Abstract
Aims
Left atrial appendage closure (LAAC) has been demonstrated to be cost-saving relative to oral anticoagulants for stroke prophylaxis in patients with non-valvular atrial fibrillation (NVAF) in the United States and Europe. This study assessed the cost-effectiveness of LAAC with the Watchman device relative to warfarin and direct oral anticoagulants (DOACs) for stroke risk reduction in NVAF from a Japanese public healthcare payer perspective.
Methods
A Markov model was developed with 70-year-old patients using a lifetime time horizon. LAAC clinical inputs were from pooled, 5-year PROTECT AF and PREVAIL trials; warfarin and DOAC inputs were from published meta-analyses. Baseline stroke and bleeding risks were from the SALUTE trial on LAAC. Cost inputs were from the Japanese Medical Data Vision database. Probabilistic and one-way sensitivity analyses were performed.
Results
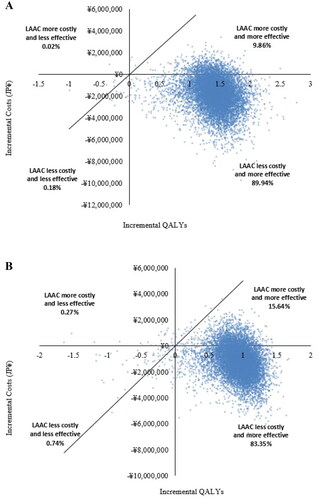
Over the lifetime time horizon, LAAC was less costly than warfarin (savings of JPY 1,878,335, equivalent to US $17,600) and DOACs (savings of JPY 1,198,096, equivalent to US $11,226). LAAC also provided 1.500 more incremental quality-adjusted life years (QALYs) than warfarin and 0.996 more than DOACs. In probabilistic sensitivity analysis, LAAC was cost-effective relative to warfarin and DOACs in 99.98% and 99.73% of simulations, respectively. LAAC dominated (had higher cumulative QALYs and was less costly than) warfarin and DOACs in 89.94% and 83.35% of simulations, respectively.
Conclusions
Over a lifetime time horizon, LAAC is cost-saving relative to warfarin and DOACs for stroke risk reduction in NVAF patients in Japan and is associated with improved quality-of-life.
PLAIN LANGUAGE SUMMARY
This study examined the cost-effectiveness of left atrial appendage closure (LAAC) compared to oral anticoagulants for stroke risk reduction among individuals with a specific type of irregular heart rhythm called non-valvular atrial fibrillation (NVAF). This study evaluated the cost-effectiveness of LAAC using the Watchman device in comparison to warfarin and direct oral anticoagulants (DOACs) from the perspective of Japan’s public healthcare system. To investigate this, a computer-based model was developed involving 70-year-old patients over their lifetime. Data from notable studies such as the PROTECT AF and PREVAIL trials (covering 5 years) for LAAC and published meta-analyses for warfarin and DOACs were incorporated into the model. Baseline stroke and bleeding risks were derived from the SALUTE trial on LAAC. Cost inputs were based on data from the Japanese Medical Data Vision database. Additionally, we performed thorough cost-effectiveness analyses, including probabilistic and one-way sensitivity assessments. Our findings revealed that, over a lifetime, LAAC was more cost-effective than both warfarin and DOACs. Further, LAAC contributed an additional 1.500 quality-adjusted life years (QALYs) compared to warfarin and 0.996 QALYs compared to DOACs. In the long-term, adopting LAAC as an alternative to warfarin and DOACs is a cost-effective strategy for reducing stroke risk in NVAF patients in Japan. Moreover, it is associated with enhanced quality-of-life. These findings hold significant implications for informing decision-making in healthcare policies and clinical practices for NVAF patients.
Introduction
Atrial fibrillation (AF) is the most common cardiac arrhythmia, affecting more than 12 million people in the Asia-Pacific regionCitation1. In Japan, the number of patients with AF is expected to exceed 1 million by 2050Citation2. AF is associated with a five-fold increase in the risk of strokeCitation3. In particular, patients with non-valvular AF (NVAF) have an annual ischemic stroke risk of 5%, which is 2–7-times higher than those without NVAFCitation3. AF and AF-related stroke place a significant economic burden on global healthcare systems. In Japan, annual medical and long-term care costs attributed to AF are estimated to be between JPY 137.5 and 191.4 billionCitation4. The cost of acute hospital care for ischemic stroke in Japan is over JPY 1 million per patientCitation5. Additional costs attributable to long-term care and productivity losses among patients with ischemic stroke are also substantialCitation6.
