Abstract
Background
Postoperative atrial fibrillation (POAF) is one of the most common complications following cardiac surgery. POAF is associated with increased hospitalization costs, but its long-term economic burden is not well defined.
Objective
To assess 30-day and 1-year incremental healthcare resource utilization (HRU) and costs associated with POAF in the United States (US).
Methods
This retrospective cohort study used claims data from the IBM Watson MarketScan database. A cohort of US adults aged 55--90 years who underwent open-heart surgery between 1 January 2017 and 31 December 2018 was used to compare patients who experienced POAF versus patients who did not (controls). The outcomes of interest were incremental HRU and costs, which were assessed during the index hospitalization and 30-day and 1-year postdischarge time periods. Inverse probability weighting was used to adjust for differences in baseline characteristics.
Results
A total of 8,020 patients met the study inclusion criteria with 5,765 patients in the control cohort (mean age, 63.4 years) and 2,255 patients in the POAF cohort (mean age, 65.8 years). After adjustment, patients with POAF had an index hospitalization that was 1.9 days longer (99% CI, 1.3-2.4 days; p < 0.001) and cost $13,919 more (99% CI, $2,828-$25,011; p < 0.001) than for patients without POAF. POAF patients also had significantly higher HRU at 30 days and 1-year postdischarge with incremental costs of $4,649 (99% CI, $1,479-$7,819; p < 0.001) and $10,671 (99% CI, $2,407-$18,935; p < 0.001), respectively.
Conclusion
POAF following open-heart surgery poses a significant economic burden up to 1 year postdischarge.
Introduction
Postoperative atrial fibrillation (POAF), defined as atrial fibrillation (AF) following surgery, is one of the most common complications of open-heart surgery. Approximately 230,000 patients undergo coronary artery bypass and/or valvular surgery each year in the United StatesCitation1. POAF affects between 10% and 60% of these patients depending on the type(s) of surgery, with higher incidence following valvular or combination surgeriesCitation2–4. POAF is characterized by intermittent and often fleeting episodes of AF that typically begin 2–4 days following surgeryCitation5. Preoperative risk factors for POAF include advanced age, male sex, history of AF, and congestive heart failureCitation5–7. Current clinical guidelines recommend the use of off-label therapies such as β-blockers, amiodarone, or sotalol for the prevention of POAFCitation8–10. Currently, there is no US Food and Drug Administration (FDA)-approved therapy for the prevention of POAF.
A meta-analysis of 35 studies showed that patients who experience POAF have 62% higher odds of having a stroke and 44% higher odds of mortality within 30 days of surgeryCitation11. Postoperative hospital stays and hospitalization costs for patients with POAF were 1–4 days longer and approximately $2,000 to $15,500 higher versus those for patients without POAFCitation12–20.
Though much has been done to characterize the short-term costs and clinical outcomes of POAF, results vary widely due to narrow and heterogenous patient populations. Much less research has been conducted to characterize healthcare resource utilization (HRU) and the long-term consequences and costs of POAF. This study aimed to bridge these gaps by using the IBM Watson MarketScan database to evaluate both the short-term (index hospitalization + 30 days postdischarge) and long-term (1-year postdischarge) HRU and costs in patients who experience POAF compared with those who do not.
Methods
Study design
This was a retrospective cohort study comprising patients between the ages of 55 and 90 years who had undergone open-heart surgery.
Data source
The IBM Watson MarketScan database is a claims database of US patients with employer-sponsored insurance that covered approximately 40 million individuals in 2018. Specific databases used in this study were the Commercial Claims and Encounters database and the Medicare Supplemental database. The MarketScan databases contain both medical and pharmacy benefit claims that are linked via unique enrollee identifiers. These data are de-identified and compliant with the Health Insurance Portability and Accountability ActCitation21. The University of Washington Human Subjects Division Institutional Review Board (IRB) determined that our study did not meet the definition of human subjects research and was exempt from IRB review.
