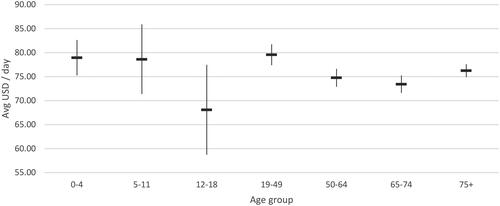
Figure 9. Average hospitalization cost per day (USD 2022), ICU without mechanical ventilation support.
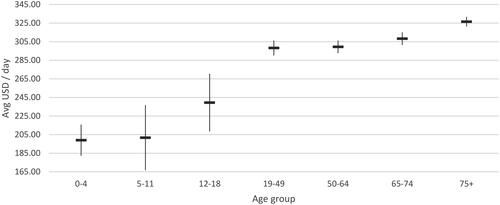
Figure 10. Average hospitalization cost per day (USD 2022), ICU with mechanical ventilation support.
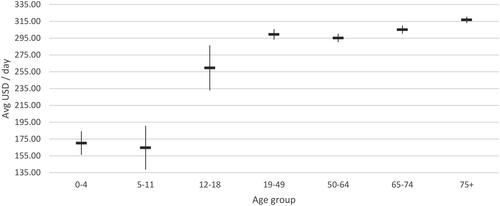
Table 3. Direct healthcare costs associated with acute COVID-19 infection, per day (USD 2022) during January 1-April 30 2022.
The average cost per day in a normal ward and ICU with MV was $75.91 and $301.22 respectively. Similar trends of without MV support were observed in the normal ward and ICU with MV support, wherein cost per day in ICU with MV support was significantly high among adult population ($259.64-316.75) compared to young children ($164.76-170.28) and relatively similar daily cost rates were reported across different age groups in normal wards with MV support.
Discussion
Our study findings indicated a substantial impact of the Omicron variant of COVID-19 on Brazil’s healthcare system, including significant number of cases, high HCRU and corresponding costs. Although the country achieved an impressive overall vaccination rate of 82.68% for partial immunization and 74.45% for full immunization, a majority (87.17%) of confirmed COVID-19 cases during the Omicron predominance were symptomatic. Among children under the age of 5, the uptake of partial or full immunization was less than 0.1%. This low rate was attributed to a delay in initiating primary vaccination for the pediatric population in the country, which started for those aged 3 years and older in 2022, and was extended to include children aged 6 months and older in 2023.Citation4
The uptake of booster dose(s) was significantly low at 38.06% for the overall age group and varied substantially across age groups. Adults aged 19-49 years reported a significantly lower booster rate of 41.34% than those aged 50 years or older (50-64 years: 63.00%; 65-74 years: 76.42%; ≥75 years: 74.79%), despite the Brazilian Ministry of Health recommending booster dose(s) for adults after four months of primary vaccination in November 2021. The recommendations for booster dose(s) were extended to adolescents in May 2022 only.Citation22 Hence, only 4.08% of adolescents were reported to have received a booster dose during the study period.
The overall hospitalization rate exhibited significant variation across different age groups with rates surpassing 17.89% among individuals aged 75 years and above. Despite adopting a conservative approach that solely accounted for inpatient HCRU and associated direct costs in public/general hospital settings, our findings revealed a significant burden of HCRU among individuals with acute COVID-19 requiring hospitalization. These findings strongly challenge the prevailing myths regarding the Omicron variant being mild and/or less severe, with no significant fatalities or healthcare resource utilization.Citation23
To our knowledge, this is the first study to comprehensively analyze clinical characteristics, vaccine uptake, and HCRU and costs associated with acute COVID-19 using nationwide real-world data from Brazil during the Omicron predominance. The quantitative analyses were conducted using a transparent and easily interpretable model framework that was specifically tailored to address the research questions at hand. Additionally, our study had a specific focus on assessing the burden of HCRU among individuals with acute COVID-19 infection requiring hospitalization, providing granular insights into outcome categories stratified by age group and different levels of care, such as normal ward versus ICU, with or without MV support.