Oral anticoagulants (OACs), such as warfarin or direct oral anticoagulants (DOACs), are pharmacological treatments for stroke prevention in patients with AFCitation7–9. Despite their effectiveness, OACs are associated with an increased risk of bleeding and high rates of patient non-adherenceCitation7–9. Percutaneous left atrial appendage closure (LAAC) is a device-based alternative to OACs for patients with NVAF who are at risk for stroke and systemic embolism. The WATCHMAN LAAC device (Boston Scientific Corporation, Marlborough, MA, USA) has been approved for use in many countries, including Japan. A pooled analysis of 5-year outcomes from two pivotal, randomized controlled clinical trials (RCTs): PROTECT AF (Watchman Left Atrial Appendage System for Embolic Protection in Patients with Atrial Fibrillation) and PREVAIL (Prospective Randomized Evaluation of the Watchman Left Atrial Appendage Closure Device in Patients with Atrial Fibrillation Versus Long-Term Warfarin), has demonstrated that LAAC with the Watchman device provides stroke risk reduction comparable to warfarin with additional reductions in major bleeding and mortalityCitation10. Previous studies have demonstrated the cost-effectiveness of LAAC with the Watchman device in the United States and EuropeCitation11–15. However, the cost-effectiveness of LAAC from the perspective of Japan’s healthcare system has not yet been examined. Therefore, this study aimed to evaluate the cost-effectiveness of LAAC with Watchman relative to warfarin and DOACs for stroke risk reduction in patients with NVAF from the perspective of the public healthcare payer in Japan.
Methods
This study was conducted in accordance with the Central Social Insurance Medical Council’s (CSIMC) guidelines on cost-effectiveness evaluationCitation16. A Markov model was developed using Microsoft Excel (Redmond, WA, USA) to assess the cost-effectiveness of LAAC with Watchman compared to warfarin or DOACs in NVAF patients from the perspective of the Japanese public healthcare payer. Patients were assumed to be 70 years of age with a mean CHADS2 score of 2.5 (baseline stroke risk of 2.5%) and a mean HAS-BLED score of 2.9 (baseline bleeding risk of 4.1%)Citation17,Citation18. The model was constructed with a lifetime (30-year) time horizon and a 3-month cycle length, based on the recommendation of Japanese health technology assessment guidelinesCitation19. Cost-effectiveness was reported as the incremental cost-effectiveness ratio (ICER) and evaluated using a willingness-to-pay threshold of JPY 5 million per quality-adjusted life-year (QALY) gained, as the use of QALY is recommended by the CSIMC guidelinesCitation16. An annual discount rate of 2% was applied to both cost and effectiveness measuresCitation16.
Patients were assigned to one of three treatment strategies upon entering the model: LAAC, warfarin, or DOACs as a class of therapy (). In the LAAC arm, patients could experience one-time, procedure-related complications, which include procedural stroke caused by air embolism (0.82%), major bleeding (0.55%), pericardial effusion (3.69%), and device embolization (0.68%)Citation20. Of the patients included in this study, 92.5% were successfully treated with LAACCitation10,Citation20. Following a successful LAAC implant, patients were assumed to receive warfarin for 45 days after the procedure, aspirin and clopidogrel from day 45 to 6 months, and aspirin thereafter. Patients who experienced an unsuccessful LAAC implant were assumed to be treated with DOACs. Patients who were not successfully implanted incurred the cost of the LAAC procedure as well as the cost of continued medications. Patients receiving warfarin or DOACs could discontinue therapy due to incidental bleeding or other non-clinical reasons.
Figure 1. Model structure for patients with NVAF treated with LAAC, warfarin, or DOACs. Abbreviations: LAAC, left atrial appendage closure; DOAC, direct oral anticoagulant.
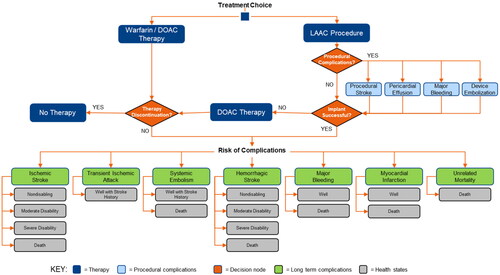
At each model cycle, we assumed that all living patients faced the risk of experiencing a major clinical event, including ischemic stroke, transient ischemic attack (TIA), systemic embolism, hemorrhagic stroke, major bleeding, myocardial infarction, and death. A previous stroke could lead to an increased risk of deathCitation21, and TIA or systemic embolism could increase the risk of subsequent stroke. The risk of stroke also increased with age. Patients who survived an ischemic or hemorrhagic stroke could experience one of three post-stroke disability categories: non-disabling, moderately disabling, or severely disabling. All events, except TIA, were assumed to have a risk of death.