Inclusion and exclusion criteria
The population of interest was patients who experienced POAF following open-heart coronary artery bypass graft (CABG) and/or valvular surgery occurring between 1 January 2017 and 31 December 2018 (Supplemental Figure 1). POAF was defined as 1 or more diagnoses of AF or atrial flutter occurring within 30 days of surgery. The date of first eligible surgery within the inclusionary time period served as the index date for each patient. Patients were required to be between 55 and 90 years of age at the time of surgery and continuously enrolled in medical and pharmacy benefits for at least 12 months prior to and after surgery. Patients with a history of permanent/persistent AF or other open-heart surgery in the 12 months prior to surgery were excluded due to the potential for these patients to receive concomitant ablation at the time of index surgery. Patients with a history of paroxysmal AF or atrial flutter were included in this study. The control group comprised patients who met all eligibility criteria and did not experience POAF. Patients who underwent surgery in 2017 and maintained continuous enrollment for 2 years after their respective index date were included in an exploratory 2-year analysis (Supplemental Figure 1). A full list of medical codes used to assess eligibility criteria is included in the Supplemental material (Supplemental Table 1).
Baseline characteristics
Baseline characteristics were assessed for each patient based on the 12-month pre-index period. Demographic characteristics included age, gender, geographical region, and payer (commercial or Medicare). Clinical characteristics of interest included several comorbidities, which were identified as indicators of POAF risk or increased HRU/costs based on medical literature review. These included paroxysmal AF, heart failure, hypertension, diabetes, and chronic obstructive pulmonary disease. In addition, the Quan Charlson Comorbidity Index (CCI) score was calculated for each patient at their index date using the diagnosis codes observed in the 12 months priorCitation22. CCI scores were stratified by 0, 1, 2, and 3+. Lastly, baseline use of anticoagulants and antiarrhythmic medications (classified by Vaughan-Williams classes I–IV) was recorded. Baseline characteristics are displayed before and after inverse probability weighting in Supplemental Table 2.
Outcomes of interest
The primary outcome was incremental HRU, which was defined as the number of hospitalizations (inpatient), outpatient medical encounters (outpatient), emergency department (ED) visits, and outpatient prescription fills (pharmacy) occurring after the index hospitalization. The index hospitalization was isolated from other inpatient claims, and its HRU was uniquely defined as the length of stay (days). Each unit of post-index HRU was further subcategorized as “AF-related,” “other cardiovascular (other CV),” or “non-cardiovascular (non-CV)” based on the primary International Classification of Diseases, 10th Revision (ICD-10) diagnosis code recorded on each claim (Supplemental Table 1). Outpatient pharmacy claims were categorized as “anticoagulant” (vitamin K antagonists and direct-acting oral anticoagulants), “antiarrhythmic” (class I–IV antiarrhythmics), and “non-cardiovascular” based on National Drug Codes (NDC) and therapeutic class identifiers.
The secondary outcome was incremental cost. For each element of HRU defined above, the associated cost recorded on each claim was extracted and delineated in the same manner described above. All costs represented the total amount paid by the payer and patient. All costs were adjusted to 2020 US dollars using the medical care component of the Consumer Price IndexCitation23. HRU and cost outcomes were captured for index hospitalization, discharge to 30 days, discharge to 1 year, and discharge to 2 years (exploratory).
Statistical analysis
Descriptive statistics were used to characterize baseline demographic and clinical characteristics of the POAF patients and controls. Continuous variables were presented using means and standard deviations and compared between the cohorts using Student t tests. Categorical variables were presented using counts and percentages and compared between the cohorts using χ2 tests.
Inverse probability weighting (IPW) was used to control for confounding baseline characteristics. Propensity scores were calculated using a logistic regression model with inclusion in the POAF cohort as the dependent variable and the following independent variables: age (categorized), sex, type of surgery (CABG, valve, CABG + valve), history of paroxysmal AF, history of heart failure, CCI score, and preoperative use of β-blockers and class III antiarrhythmics. Adjustments for these characteristics were determined a priori based on a literature review of potential POAF risk factors and confoundersCitation5–7. Patients were then weighted by the inverse of their propensity scores and the standardized difference in each characteristic was calculated before and after weighting to determine the success of IPW. This process was repeated separately for the 2-year exploratory analysis cohort.
Weighted regression was used to estimate HRU and costs. Generalized linear models with a Poisson, normal, or gamma distribution, dependent on the distribution of each outcome measure, and an identity link were used to examine incremental differences between the POAF and control cohorts. Wald tests with robust standard errors were used to determine the statistical significance of regression coefficients. All tests were 2-sided and used a stringent alpha level of 0.01 to adjust for multiple comparisons.