As previously published COVID-19 burden of illness studies in Brazil had different scope and study duration, it was difficult to perform a head-to-head comparison of our findings with such published results.Citation9–14 For instance, Cardoso et al. 20239 estimated COVID-19 related hospitalization costs across different pathways, including emergency plus ward, ward plus ICU, emergency plus ICU, and only ICU, during the period of March 2020 to August 2020 in a public academic hospital setting. They reported a median LOS of 9 days [inter quartile range (IQR): 5, 20] in only ICU, without providing stratification by MV support or age group.Citation9 In contrast, our study, conducted during a different study period, reported a LOS of 13.08 days (ranging from 9.57 days in 12-18 years to 14.91 days in 50-64 years) among ICU patients without MV support and a LOS of 15.68 days (ranging from 14.28 days in ≥75 years to 17.36 days in 12-18 years) with MV support. Furthermore, they estimated a daily cost in only ICU at international $726 (IQR: I$704-742).Citation9 Conversely, our study estimated a relatively lower average cost per day in the ICU without MV support at $302.81 (range: $199.09 in 0-4 years to $326.50 in ≥75 years) and at $301.22 (range: $164.76 in 5-11 years to $316.75 in ≥75 years) with MV support. The potential reason for the underestimation of daily costs reported in our study was that the inpatient costs reported in the SIH-SUS government database reflected an overall public health setting, in contrast to the costs reported for an individual academic public hospital by Cardoso et al. 2023.
Another study by Oliveira et al. 2023 [10] estimated cost of illness among patients with COVID-19 in three Brazilian public hospitals during a study period of March 2020 to September 2020. They estimated the median direct medical costs per admission for COVID-19 in normal wards at $1,188.8 (IQR: $582.9-2,595.9), while in the ICU, the costs amounted to $3,213.9 (IQR: $1,566.1-6,653.5). They also estimated costs by stratifying LOS into different ranges, from 0 days to 60-69 days, which varied substantially, ranging from $684.5 to $27,768.2 in normal wards and from $768.7 to $36,430.0 in the ICU.Citation10 Due to the differences in approach, study duration and reported outcomes between the two studies, a direct comparison was not feasible.
However, when applying the minimum and maximum ranges of LOS across age groups to the daily costs of all ages in our study, the estimated costs in normal wards with and without MV support would range from $519.22 to $757.58 and from $633.46 to $1,093.69, respectively. On the other hand, our estimated costs for ICU with and without MV support would fall within the range of $4,301.42 to $5,229.18 and $2,897.89 to $4,514.90, respectively. Notably, our estimated ICU ranges aligned relatively well with their average rates, while our estimates for normal wards were relatively closer to the lower bounds when compared with their respective normal ward estimates. This disparity may be attributed to variations in study duration, differences in cost estimation methodologies, as well as the relatively lower daily costs observed for normal wards with MV support compared to those without MV support in our study. These findings underscore the importance of conducting further investigations into the reported costs and rates within public/general hospital settings.
Although our study provided valuable insights for public health decision-making, the findings of this study should, however, be interpreted within the context of pertaining limitations.
This study was restricted to publicly available COVID-19 data at the time of the study when Omicron variant was predominant in Brazil, and solely captured the epidemiology and HCRU related data for a span of four months, from January 1, 2022, to April 30, 2022. Hence, the study findings may not be generalizable to other COID-19 variants, time periods or countries.
Additionally, it relied exclusively on publicly available data, which are subject to different biases and errors, and hence may undermine the reliability and validity of the study findings. However, these Brazilian public health and national surveillance databases are well-established and have been widely used in other nation-wide retrospective studies for various infectious diseases including Zika virus, tuberculosis, and COVID-19.Citation24–27
Furthermore, our assessment was confined to a limited set of clinical characteristics, encompassing confirmed COVID-19 cases, symptomatic instances, and vaccine uptake within the study population, categorized by age groups. The databases lacked COVID-19-specific demographic attributes such as gender, race, employment and/or income status, as well as other clinical characteristics including comorbidities and medication usage. This limitation hindered a comprehensive assessment of the clinical burden experienced by the study population. The scope of study was confined to the context of public and/or general hospital settings, given the significant reliance of approximately 80% of the Brazilian population on these facilities.Citation28 Hence, the study utilized HCRU data and the corresponding daily cost values derived from the SIH-SUS and SIA-SUS databases. Nonetheless, the analysis did not encompass the consideration of HCRU and expenditures incurred in private hospitals and other healthcare environments, which typically exhibit higher costs and charges when compared to public and general hospitals. Furthermore, given the substantial variations observed in the LOS and daily costs across normal ward and ICUs, both with and without MV support, the study refrained from calculating the average cost burden per patient or offering a holistic burden assessment for the study cohort. Instead, the study’s approach was centered around detailing LOS and daily cost rates along with 95% CI within wards across various age groups.