All clinical inputs used in the analysis are specified in . LAAC procedural complications and post-procedural major event rates were extracted from the pooled PROTECT AF and PREVAIL RCTs at 5-years follow-upCitation10,Citation15. The input data on warfarin and DOACs were derived from published meta-analyses of clinical trialsCitation22,Citation26. The event probabilities were extrapolated over the lifetime horizon for each of the treatment strategies. The transition probabilities were calculated by multiplying the baseline risk by the relative risk (RR) for each event. Mortality rates for unrelated causes were retrieved from Japan life tablesCitation34.
Table 1. Clinical inputs used in the model.
The baseline risks of stroke and bleeding were estimated using data from the SALUTE clinical trial of LAAC with the Watchman device for Japanese patients with NVAFCitation17. This clinical trial was chosen to inform the baseline characteristics of patients in our model due to the study’s location and Japanese NVAF patient population. CHADS2 (congestive heart failure, hypertension, age ≥ 75 years, diabetes mellitus, previous stroke or TIA) and HAS-BLED (hypertension, abnormal renal/liver function, stroke, bleeding history or predisposition, labile international normalized ratio, age > 65 years, drugs/alcohol concomitantly) scores were used in estimating the risk of stroke and bleeding, respectively. Rates of ischemic and hemorrhagic stroke increased with age by 1.4- and 1.97-fold per decade, respectivelyCitation35,Citation36. The event of either a TIA or systemic embolism increased the likelihood of having a second ischemic event 2.6-foldCitation36.
In the model, patient quality-of-life (QoL) was measured by health utility, with values ranging between 0 and 1, with 0 representing death and 1 representing perfect health. The utility values for well with warfarin, well with DOACs, and well with LAAC were retrieved from published literature ()Citation37–42. Baseline utilities were calculated by multiplying utility weights for all well-based health states by an underlying baseline utility of 0.84, representing the QoL of the Japanese population at 70 years of ageCitation37.
Table 2. Health state utilities used in the model.
Stroke severity was reported using the modified Rankin Scale (MRS) score and classified into three categories: non-disabling (MRS 0–2), moderately disabling (MRS 3), and severely disabling (MRS 4–5). The health utility values for each category of stroke severity were drawn from a study on stroke patients in JapanCitation38. Additionally, we assumed that undergoing the LAAC procedure would have a short-term negative impact on QoL; thus, a disutility value of −0.0315 was applied for 2 weeks post-procedureCitation39. Acute clinical events could also affect patient QoL and, therefore, disutilities were applied for stroke, major bleeding, systemic embolism, TIA, and myocardial infarction ().
The model incorporated therapy costs, costs of relevant clinical events, and costs of long-term care following a disabling stroke (). The LAAC costs included the cost of the procedure as well as the costs of procedure-related complications. The costs of warfarin therapy included the pharmaceutical cost and the cost related to international normalized ratio (INR) monitoringCitation45,Citation46. The cost of DOAC therapy was an average cost of the three approved drugs in Japan: dabigatran, rivaroxaban, and apixabanCitation45. Drug doses were based on a previously published prospective registry in JapanCitation47. Clinical event costs were calculated using claims data from the Japanese Medical Data Vision database, which contains medical claims for 16 million people from more than 270 acute hospitals in Japan that participate in the national accounting system for medical costs of inpatient care, called the Japanese Diagnosis Procedure Combination/Per-Diem Payment SystemCitation44. All costs were in 2020 Japanese Yen and converted to US dollars (USD) based on the 2020 average currency exchange rate.
Table 3. Cost inputs used in the model.
Model parameter uncertainty was assessed using one-way sensitivity analysis (OWSA) and probabilistic sensitivity analysis (PSA) at 30 years. In the OWSA, model inputs were varied within the range of 95% confidence intervals (CIs), when available, and by ±20% of the base-case value when the CIs were not publicly available. Additionally, we assumed the success rate of the LAAC procedure could reach an upper limit of 100% based on the findings from the SALUTE trial. The discount rate was varied by 0–4%.
PSA was undertaken to assess the impact of individual parameter uncertainty on model results. A beta distribution was assumed for baseline risk, health state utilities, and the risk of procedural complications. Gamma, log-normal, and Dirichlet distributions were assumed for costs, the RR of each major event, and stroke severity, respectively. Random dispersion was specified using the reported standard error or an assumed standard error of 10% when the standard error was not available in the literature. The PSA followed a standard Monte Carlo approach consisting of 10,000 randomly drawn simulations of parameter values.
Results
Base-case analysis
In the base-case analysis, LAAC patients had more QALYs than warfarin patients by year 2 (1.582 vs 1.577). Such an advantage of the LAAC group continued throughout the lifetime horizon. LAAC became cost-effective with an ICER of JPY 3,867,297/QALY (US $36,236/QALY) at year 8, and dominant (more effective and less costly) at year 13, as compared to warfarin (). These trends continued thereafter over the remainder of the time horizon ().
Table 4. Annual cumulative cost, QALY, and ICER results (JPY/QALY) per patient.