Results
Patient characteristics
A total of 2,255 patients were identified for the POAF cohort and 5,765 patients for the control cohort (Supplemental Figure 2). Baseline characteristics are presented in . Overall, both cohorts had equally large proportions of males (76%). The mean age of POAF patients was 65.8 years and the mean age of control patients was 63.4 years. POAF patients were more likely to undergo valvular surgery or a combination of surgeries and have a history of paroxysmal AF or heart failure. After IPW, all adjusted baseline characteristics’ standardized differences were <0.1, which were determined to be indicative of successful weighting (Supplemental Figure 3)Citation24. The baseline characteristics for the 2-year continuous enrollment cohort (Supplemental Table 3) largely reflect the same trends seen in the 1-year cohort. The HRU and costs presented in the following sections are adjusted. Unadjusted HRU and costs are included in the Supplemental material (Supplemental Figure 4).
Table 1. Baseline demographic and clinical characteristics.
Healthcare resource utilization
Average short-term HRU (index hospitalization + 30 days postdischarge) is presented in Supplemental Table 4. For the index hospitalization, patients in the POAF cohort had a mean length of stay of 9.3 days and patients in the control cohort had a mean length of stay of 7.4 days. The index hospitalization was on average 1.9 days longer in the POAF group compared with the control group (99% CI, 1.3–2.4 days; p < 0.001). Postdischarge, POAF patients experienced almost twice as many 30-day readmissions compared with control patients (0.18 vs 0.099 readmissions per patient; p < 0.001). POAF patients also had significantly more ED visits (0.14 vs 0.092 visits per patient; p < 0.001), outpatient medical visits (5.80 vs 4.72 claims per patient; p < 0.001), and pharmacy prescription fills (8.23 vs 7.30 fills per patient; p < 0.001). The POAF group also had significantly higher HRU in all subcategories except noncardiovascular pharmacy claims (Supplemental Table 4).
Average long-term HRU (discharge to 1 year) (Supplemental Table 5) followed the same trend seen in short-term HRU. POAF patients experienced significantly more hospitalizations (0.49 vs 0.35 readmissions per patient; p < 0.001), ED visits (0.62 vs 0.48 visits per patient; p < 0.001), outpatient medical visits (41.40 vs 36.40 claims per patient; p < 0.001), and pharmacy prescription fills (51.60 vs 46.60 fills per patient; p < 0.001) versus control patients. The POAF group had significantly higher HRU in all subcategories except for non-AF-related (i.e. other) cardiovascular ED claims and noncardiovascular pharmacy claims. The differences between groups were particularly pronounced for AF-related claims compared with claims in other subcategories (Supplemental Table 5).
In the 2-year exploratory analysis, statistically significant differences were maintained across all care settings, with POAF patients having more hospitalizations (0.67 vs 0.50 readmissions per patient; p < 0.01), ED visits (1.03 vs 0.82 visits per patient; p < 0.01), outpatient medical claims (65.76 vs 56.23 claims per patient; p < 0.001), and pharmacy prescription fills (91.30 vs 83.51 fills per patient; p < 0.001) versus control patients. However, statistically significant differences dropped off for most subcategories with the exception of AF-related and anticoagulant claims (Supplemental Table 6).
Direct healthcare costs
Average short-term costs (index hospitalization + 30 days postdischarge) are presented in . After adjustment, POAF patients had a mean index hospitalization cost of $126,111 compared with $112,192 in the control group, translating to an incremental cost of $13,919 (99% CI, $2,828–$25,011; p < 0.001). Total 30-day costs, excluding the index hospitalization, were $12,193 and $7,545 in the POAF and control groups, respectively, with an incremental cost of $4,649 (99% CI, $1,479–$7,819; p < 0.001). POAF patients accrued significantly higher 30-day readmissions costs ($9,266 vs $5,455; p < 0.001), ED costs ($154 vs $93; p < 0.001), outpatient medical costs ($2,243 vs $1,588; p < 0.001), and pharmacy prescription costs ($530 vs $408; p < 0.001) ().
Table 2. 30-day costs.
