Abstract
Aims
Thailand’s national smoking cessation services (FAH-SAI clinics) were founded in 2010. A cost-effectiveness analysis (CEA) is needed to inform policymakers of the allocation and prioritization of the limited budget to maximize the value for money of reimbursing these services. Chronic obstructive pulmonary disease (COPD) patients would benefit from smoking cessation services. Therefore, this study aimed to assess the cost-effectiveness of these multidisciplinary services compared to the usual care among COPD patients in Thailand from a societal perspective.
Methods
We conducted a CEA from a societal perspective using a Markov model to simulate lifetime costs and quality-adjusted life years (QALYs) gained by each smoking cessation intervention over the patient’s lifetime. We derived the effectiveness of the smoking cessation services from a multicenter, longitudinal study of smoking cessation services in Thailand and estimated the natural quit rate, transition probabilities, health utility, and cost data from the published literature. Costs and outcomes were discounted at 3%. Sensitivity analyses were performed.
Results
Compared to the usual care, FAH-SAI clinics were associated with higher costs (4,207 THB (US$133)) and improved QALYs (0.11), with an incremental cost-effectiveness ratio of 37,675 THB/QALY (US$1,187/QALY). The effectiveness of FAH-SAI clinics was a key driver of the cost-effectiveness results. At the willingness-to-pay (WTP) threshold of 160,000 THB (US$5,042) per QALY gained, the probability of being cost-effective was 96.5%.
Conclusions
FAH-SAI clinics were cost-effective under Thailand’s WTP threshold. Our results could inform policymakers in allocating resources to support smoking cessation services for COPD patients in Thailand.
Introduction
Cigarette smoking is one of the leading global causes of premature death, causing more than 8 million deaths a yearCitation1. Smoking is associated with increased healthcare costs for tobacco-related diseases and contributes to lost human capital due to morbidity and mortality worldwideCitation2. Also, smoking is strongly associated with negative long-term labor market outcomes, such as reduced labor market earnings, which significantly contribute to the overall economic burden of smokingCitation3. To minimize the global burden of tobacco use and country-level tobacco demand, the World Health Organization (WHO) introduced the first international global health treaty, the WHO Framework Convention on Tobacco Control (FCTC), in 2005, followed by releasing tobacco control measures, namely MPOWER, in 2008. The MPOWER guidelines include: Monitoring tobacco use, Protecting people from tobacco smoke, Offering help to quit smoking, Warning people about the dangers of tobacco, Enforcing bans on tobacco advertising and sponsorship, and Raising tobacco taxesCitation4. Offering smoking cessation interventions could improve health-related quality-of-life and reduce the impact of costly chronic diseases, and is considered cost-saving for health systemsCitation5–8; however, it still has not been implemented in certain countriesCitation9. Thus, increasing its visibility, acceptability, and accessibility is essential.
Thailand is an example of a country known for its high standards concerning tobacco controlCitation10. In 2010, Thailand’s national smoking cessation services, known as FAH-SAI clinics, were founded in line with the MPOWER guidelinesCitation11. FAH-SAI clinics offer free and comprehensive smoking cessation services to all Thai citizens. This program currently operates in 552 healthcare facilities across 77 provinces in Thailand. Trained nurses primarily manage the clinics and can consult with physicians if necessary. The interventions and activities follow a standardized protocol developed by the Ministry of Health and National Alliance for Tobacco-Free Thailand, based on the well-established 5As model for smoking cessation (Ask, Advise, Assess, Assist, and Arrange). These activities encompass identifying and documenting tobacco use status, evaluating the severity of nicotine dependence, advising patients to quit smoking, assessing their willingness to quit, and assisting them through counseling techniques and pharmacological methods, such as nicotine replacement therapy, herbs, and traditional therapy. While the core interventions remain consistent, there may be slight variations across settings due to local context and the availability of human resources. For example, home visits could be arranged in certain settings, while group counseling might be the primary approach in others. Typically, each counseling session lasts between 15 and 30 minutes, with follow-ups scheduled at months 1, 3, and 6Citation12.