Table 5. Base-case QALY, cost, and ICER results (JPY/QALY) per patient at 30 years.
LAAC patients had more QALYs than DOAC patients from year 4 (3.067 vs 3.056) through year 30 (10.845 vs 9.849). LAAC was cost-effective relative to DOACs at year 10, with an ICER of JPY 3,961,249/QALY (US $37,116/QALY), and less costly by year 15 (). LAAC became dominant relative to DOACs by year 15 and remained so throughout the rest of the modeled time horizon ().
OWSA: 15 most impactful variables to ICER estimate
The OWSA results are shown in the form of a tornado diagram indicating the expected ICER range for LAAC versus warfarin () and for LAAC versus DOACs (). For each case, the 15 most impactful variables to the ICER estimate at 30 years are depicted in descending order of influence.
Figure 2. Tornado diagrams of one-way sensitivity analyses at 30 years for (a) LAAC versus warfarin and (b) LAAC versus DOACs. Abbreviations: DOAC, direct oral anticoagulant; LAAC, left atrial appendage closure; MRS, modified Rankin Scale score; OAC, oral anticoagulant; QoL, quality-of-life; RR, relative risk.
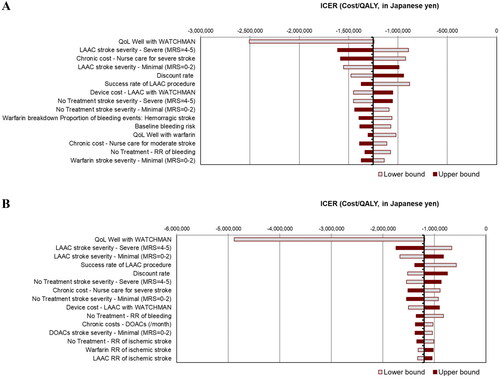
Over the lifetime horizon, the OWSA of LAAC versus warfarin showed that model results were most sensitive to variations in the health utility value used for well with LAAC. Other impactful variables included the percentage of LAAC patients who experienced a severely disabling stroke and the long-term care costs for these patients. Similarly, OWSA demonstrated that the same variables were most impactful when comparing LAAC with DOACs. Although the variations of ICER are, more or less, brought by the variable uncertainty, we can interpret the cost-effectiveness of LAAC versus warfarin or DOACs to be quite robust since all the ICER estimates remained dominant, thus negative values, when the input parameters were varied within the prespecified ranges.
PSA results
The scatterplots constructed from PSA simulations demonstrated that LAAC was cost-effective relative to DOACs and warfarin over a lifetime time horizon in 99.73% and 99.98% of simulations, respectively, using a willingness-to-pay threshold of JPY 5 million per QALY gained (). LAAC dominated (had higher cumulative QALYs and lower costs than) DOACs and warfarin in 83.35% and 89.94% of simulations, respectively.
Discussion
To the best of our knowledge, this is the first study from the perspective of the public healthcare payer in Japan to evaluate the cost-effectiveness of LAAC therapy with the Watchman device relative to warfarin and DOACs for patients with NVAF. Using 5-year, pooled data from the PROTECT and PREVAIL RCTs and data from the SALUTE trial, our results indicate that LAAC with the Watchman device is a cost-effective and dominant (more effective and less costly) treatment strategy relative to both warfarin and DOACs over a lifetime horizon. LAAC was associated with lower overall costs compared to warfarin and DOACs in years 13 and 15, respectively, with cost savings accrued thereafter. Additionally, LAAC patients had higher QoL scores and experienced fewer strokes leading to disability than patients treated with warfarin or DOACs.
Sensitivity analyses suggested the results of the base case analysis were robust and relatively insensitive to variation in individual model parameters at 30 years. Using the CSIMC’s recommended willingness-to-pay threshold of JPY 5 million per QALY, LAAC was cost-effective relative to warfarin in 99.98% and dominated in 89.94% of total simulations. Relative to DOACs, LAAC was cost-effective and dominated in 99.73% and 83.35% of total simulations, respectively.
Some previously published studies have explored the economic value of LAAC with the Watchman device compared with warfarin and DOACs in different patient populations and/or healthcare systemsCitation11–15. In general, the results of the current study are consistent with these previously published findings. Such consistency might be the result of the similarity of the modeling structure between this study and previously published studies that were analyzed from a US payer perspectiveCitation11–15. Our model, however, was updated to reflect Japanese clinical practice by considering DOACs to be the standard of care for patients who experienced an unsuccessful LAAC implant.