Average long-term costs (discharge to 1 year) are presented in . At 1 year, POAF patients accrued an average of $52,232 of postdischarge costs compared with $41,561 in the control group, with an incremental cost of $10,671 (99% CI, $2,407–$18,935; p < 0.001). One-year readmission costs were $23,941 compared with $18,604 for the POAF and control groups, respectively. However, the incremental difference between these groups for readmission of $5,337 was not statistically significant. POAF patients had significantly higher ED costs ($626 vs $468; p < 0.001), outpatient medical costs ($21,301 vs $17,380; p < 0.01), and pharmacy prescription costs ($6,364 vs $5,108; p < 0.001). Incremental costs in the AF-related subcategory were significant in all care settings ().
Table 3. 1-year costs.
At 2 years, POAF patients accrued total postdischarge costs of $70,580 compared with $64,548 in control patients. The total incremental cost associated with POAF narrowed to $6,032, which was not significant. The only incremental costs that reached statistical significance at 2 years were overall pharmacy costs, anticoagulant costs, and inpatient and outpatient AF-related costs (Supplemental Table 7).
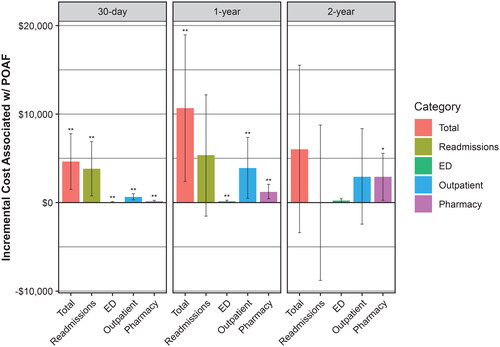
To illustrate the time trend of total incremental costs associated with POAF, the total and categorized incremental costs were plotted at the 30-day, 1-year, and 2-year postdischarge time points (). At 30 days postdischarge, the majority (82%) of incremental costs were due to 30-day readmissions. At 1 year postdischarge, readmissions remained the largest contributor (50%), but outpatient medical (37%) and pharmacy (12%) costs were also substantial contributors to total incremental costs. At 2 years postdischarge, readmissions costs equalized between the cohorts, and outpatient medical (49%) and pharmacy (48%) costs made up the bulk of the total incremental costs.
Discussion
Results summary
In this study, the incremental HRU and costs associated with POAF following open-heart surgery were assessed. The differences observed in baseline characteristics between the POAF and control cohorts were consistent with the medical literatureCitation25. POAF patients were generally older, underwent more valvular surgeries, and had more POAF risk factors such as a history of paroxysmal AF and heart failure. After controlling for these potential confounders, POAF patients had significantly longer index hospitalizations and utilized more healthcare resources across all care settings at 30 days, 1 year, and 2 years after discharge compared with control patients. The difference between the cohorts was most pronounced for AF-related claims and at the earlier time points.
POAF patients also incurred higher healthcare costs at all time points and across all care settings. Notably, while incremental HRU and costs generally increased from the 30-day to 1-year time points, the incremental differences did not grow in proportion to the time elapsed. This suggests that over time, the differences in HRU and costs between the POAF and control cohorts regress to a mean. This trend is corroborated by the exploratory 2-year data where total incremental costs at 2 years decreased compared with 1-year costs. Overall, these results suggest that the HRU and cost burden of POAF is greatest during and proximal to the index hospitalization. It is important to note that the higher HRU and costs in the POAF cohort may reflect greater, more complex comorbidities and downstream complications of POAF rather than a reflection of the POAF itself. While POAF patients did consistently incur higher AF-specific costs at all time points, supporting the association between POAF and recurrent AFCitation26, these costs constituted a fraction of all incremental costs.
A closer look at the main contributors to total incremental healthcare costs associated with POAF revealed that 30-day readmissions constituted the majority of healthcare costs in the first 30 days postdischarge. This finding is of particular importance for cardiac surgery centers, as 30-day readmission following CABG is a key quality metric monitored by the Centers for Medicare & Medicaid Services with significant implications for quality ratings and reimbursement penaltiesCitation27. While the increased 30-day readmissions cannot be directly and entirely attributed to POAF or the treatment thereof, these findings do stress the need for more effective prevention strategies for POAF. The differences in hospital admissions between the 2 cohorts became less significant over time and the main sources of economic burden were eventually from outpatient medical and pharmacy claims. This suggests that POAF patients require the most resource-intensive care in the immediate postoperative period and may suggest that HRU gradually stabilizes to a point where increased outpatient monitoring and medication management are sufficient to prevent significant differences in rehospitalization.