Nevertheless, the number of smoking-attributable deaths remains high. Approximately 50,000 Thai people die yearly from smoking-related diseasesCitation13. In 2017, the smoking rate of the Thai adult population was 19%Citation14 and the total economic costs attributable to smoking were estimated at 101.14 billion Thai Baht (THB) (US$2.98 billion), equivalent to 0.65% of the gross domestic product (GDP) and accounting for 17.41% of Thailand’s total healthcare expenditureCitation15.
Despite FAH-SAI clinics being founded for more than a decade, the medicines used for the smoking cessation services and the service fee have not been reimbursed. These problems will be addressed strategically if health policymakers have sufficient information regarding the cost-effectiveness of the services to support the decision to allocate resources for FAH-SAI clinics. Although the cost-effectiveness of FAH-SAI clinics has been shown among smokers with cardiovascular diseasesCitation16, its cost-effectiveness of these clinics among smokers with COPD remains unknown.
A number of economic evaluations have been conducted to evaluate the cost-effectiveness of pharmacotherapy for smoking cessation in various settingsCitation5,Citation8,Citation16 and in the Thai general populationCitation6,Citation7, which showed promising results on cost-effectivenessCitation5,Citation8,Citation17, with some studies showing cost savingsCitation6,Citation7,Citation16 in the long run as a result of death and tobacco-related diseases that were averted. However, these pharmacotherapy smoking cessation interventions are still not reimbursable, possibly due to the high budget impact required to cover a large population. The public payers could not afford the upfront costs of these interventions in the short run if the treatments were reimbursable to every smoker in Thailand. Thus, it is necessary to target smoking cessation services in a specific population group, such as Thai smokers with chronic obstructive pulmonary disease (COPD), to ensure the interventions’ feasibility, affordability, and sustainability.
To inform policymakers of the value of FAH-SAI clinics, a cost-effectiveness analysis (CEA) of the clinics is needed. The cost-effectiveness evidence would also help policymakers in allocating and prioritizing the limited budget to maximize the value for money of reimbursing these services.
Hence, we aimed to conduct a CEA to determine the incremental cost-effectiveness ratio (ICER) of FAH-SAI clinics compared to usual care among Thai COPD patients. We selected COPD because it is a significant condition entailing a high economic burden of 11.40 billion Thai Baht (THB) (US$ 0.34 billion) in 2009, and 80% of COPD deaths in Thailand were caused by smokingCitation13,Citation18. Hence, COPD patients are a cohort who would benefit highly from FAH-SAI clinics.
Our research serves as a valuable contribution to the global literature on smoking cessation strategies, offering insights into the cost-effectiveness of targeted interventions within a specific context. Additionally, this economic evaluation will serve as crucial evidence for guiding policy decisions in Thailand and similar countries, which have limited resources, similar health systems, high smoking rates, and comparable contexts. This is particularly relevant for low- and middle-income countries (LMICs) facing challenges in providing affordable smoking cessation services to their COPD patients. For example, countries like the Philippines, Malaysia, and Vietnam exhibit similar cultural norms and socio-demographic characteristics. Their smoking prevalence rates in 2020 reflected this similarity, with Malaysia at 22.5%, the Philippines at 22.9%, and Vietnam at 24.8% – all closely resembling Thailand's rate of 22.1%Citation19.
Methods
Overview
We conducted a CEA study to assess the cost-effectiveness of FAH-SAI clinics compared to usual care from a societal perspective. Usual care includes the provision of brief advice and smoking cessation treatments without follow-up care. We report this CEA according to the 2022 Consolidated Health Economic Evaluation Reporting Standards (CHEERS)Citation20.
We developed a Markov model to simulate the lifetime costs and outcomes associated with each smoking cessation option using a 1-year cycle length. We simulated a hypothetical cohort of Thai smokers aged 72 years who smoke 10–20 cigarettes per day and live with different stages of COPD based on post-bronchodilator forced expiratory volume in 1 second (FEV1), as defined by Global Initiative for Chronic Obstructive Lung Disease (GOLD) guidelinesCitation21. The ICER is expressed as the incremental costs per incremental quality-adjusted life years (QALYs) gained.