The AF treatment guidelines from the Japanese Circulation Society recommend the use of warfarin and DOACs for stroke prophylaxis in patients with NVAFCitation7. However, a recently published study reported that many Japanese patients receiving warfarin may have inadequate prothrombin time–international normalized ratio (PT-INR) monitoring and control, which could lead to poor adherence and an increased risk of strokeCitation49. Although patients receiving DOACs do not require regular PT-INR monitoring, long-term adherence remains an issue to be further investigatedCitation30. Further, both warfarin and DOACs are associated with an increased risk of bleeding, which can lead to increased morbidity and mortality. The aging population in Japan is especially prone to increased risk of bleeding, lower body weight, and abnormal renal function, all of which represent additional burdens and challenges for physicians treating NVAF patients in JapanCitation17.
LAAC with the Watchman device is a one-time procedure aimed at providing NVAF patients with lifelong stroke risk reduction. It is not subject to the issue of patient adherence, offering an alternative for patients deemed unsuitable for long-term oral anticoagulation. The SALUTE trial achieved similar results to the large-scale, long-term RCTs, confirming the safety and efficacy of the Watchman device in Japanese NVAF patientsCitation17.
According to the International Monetary Fund, Japan’s population is aging and shrinkingCitation50. The Japanese government projects that by 2060, there will be nearly one elderly person for each person of working age. As healthcare budgets come under increasing pressure due to aging populations, economic evaluations such as this cost-effectiveness analysis of LAAC will provide the necessary evidence to help decision-makers determine which treatments can provide good value for money.
Limitations of this model include the fact that the Markov model was based on clinical inputs derived from published clinical trials and meta-analyses, which may differ from clinical outcomes achieved in the real world, including treatment administration, patient adherence, and procedural complication rates. A discrepancy between RCTs and real-world practice raises concerns about the generalizability of the modeling results. For example, it is likely that patients have substantially better adherence to medical therapy in clinical trials than in a real-world setting of care. However, given the clinical inputs in this model were from clinical trials and meta-analyses, the findings are conservative and likely disfavor LAAC by overestimating the real-world effectiveness of warfarin and NOACs. A lack of direct treatment comparison is another issue to be considered. In this study, LAAC patients were compared to DOAC patients through indirect comparison methods since data from head-to-head RCTs comparing LAAC and DOACs do not yet exist. Further, a lack of local clinical evidence collected from patients in Asia is a limitation to be considered. While data from the WASP registry of LAAC with the Watchman device in Asian patients is available, the pivotal RCTs used in this study are preferred for a couple of reasonsCitation51. RCTs are highly controlled and hold a higher standard than registry studies. Further, the WASP registry only had one treatment arm, therefore contributing to potential selection bias if we had adopted the single-arm WASP registry results using comparison groups from the RCTs. Although the aforementioned limitations exist, we believe using data from multiple published clinical trials allowed us to capture a comprehensive overview of the relevant population for our study, even though some of the individual trials might have limited sample sizes.
The validity of imputation is another issue of concern. Although clinical inputs were derived from studies with different lengths of follow-up, all were extrapolated over the lifetime horizon. Another limitation to consider is the appropriateness of the assumption that, following a successful LAAC procedure, all patients would continue to be successfully treated for the rest of their lives. This might be slightly too optimistic, and more pessimistic scenario analyses remain for further investigation. Finally, the cycle length of the model was 3 months and the model allowed only one clinical event per cycle, which means that only one clinical event could occur every 3 months for patients in the model. In reality, however, AF patients may experience more than one clinical event every 3 months. Hence a question remains as to whether we should make the model more complex to reflect the real world more precisely. However, it should be noted that this model is an adaptation of a previously published model that assumed the same cycle length based on the published clinical trial data.
Conclusions
Consistent with the results of previous CEAs conducted in the US and EuropeCitation11–15, this study demonstrated that LAAC with the Watchman device was cost-effective and cost-saving from a Japanese public healthcare payer perspective relative to warfarin and DOACs for stroke risk reduction in NVAF. LAAC with the Watchman device may represent an opportunity for long-term savings to the healthcare system in Japan. These findings should be taken into consideration by local payers and clinical societies when formulating policies and updating practice guidelines.
Transparency
Author contributions
All authors were involved in the conception and design of this study, or analysis and interpretation of the data; drafting of the paper or revising it critically for intellectual content; and final approval of the version to be published. All authors agree to be accountable for all aspects of the work.
Reviewer disclosures
A reviewer on this manuscript has disclosed that they retired from Pfizer 15 years ago and during this time they worked on outcomes studies with apixaban. They also actively participate in several projects with colleagues in Japan for several drug products. It was this experience that enabled them to review.
The other peer reviewers on this manuscript have no other relevant financial relationships or otherwise to disclose.
IRB information
Since this study does not involve human participants, neither institutional review board approval nor participant consent was required.
Acknowledgements
The authors wish to acknowledge the contributions of Sato Takahiro as a reviewer of this manuscript.
Declaration of funding
This study was funded by Boston Scientific and Boston Scientific Japan.