Strengths and limitations
To our knowledge, this study is the first to utilize a nationwide claims database to assess the economic burden of POAF. The use of MarketScan allowed for a patient sample representative of the commercially insured United States population, rather than a sample from a single center or a cluster of centers. Furthermore, the delineation of outcomes into categories and subcategories allowed for close examination of the composition of the incremental HRU and costs in POAF patients. Evaluation of 1-year and 2-year outcomes also helped elucidate the long-term burden of POAF, which has not been well characterized by previous studies.
The study had several limitations. First, the use of claims data inherently limited the ability to control for potential confounders that were not adequately captured via ICD-10 codes, particularly procedural findings, measurements, or intraoperative characteristics such as left atrial size, left ventricular ejection fraction, body mass index, and operating room time. Among the comorbidities that we were able to control for, disease severity could not be accounted for. Second, the use of ICD-10 codes allowed us to determine the occurrence of POAF, but not characterize the duration or severity of POAF. Third, the requirement of at least 1 year of continuous follow-up may have introduced survivor bias by limiting the study cohort to those who were healthy enough to survive at least 1 year after surgery. Fourth, the 1-year pre-index period used to assess baseline characteristics may not have been long enough to capture all relevant comorbidities, particularly those that may be well controlled.
Study implications
To date, there is no FDA-approved medication or procedure for the prevention of POAF. Off-label β-blockers are frequently used preoperatively for prophylaxisCitation8–10. However, the incidence of POAF remains undeniably highCitation2–4. In the present study, the incidence of POAF was 28%, representing almost one-third of cardiac surgery patients. Furthermore, the use of β-blockers was remarkably similar between the POAF and control cohorts (52%), suggesting that there is still a significant unmet need for more effective preventive therapies for POAF. This study demonstrated both the short-term and long-term economic burden of POAF to payers and helps provide impetus for further research and development of novel prevention modalities for POAF.
Conclusion
This retrospective claims analysis demonstrated that patients who underwent open-heart surgery and experienced POAF had significantly higher HRU and costs during the index hospitalization, at 30 days, and at 1-year postdischarge compared with patients who did not experience POAF. These effects were particularly pronounced for 30-day readmissions, AF-related claims, and at earlier time points.
Transparency
Author contributions
Conception and design, or analysis and interpretation of the data: TJP, RH, PG, DS, and BD contributed to conception, design, analysis, and interpretation of the data. WF, JP, and MR contributed to the interpretation of the data.
Drafting of the paper or revising it critically for intellectual content: All authors.
Final approval of the version to be published: All authors.
All authors agree to be accountable for all aspects of the work.
Previous presentations
Parts of this manuscript have been previously presented at ISPOR 2022.
Supplemental Material
Download MS Word (420.1 KB)Acknowledgements
Medical writing support was provided by Lisa Feder, of Peloton Advantage, LLC, an OPEN Health company, Parsippany, NJ, and was funded by AbbVie. Data management support was provided by Genesis Research and Sara Higa.
Declaration of funding
Allergan (before acquisition by AbbVie) funded this study and participated in the study design, research, analysis, data collection, interpretation of data, reviewing, and approval of the publication. All authors had access to relevant data and participated in the drafting, review, and approval of this publication. No honoraria or payments were made for authorship.
Declaration of financial/other relationships
TJP received funding via an Allergan training grant to support research for this study, is an employee of AbbVie, and may hold AbbVie stock.
DS and WGF are employees of AbbVie and may hold AbbVie stock.
RH Conflicts of interest: none
PG is a former employee of AbbVie and may hold AbbVie stock.
JP is supported by R01AG074185 from the National Institutes of Aging. He also receives grants for clinical research from Abbott, American Heart Association, Association for the Advancement of Medical Instrumentation, Bayer, Boston Scientific, iRhythm, and Philips and is a consultant to Abbott, AbbVie, ARCA biopharma, Bayer, Boston Scientific, Bristol Myers Squibb (Myokardia), ElectroPhysiology Frontiers, Element Science, Itamar Medical, LivaNova, Medtronic, Milestone, Philips, ReCor, Sanofi, and Up-to-Date.