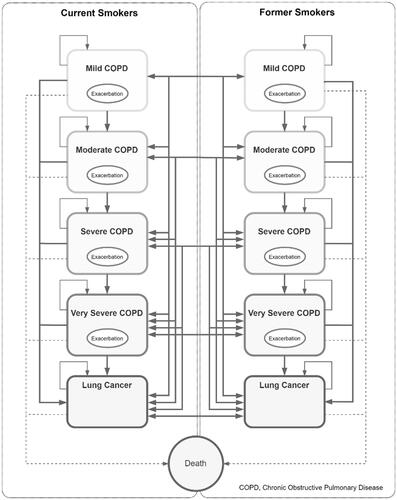
The six-health state model consists of mild COPD, moderate COPD, severe COPD, very severe COPD, lung cancer, and death. Since the smoking status of the cohort was also considered, the model was built as two identical sub-models for current and former smokers (). The participants could transition between COPD severity stages or between current and former smokers. Initially, all patients entered the model as current smokers in one of four COPD severity states before transitioning to other health states. The patients were distributed to mild COPD (36.0%), moderate COPD (32.0%), severe COPD (23.0%), and very severe COPD (9.0%)Citation22. They either remained in the same state or transitioned to more severe COPD stages or lung cancer in the next cycle. Regression was not allowed, and death was considered an absorbing state. The risk of death was calculated based on the age-specific mortality rate (ASMR) for the Thai populationCitation23 and adjusted for the relative risk of mortality from COPDCitation24. The mortality probability was further adjusted to account for male-to-female ratio, disease severity, lung cancer, and smoking status. Transition probabilities were conditional on the sex and smoking status of the cohort. In addition to the transition between the states, the participants in any of the four COPD disease states were at risk of an exacerbation event each year which may be severe or non-severe, with associated differences in costs and utilities. The model accounted for the relapse rate, natural quit rate, and half-cycle corrections.
Our model assumes that smokers make a single quit attempt only in the first cycle, meaning those who fail or relapse are considered smokers for the rest of their lives. We chose to simulate a single quit attempt during the initial year due to the potential interest of Thailand's National Health Security Office (NHSO) in assessing the cost-effectiveness of FAH-SAI clinics. Typically, this service is anticipated to be reimbursed on a one-time basis.
Model inputs
Model input parameters were obtained from published literature and Thailand-specific data sources. The input parameters are shown in and Supplementary Table S1.
Table 1. Input parameters for base-case analysis.
Effectiveness and relapse rates
The effectiveness of FAH-SAI clinics was based on a 6-month continuous smoking abstinence rate (CAR) derived from a multicentered prospective cohort study of FAH-SAI clinics across Thailand. The effectiveness was 17.3% (SE = 5.2%)Citation12, while the effectiveness of usual care was 7.8% (SE = 2.6%)Citation6.
Our model accounted for relapse rates. As data regarding the relapse rates in COPD patients are limited, we applied the rates obtained from the general population to patients with COPD. The relapse rates were assumed to be higher in the first 5 years compared to subsequent years and were calculated from a longitudinal study that examined the prevalence and predictors of late relapse/sustained abstinence in adult former smokersCitation25. The relapse rates for subsequent years were derived from a 35-year prospective observational studyCitation26. We chose these sources because they were community-based studies with large sample sizes. The relapse rates varied by time since quitting, namely years 1–5, years 6–10, and years 11 and over ().
Transition probabilities
Transition probabilities of COPD were retrieved from a study on COPD progression using a dynamic population modelCitation27. The incidence of lung cancer among COPD patients was obtained from a cohort study that investigated the risk of lung cancer among patients with COPDCitation28.