Declaration of financial/other relationships
At the time of writing, YZ and SLA were full-time employees of Boston Scientific and MS was a full-time employee of Boston Scientific Japan. At the time of writing, MBG was a full-time employee of Ipsos Healthcare, a consulting firm that received fees from Boston Scientific. AMM is a full-time employee of Boston Scientific and Virginia Priest is a full-time employee of Boston Scientific Asia Pacific. SI and HS are full-time employees of CRECON Medical Assessment, a consulting firm that received fees from Boston Scientific. VYR has received consulting fees from Boston Scientific. IK, HH, KI, MY, DRH, and RLA have no relevant disclosures to report.
References
- Rahman F, Kwan GF, Benjamin EJ. Global epidemiology of atrial fibrillation. Nat Rev Cardiol. 2014;11(11):639–654. doi: 10.1038/nrcardio.2014.118.
- Inoue H, Fujiki A, Origasa H, et al. Prevalence of atrial fibrillation in the general population of Japan: an analysis based on periodic health examination. Int J Cardiol. 2009;137(2):102–107. doi: 10.1016/j.ijcard.2008.06.029.
- Fuster V, Rydén LE, Cannom DS, et al. American college of cardiology; American heart association task force on practice guidelines; European society of cardiology committee for practice guidelines; writing committee to revise the 2001 guidelines for the management of patients with atrial fibrillation. ACC/AHA/ESC 2006 guidelines for the management of patients with atrial fibrillation–executive summary: a report of the American college of cardiology/American heart association task force on practice guidelines and the European society of cardiology committee for practice guidelines (writing committee to revise the 2001 guidelines for the management of patients with atrial fibrillation). J Am Coll Cardiol. 2006;48(4):854–906. doi: 10.1016/j.jacc.2006.07.009.
- Kamae I, Kitamura A, Sakurai M, et al. Economic burden of atrial fibrillation in Japan. Value Health. 2018;21(Suppl 2):S70. doi: 10.1016/j.jval.2018.07.530.
- Yoneda Y, Uehara T, Yamasaki H, et al. Hospital-based study of the care and cost of acute ischemic stroke in Japan. Stroke. 2003;34(3):718–724. doi: 10.1161/01.STR.0000056171.55342.FF.
- Hattori N, Hirayama T, Katayama Y. Medical care for chronic-phase stroke in Japan. Neurol Med Chir. 2012;52(4):175–180. doi: 10.2176/nmc.52.175.
- Group JJW. Guidelines for pharmacotherapy of atrial fibrillation (JCS2013). Circ J. 2014;78(8):1997–2021.
- Ponikowski P, Voors AA, Anker SD, et al. 2016 ESC guidelines for the diagnosis and treatment of acute and chronic heart failure: the task force for the diagnosis and treatment of acute and chronic heart failure of the European society of cardiology (ESC). developed with the special contribution of the heart failure association (HFA) of the ESC. Eur Heart J. 2016;37(27):2129–2200. doi: 10.1093/eurheartj/ehw128.
- January CT, Wann LS, Calkins H, et al. AHA/ACC/HRS focused update of the 2014 AHA/ACC/HRS guideline for the management of patients with atrial fibrillation: a report of the American college of cardiology/American heart association task force on clinical practice guidelines and the heart rhythm society. J Am Coll Cardiol. 2019;74(1):104–132. 2019 doi: 10.1016/j.jacc.2019.01.011.
- Reddy VY, Doshi SK, Kar S, et al. 5-Year outcomes after left atrial appendage closure: from the PREVAIL and PROTECT AF trials. J Am Coll Cardiol. 2017;70(24):2964–2975. doi: 10.1016/j.jacc.2017.10.021.
- Reddy VY, Akehurst RL, Armstrong SO, et al. Time to cost-effectiveness following stroke reduction strategies in AF: warfarin versus NOACs versus LAA closure. J Am Coll Cardiol. 2015;66(24):2728–2739. doi: 10.1016/j.jacc.2015.09.084.
- Reddy VY, Akehurst RL, Armstrong SO, et al. Cost effectiveness of left atrial appendage closure with the Watchman device for atrial fibrillation patients with absolute contraindications to warfarin. Europace. 2016;18(7):979–986. doi: 10.1093/europace/euv412.
- Reddy VY, Akehurst RL, Amorosi SL, et al. Cost-effectiveness of left atrial appendage closure with the Watchman device compared with warfarin or non-vitamin K antagonist oral anticoagulants for secondary prevention in nonvalvular atrial fibrillation. Stroke. 2018;49(6):1464–1470. doi: 10.1161/STROKEAHA.117.018825.
- Freeman JV, Hutton DW, Barnes GD, et al. Cost-effectiveness of percutaneous closure of the left atrial appendage in atrial fibrillation based on results from PROTECT AF versus PREVAIL. Circ Arrhythm Electrophysiol. 2016;9(6):e003407.