It is worth mentioning that this study focused solely on inpatient HCRU and associated per day costs. Consequently, healthcare costs incurred in outpatient settings, ambulatory services, pharmacy fillings, and indirect costs such as lost wages and work productivity, caregiver burden etc. were beyond the scope of this study and were not taken into consideration. As a result, the study findings may have underestimated the true healthcare burden attributable to COVID-19 in Brazil during the period of Omicron predominance. Further research, expanding the overall healthcare utilization and estimating direct and indirect costs over an extended study period, is warranted to accurately quantify the burden of COVID-19 Omicron variant in the country.
Conclusions
This study quantified the burden of COVID-19 in terms of symptomatic cases, HCRU and associated direct costs related to acute cases of COVID-19 requiring hospitalization when the Omicron was predominant in Brazil. These results serve to provide insights for future researchers into HCRU and associated costs within the context of the Brazilian public health setting. Our study also examined the vaccine uptake for both primary and booster series among various age groups, which indicated that despite achieving a commendable vaccine uptake nationwide, most of the confirmed COVID-19 cases during the Omicron predominant period were symptomatic. The findings highlight the significant demand for healthcare resources required to effectively manage the COVID-19 pandemic and underscore the critical need to prioritize and enhance booster immunization efforts for the eligible population.
Transparency
Author contributions
All authors contributed to the study conception, design, data acquisition, analysis, interpretation, and drafting and revising the manuscript.
Authorship
All named authors meet the International Committee of Medical Journal Editors (ICMJE) criteria for authorship for this article, take responsibility for the integrity of the work as a whole, and have given their approval for this version to be published.
Acknowledgements
None stated.
Declaration of financial/other relationships
JY, MHK, AD, TS, PH, and JRS are employees of Pfizer and may hold stock or stock options. GC and ML are employees of Analytix, which was a paid consultant to Pfizer in connection with the development of this study. SV is an employee of HealthEcon Consulting, Inc. and an external consultant for Pfizer who has received consulting fees from Pfizer in connection with the development of this manuscript.
Additional information
Funding
References
- World Health Organization: Brazil COVID-19 cases and deaths. 2023. [cited 2023 Apr 26]. Available from: https://covid19.who.int/region/amro/country/br.
- ECLAC. Preliminary overview of the economies of Latin America and the Caribbean. 2021.
- Sott MK, Bender MS, da Silva Baum K. Covid-19 outbreak in Brazil: health, social, political, and economic implications. Int J Health Serv. 2022;52(4):442–454. doi: 10.1177/00207314221122658.
- Spinardi J, Dantas AC, Carballo C, et al. Narrative review of the evolution of COVID-19 vaccination recommendations in countries in Latin America, Africa and the Middle East, and Asia. Infect Dis Ther. 2023;12(5):1237–1264. doi: 10.1007/s40121-023-00804-2.
- Henrique ALB. COVID-19 vaccination in Brazil is a success, despite the failure of the federal government 2022. Available from: https://oneill.law.georgetown.edu/covid-19-vaccination-in-brazil-is-a-success-despite-the-failure-of-the-federal-government/.
- Martins-Filho PR, Marques RS, Tavares CSS, et al. The role of vaccination in preventing deaths during the omicron-driven tsunami in Brazil. Disaster Med Public Health Prep. 2022:1–2. doi: 10.1017/dmp.2022.146.
- Taylor L. Covid-19: Brazil sees omicron cases soar but data blackout obscures true impact. BMJ. 2022;376:o133. doi: 10.1136/bmj.o133.
- Adamoski D, Baura VA, Rodrigues AC, et al. SARS-CoV-2 delta and omicron variants surge in Curitiba, Southern Brazil, and its impact on overall COVID-19 lethality. Viruses. 2022;14(4):809. doi: 10.3390/v14040809.
- Cardoso RB, Marcolino MAZ, Marcolino MS, et al. Comparison of COVID-19 hospitalization costs across care pathways: a patient-level time-driven activity-based costing analysis in a Brazilian hospital. BMC Health Serv Res. 2023;23(1):198. doi: 10.1186/s12913-023-09049-8.
- Oliveira LA, Lucchetta RC, Mendes AM, et al. Cost of illness in patients with COVID-19 admitted in three Brazilian public hospitals. Value Health Reg Issues. 2023;36:34–43. doi: 10.1016/j.vhri.2023.02.004.