MAR Conflicts of interest: none
BD Conflicts of interest: none
Reviewer disclosures
Peer reviewers on this manuscript have no relevant financial or other relationships to disclose.
Data sharing statement
AbbVie is committed to responsible data sharing regarding the clinical trials we sponsor. This includes access to anonymized, individual, and trial-level data (analysis data sets), as well as other information (e.g. protocols, clinical study reports, or analysis plans), as long as the trials are not part of an ongoing or planned regulatory submission. This includes requests for clinical trial data for unlicensed products and indications.
These clinical trial data can be requested by any qualified researchers who engage in rigorous, independent, scientific research, and will be provided following review and approval of a research proposal, Statistical Analysis Plan (SAP), and execution of a Data Sharing Agreement (DSA). Data request can be submitted at any time after approval in the US and Europe and after acceptance of this manuscript for publication. The data will be accessible for 12 months, with possible extensions considered. For more information on the process or to submit a request, visit the following link: https://vivli.org/ourmember/abbvie/ then select “Home.”
References
- Fernandez FG, Shahian DM, Kormos R, et al. The Society of Thoracic Surgeons national database 2019 annual report. Ann Thorac Surg. 2019;108(6):1625–1632. doi: 10.1016/j.athoracsur.2019.09.034.
- Villareal RP, Hariharan R, Liu BC, et al. Postoperative atrial fibrillation and mortality after coronary artery bypass surgery. J Am Coll Cardiol. 2004;43(5):742–748. doi: 10.1016/j.jacc.2003.11.023.
- Creswell LL, Schuessler RB, Rosenbloom M, et al. Hazards of postoperative atrial arrhythmias. Ann Thorac Surg. 1993;56(3):539–549. doi: 10.1016/0003-4975(93)90894-n.
- Maisel WH, Rawn JD, Stevenson WG. Atrial fibrillation after cardiac surgery. Ann Intern Med. 2001;135(12):1061–1073. doi: 10.7326/0003-4819-135-12-200112180-00010.
- Funk M, Richards SB, Desjardins J, et al. Incidence, timing, symptoms, and risk factors for atrial fibrillation after cardiac surgery. Am J Crit Care. 2003;12(5):424–433. quiz 434-5. doi: 10.4037/ajcc2003.12.5.424.
- Mathew JP, Fontes ML, Tudor IC, et al. A multicenter risk index for atrial fibrillation after cardiac surgery. JAMA. 2004;291(14):1720–1729. doi: 10.1001/jama.291.14.1720.
- Banach M, Rysz J, Drozdz JA, et al. Risk factors of atrial fibrillation following coronary artery bypass grafting: a preliminary report. Circ J. 2006;70(4):438–441. doi: 10.1253/circj.70.438.
- Frendl G, Sodickson AC, Chung MK, et al. 2014 AATS guidelines for the prevention and management of perioperative atrial fibrillation and flutter for thoracic surgical procedures. J Thorac Cardiovasc Surg. 2014;148(3):e153-93–e193. doi: 10.1016/j.jtcvs.2014.06.036.
- Bradley D, Creswell LL, Hogue CW, Jr., et al. Pharmacologic prophylaxis: American College of Chest Physicians guidelines for the prevention and management of postoperative atrial fibrillation after cardiac surgery. Chest. 2005;128(2 suppl):39s–47s. doi: 10.1378/chest.128.2_suppl.39s.
- Muehlschlegel JD, Burrage PS, Ngai JY, et al. Society of Cardiovascular Anesthesiologists/European Association of Cardiothoracic Anaesthetists practice advisory for the management of perioperative atrial fibrillation in patients undergoing cardiac surgery. Anesth Analg. 2019;128(1):33–42. doi: 10.1213/ANE.0000000000003865.
- Lin MH, Kamel H, Singer DE, et al. Perioperative/postoperative atrial fibrillation and risk of subsequent stroke and/or mortality. Stroke. 2019;50(6):1364–1371. doi: 10.1161/STROKEAHA.118.023921.
- Hernández-Leiva E, Alvarado P, Dennis RJ. Postoperative atrial fibrillation: evaluation of its economic impact on the costs of cardiac surgery. Braz J Cardiovasc Surg. 2019;34(2):179–186. doi: 10.21470/1678-9741-2018-0218.