- Reddy VY, Akehurst RL, Gavaghan MB, et al. Cost-effectiveness of left atrial appendage closure for stroke reduction in atrial fibrillation: analysis of pooled, 5-year, long-term data. J Am Heart Assoc. 2019;8(13):e011577.
- Health Labour Sciences Research Grant (Policy Integrated Science Research Project). “Evaluation Methods for Policy Application of Medical Economic Evaluation and Standardization of Data and Construction of the System” (Research Representative: Takashi Fukuda). The analysis guideline for cost-effectiveness evaluation by the Central Social Insurance Medical Council. 2015. https://www.mhlw.go.jp/file/05-Shingikai-12404000-Hokenkyoku -Iryouka/0000104722.pdf.
- Aonuma K, Yamasaki H, Nakamura M, et al. Percutaneous WATCHMAN left atrial appendage closure for japanese patients with nonvalvular atrial fibrillation at increased risk of thromboembolism - first results from the SALUTE trial. Circ J. 2018;82(12):2946–2953. doi: 10.1253/circj.CJ-18-0222.
- Naganuma M, Shiga T, Sato K, et al. Clinical outcome in Japanese elderly patients with non-valvular atrial fibrillation taking warfarin: a single-center observational study. Thromb Res. 2012;130(1):21–26. doi: 10.1016/j.thromres.2011.11.005.
- Core2 Health. Guideline for preparing cost-effectiveness evaluation to the Central Social Insurance Medical Council. Version 2.0; [cited 2022 May 20]. Available from: https://c2h.niph.go.jp/tools/guideline/guideline_en.pdf.
- Boston Scientific. WATCHMAN FLX™ [package insert]; [cited 2021 Sep 2]. Available from:https://www.bostonscientific. com/content/dam/Manuals/us/current-rev-en/50816633-01A_Watchman_IFU_en_s.pdf.
- Dennis MS, Burn JP, Sandercock PA, et al. Long-term survival after first-ever stroke: the oxfordshire community stroke project. Stroke. 1993;24(6):796–800. doi: 10.1161/01.str.24.6.796.
- Hart RG, Pearce LA, Aguilar MI. Meta-analysis: antithrombotic therapy to prevent stroke in patients who have nonvalvular atrial fibrillation. Ann Intern Med. 2007;146(12):857–867. doi: 10.7326/0003-4819-146-12-200706190-00007.
- Freeman JV, Zhu RP, Owens DK, et al. Cost-effectiveness of dabigatran compared with warfarin for stroke prevention in atrial fibrillation. Ann Intern Med. 2011;154(1):1–11. doi: 10.7326/0003-4819-154-1-201101040-00289.
- Connolly SJ, Ezekowitz MD, Yusuf S, et al. Dabigatran versus warfarin in patients with atrial fibrillation. N Engl J Med. 2009;361(12):1139–1151. doi: 10.1056/NEJMoa0905561.
- Granger CB, Alexander JH, McMurray JJ, et al. Apixaban versus warfarin in patients with atrial fibrillation. N Engl J Med. 2011;365(11):981–992. doi: 10.1056/NEJMoa1107039.
- Ruff CT, Giugliano RP, Braunwald E, et al. Comparison of the efficacy and safety of new oral anticoagulants with warfarin in patients with atrial fibrillation: a meta-analysis of randomised trials. Lancet. 2014;383(9921):955–962. doi: 10.1016/S0140-6736(13)62343-0.
- Ezekowitz MD, Bridgers SL, James KE, et al. For the veterans affairs stroke prevention in nonrheumatic atrial fibrillation investigators. Warfarin in the prevention of stroke associated with nonrheumatic atrial fibrillation. N Engl J Med. 1992;327(20):1406–1412. doi: 10.1056/NEJM199211123272002.
- Singer DE, Hughes RA, Gress DR, et al. The effect of low-dose warfarin on the risk of stroke in patients with nonrheumatic atrial fibrillation. N Engl J Med. 1990;323(22):1505–1511. doi: 10.1056/NEJM199011293232201.
- Tokano T, Nakazato Y, Kato E, et al. The efficacy and safety of novel anticoagulant “Dabigatran” in patients with atrial fibrillation – three-year experience. Heart. 2015;47:563–569.
- Shiga T, Naganuma M, Nagao T, et al. Persistence of non-vitamin K antagonist oral anticoagulant use in Japanese patients with atrial fibrillation: a single-center observational study. J Arrhythm. 2015;31(6):339–344. doi: 10.1016/j.joa.2015.04.004.
- Stroke prevention in atrial fibrillation investigators. Stroke prevention in atrial fibrillation study: final results. Circulation. 1991;84(2):527–539.