- Beck da Silva Etges AP, Bertoglio Cardoso R, Marcolino MS, et al. The economic impact of COVID-19 treatment at a hospital-level: investment and financial registers of Brazilian hospitals. J Health Econ Outcomes Res. 2021;8(1):36–41. doi: 10.36469/jheor.2021.22066.
- Miethke-Morais A, Cassenote A, Piva H, et al. COVID-19-related hospital cost-outcome analysis: the impact of clinical and demographic factors. Braz J Infect Dis. 2021;25(4):101609. doi: 10.1016/j.bjid.2021.101609.
- Nascimento I, Oliveira ALM, Diniz PHC, et al. Hospitalization, mortality and public healthcare expenditure in Brazil during the COVID-19 crisis: vulnerabilities in the spotlight. Sao Paulo Med J. 2022;140(2):290–296. doi: 10.1590/1516-3180.2021.0496.23072021.
- Sousa FF, Vieira BB, Reis ADC. Cost analysis of hospitalization for COVID-19 in a Brazilian public teaching hospital. Value Health Reg Issues. 2023;34:48–54. doi: 10.1016/j.vhri.2022.10.006.
- The Brazilian Unified Health System “Sistema Único de Saúde”. [cited 2023 Apr 10]. Available from: https://www.saude.mg.gov.br/sus.
- SUS Hospital Information System – SIH/SUS. [cited 2023 Apr 20]. Available from: https://ces.ibge.gov.br/base-de-dados/metadados/ministerio-da-saude/sistema-de-informacoes-hospitalares-do-sus-sih-sus.html.
- SUS Ambulatory Information System – SIA/SUS. [cited 2023 Apr 20]. Available from: https://ces.ibge.gov.br/base-de-dados/metadados/ministerio-da-saude/sistema-de-informacoes-ambulatoriais-do-sus-sia-sus.html.
- The Síndrome Respiratória Aguda Grave (SRAG). [cited 2023 Apr 20]. Available from: https://infoms.saude.gov.br/extensions/covid-19_html/covid-19_html.html.
- COVID-19 cases 2023. [cited 2023 Apr 20]. Available from: https://covid.saude.gov.br/.
- Brazilian Institute of Geography and Statistics (IBGE). [cited 2023 Apr 24]. Available from: https://www.ibge.gov.br/estatisticas/sociais/populacao.html.
- Fiocruz Genomic Network. [cited 2023 Apr 24]. Available from: https://www.genomahcov.fiocruz.br/en/.
- Ministério da Saúde amplia dose de reforço contra a Covid-19 para adolescentes. [cited 2023 May 27]. Available from: https://www.gov.br/saude/pt-br/assuntos/noticias/2022/maio/ministerio-da-saude-amplia-dose-de-reforco-contra-a-covid-19-para-adolescentes.
- World Health Organization. The Omicron variant: sorting fact from myth 2022. [cited 2023 May 1]. Available from: https://www.who.int/europe/news/item/19-01-2022-the-omicron-variant-sorting-fact-from-myth.
- Bigoni A, Malik AM, Tasca R, et al. Brazil’s health system functionality amidst of the COVID-19 pandemic: an analysis of resilience. Lancet Reg Health Am. 2022;10:100222. doi: 10.1016/j.lana.2022.100222.
- de Oliveira WK, de França GVA, Carmo EH, et al. Infection-related microcephaly after the 2015 and 2016 zika virus outbreaks in Brazil: a surveillance-based analysis. Lancet. 2017;390(10097):861–870. doi: 10.1016/S0140-6736(17)31368-5.
- Chenciner L, Annerstedt KS, Pescarini JM, et al. Social and health factors associated with unfavourable treatment outcome in adolescents and young adults with tuberculosis in Brazil: a national retrospective cohort study. Lancet Glob Health. 2021;9(10):e1380–e90. doi: 10.1016/S2214-109X(21)00300-4.
- Bastos LSL, Aguilar S, Rache B, et al. Primary healthcare protects vulnerable populations from inequity in COVID-19 vaccination: an ecological analysis of nationwide data from Brazil. Lancet Reg Health Am. 2022;14:100335. doi: 10.1016/j.lana.2022.100335.
- Mnistry of Health Brazil. Largest public health system in the world, SUS completes 31 years 2022. [cited 2023 Aug 20]. Available from: https://www.gov.br/saude/pt-br/assuntos/noticias/2021/setembro/maior-sistema-publico-de-saude-do-mundo-sus-completa-31-anos.