- Liu X, Zhang K, Wang W, et al. Dexmedetomidine sedation reduces atrial fibrillation after cardiac surgery compared to propofol: a randomized controlled trial. Crit Care. 2016;20(1):298. doi: 10.1186/s13054-016-1480-5.
- Ebinger JE, Porten BR, Strauss CE, et al. Design, challenges, and implications of quality improvement projects using the electronic medical record: case study: a protocol to reduce the burden of postoperative atrial fibrillation. Circ Cardiovasc Qual Outcomes. 2016;9(5):593–599. doi: 10.1161/CIRCOUTCOMES.116.003122.
- LaPar DJ, Speir AM, Crosby IK, et al. Postoperative atrial fibrillation significantly increases mortality, hospital readmission, and hospital costs. Ann Thorac Surg. 2014;98(2):527–533; discussion 533. doi: 10.1016/j.athoracsur.2014.03.039.
- Redle JD, Khurana S, Marzan R, et al. Prophylactic oral amiodarone compared with placebo for prevention of atrial fibrillation after coronary artery bypass surgery. Am Heart J. 1999;138(1):144–150. doi: 10.1016/s0002-8703(99)70260-7.
- Shirzad M, Karimi A, Tazik M, et al. Determinants of postoperative atrial fibrillation and associated resource utilization in cardiac surgery. Rev Esp Cardiol. 2010;63(9):1054–1060. doi: 10.1016/S0300-8932(10)70227-X.
- Shams Vahdati S, Samadikhah J, Hakim SH, et al. Comparison of the length of hospital stay between the patients with atrial fibrillation treated with amiodarone and patients with normal sinus rhythm after coronary artery bypass graft. J Cardiovasc Thorac Res. 2012;4(1):17–20.
- Kerstein J, Soodan A, Qamar M, et al. Giving IV and oral amiodarone perioperatively for the prevention of postoperative atrial fibrillation in patients undergoing coronary artery bypass surgery: the GAP study. Chest. 2004;126(3):716–724. doi: 10.1378/chest.126.3.716.
- Almassi GH, Wagner TH, Carr B, et al. Postoperative atrial fibrillation impacts on costs and one-year clinical outcomes: the Veterans Affairs Randomized On/Off Bypass Trial. Ann Thorac Surg. 2015;99(1):109–114. doi: 10.1016/j.athoracsur.2014.07.035.
- IBM MarketScan research databases for life sciences researchers. Somers (NY): IBM Corporation; 2021. Available from: https://www.ibm.com/downloads/cas/OWZWJ0QO.
- Quan H, Sundararajan V, Halfon P, et al. Coding algorithms for defining comorbidities in ICD-9-CM and ICD-10 administrative data. Med Care. 2005;43(11):1130–1139. doi: 10.1097/01.mlr.0000182534.19832.83.
- U.S. Bureau of Labor Statistics. Consumer Price Index for all urban consumers (CPI-U): U.S. city average, by expenditure category. Washington (DC): U.S. Bureau of Labor Statistics; 2022. Available from: https://www.bls.gov/news.release/cpi.t01.htm.
- Normand ST, Landrum MB, Guadagnoli E, et al. Validating recommendations for coronary angiography following acute myocardial infarction in the elderly: a matched analysis using propensity scores. J Clin Epidemiol. 2001;54(4):387–398. doi: 10.1016/s0895-4356(00)00321-8.
- Dobrev D, Aguilar M, Heijman J, et al. Postoperative atrial fibrillation: mechanisms, manifestations and management. Nat Rev Cardiol. 2019;16(7):417–436. doi: 10.1038/s41569-019-0166-5.
- Ahlsson A, Fengsrud E, Bodin L, et al. Postoperative atrial fibrillation in patients undergoing aortocoronary bypass surgery carries an eightfold risk of future atrial fibrillation and a doubled cardiovascular mortality. Eur J Cardiothorac Surg. 2010;37(6):1353–1359. doi: 10.1016/j.ejcts.2009.12.033.
- Centers for Medicare and Medicaid Services. Hospital Readmissions Reduction Program (HRRP). Baltimore (MD): Centers for Medicare and Medicaid Services; 2021. Available from: https://www.cms.gov/Medicare/Medicare-Fee-for-Service-Payment/AcuteInpatientPPS/Readmissions-Reduction-Program.