- Petersen P, Boysen G, Godtfredsen J, et al. Placebo-controlled, randomized trial of warfarin and aspirin for prevention of thromboembolic complications in chronic atrial fibrillation: the copenhagen AFASAK study. Lancet. 1989;1(8631):175–179. doi: 10.1016/s0140-6736(89)91200-2.
- Connolly SJ, Laupacis A, Gent M, et al. Canadian atrial fibrillation anticoagulation (CAFA) study. J Am Coll Cardiol. 1991;18(2):349–355. doi: 10.1016/0735-1097(91)90585-w.
- MHLW Ministry of Health, Labor and Welfare. Abridged life table 2017; [cited 2019 Jun 17]. Available from:https://www.mhlw.go.jp/toukei/saikin/hw/life/life17/index.html.
- Risk factors for stroke and efficacy of antithrombotic therapy in atrial fibrillation analysis of pooled data from five randomized controlled trials. Arch Intern Med. 1994;154:1449–1457.
- Ariesen MJ, Claus SP, Rinkel GJ, et al. Risk factors for intracerebral hemorrhage in the general population: a systematic review. Stroke. 2003;34(8):2060–2065. doi: 10.1161/01.STR.0000080678.09344.8D.
- Shiroiwa T, Fukuda T, Ikeda S, et al. Japanese population norms for preference-based measures: EQ-5D-3L, EQ-5D-5L, and SF-6D. Qual Life Res. 2016;25(3):707–719. doi: 10.1007/s11136-015-1108-2.
- Noto S, Yanagi H, Tomura S. Measuring utilities for various functional outcomes after stroke. Comparison of rating scale and time trade-off methods. Jpn J Public Health. 2002;49:1205–1216.
- Reddy VY, Sievert H, Halperin J, et al. Percutaneous left atrial appendage closure vs warfarin for atrial fibrillation: a randomized clinical trial. JAMA. 2014;312(19):1988–1998. doi: 10.1001/jama.2014.15192.
- Gage BF, Scott JD, Owens DK. Marginal cost-utility of warfarin and aspirin in elderly patients with non-valvular atrial fibrillation. Med Decis Making. 1993;13:386.
- O’Brien CL, Gage BF. Costs and effectiveness of ximelagatran for stroke prophylaxis in chronic atrial fibrillation. JAMA. 2005;293(6):699–706. doi: 10.1001/jama.293.6.699.
- Sullivan PW, Arant TW, Ellis SL, et al. The cost effectiveness of anticoagulation management services for patients with atrial fibrillation and at high risk of stroke in the US. Pharmacoeconomics. 2006;24(10):1021–1033. doi: 10.2165/00019053-200624100-00009.
- The Ministry of Health, Labor and Welfare. April 1, 2020 Notification. Available from: https://www.mhlw.go.jp/content/12400000/000602947.pdf.
- Medical Data Vision Co., Ltd.; [cited 2020 Mar 30]. Available from: https://www.mdv.co.jp/mdv_database/english/.
- Medical treatment fee point, April 2020. Tokyo: Igakutsushinsya Co. Ltd. 2020.
- Kodani E, Atarashi H, Inoue H. J‐RHYTHM registry investigators. Impact of blood pressure control on thromboembolism and major hemorrhage in patients with nonvalvular atrial fibrillation: a subanalysis of the J-RHYTHM registry. J Am Heart Assoc. 2016;5:e004075.
- Yamashita Y, Uozumi R, Hamatani Y, et al. Current status and outcomes of direct oral anticoagulant use in real-world atrial fibrillation patients – fushimi AF registry. Circ J. 2017;81(9):1278–1285. doi: 10.1253/circj.CJ-16-1337.
- The Ministry of Health, Labor and Welfare. Survey of long-term care benefit expenditure, August 2020. https://www.e-stat.go.jp/stat-search/files?page=1&layout=datalist&toukei=00450049&tstat=000001123535&cycle=1&year=20200&month=23070908&tclass1=000001123536&tclass2=000001144553&result_back=1&tclass3val=0.
- Hirano T, Kaneko H, Mishina S, et al. Suboptimal anticoagulant management in japanese patients with nonvalvular atrial fibrillation receiving warfarin for stroke prevention. J Stroke Cerebrovasc Dis. 2017;26(10):2102–2110. doi: 10.1016/j.jstrokecerebrovasdis.2017.04.030.
- Fund. Japan: Demographic Shift Opens Door to Reforms. February International Monetary. 2020; [cited 2020 Feb 29]. Available from: https://www.imf.org/en/News/Articles/2020/02/10/na021020-japan-demographic-shift-opens-door-to-reforms.
- Phillips KP, Santoso T, Sanders P, et al. Left atrial appendage closure with WATCHMAN in asian patients: 2 year outcomes from the WASP registry. Int J Cardiol Heart Vasc. 2019;23:100358. doi: 10.1016/j.ijcha.2019.100358.