Costs
The costs included direct medical and direct non-medical costs as recommended by Thailand’s Health Technology Assessment (HTA) guidelineCitation35 for CEAs conducted from the societal perspective. It is important to note that the societal perspective, according to the Thailand HTA guideline, omits indirect costs from the evaluation. Direct medical costs associated with the model health states included costs incurred during an exacerbation, costs of maintenance therapies, and drug costs, which were derived from a recent Thai study that determined the cost of illness for COPDCitation22. The cost of lung cancer was taken from a study that quantified treatment and healthcare costs of lung cancer among 27,896 Thai people with lung cancerCitation29. Direct non-medical costs of COPD, including food and transportation, were adapted from the standard costing database in ThailandCitation36. The total cost of smoking cessation interventions included consultation fees and medication costs. The cost of usual care included only consultation fees. The intervention and usual care costs were adapted from a study that estimated the cost-effectiveness of a smoking cessation intervention in COPD patients in ThailandCitation17.
All costs in this study were adjusted to 2021 Thai baht using Thailand’s medical and personal care consumer price indexCitation37. The exchange rate used to convert Thai baht to US dollars was 31.73 baht per US dollarCitation38.
Utilities
Utilities used in this analysis were obtained from published literatureCitation30,Citation31. During cycles in which COPD patients experience an exacerbation, we assumed that the overall utility value was reduced by 15% for non-severe exacerbationsCitation6,Citation32 and by 50% for severe exacerbationsCitation6,Citation33.
Discounting
In the base-case analysis, all costs and outcomes were discounted at a rate of 3%, following the recommendations of Thailand’s HTA guidelinesCitation35.
Base case analysis
We estimated the incremental costs, incremental QALYs, and ICER to determine whether Thailand’s national smoking cessation services are a cost-effective intervention. The cost-effectiveness interpretation was based on a standard willingness-to-pay (WTP) threshold at 160,000 THB (US$5,042) per QALY gained used in ThailandCitation35.
Sensitivity analyses
A one-way sensitivity analysis was performed to investigate the effects of altering input parameters by varying ±20% and presented as a tornado plot. A probabilistic sensitivity analysis (PSA) using 1,000 Monte Carlo simulations was conducted to consider the uncertainty associated with the parameters used in the model and the impact on the results. A cost-effectiveness acceptability curve was plotted based on the results derived from the PSA simulations.
Results
Costs and outcomes
Our base-case analysis showed that the lifetime costs for FAH-SAI clinics were 65,252 THB (US$2,056) per patient and 61,045 THB (US$1,924) per patient for the usual care. The services generated more health benefits than the usual care (5.53 vs 5.37 QALYs). From the difference in costs of 4,207 THB (US$133) and QALYs gained of 0.11, the ICER was estimated at 37,675 THB (US$1,187) per QALYs. The estimated incremental costs and incremental QALYs for both approaches are shown in .
Table 2. Incremental cost and incremental QALYs associated with the national smoking cessation clinic compared with the usual care.
Sensitivity analyses
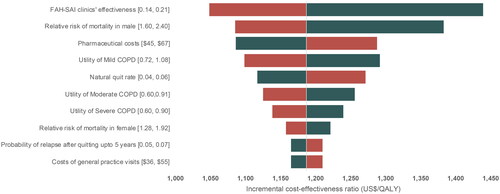
A tornado diagram () was created based on the results of one-way sensitivity analyses, showing that the effectiveness of FAH-SAI clinics was the key driver. The ICER decreased from 45,677 THB (US$1,440) to 33,277 THB (US$1,049) when the effectiveness rose from 0.14 to 0.21. The second most influential parameter was the relative risk of male mortality, with the ICER ranging from 43,879 THB (US$1,383) to 34,455 THB (US$1,086) when this parameter varied between 1.60 and 2.40. Despite this variation, the ICERs remained below the WTP threshold for Thailand.
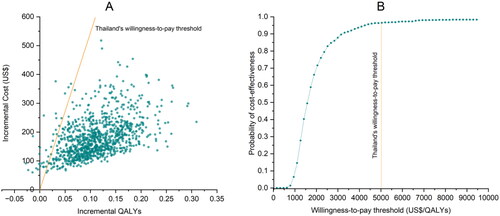
Results from PSA showed that FAH-SAI clinics were cost-effective compared to the usual care, given the WTP threshold of 160,000 THB (US$5,042) (). At the maximum willingness to pay threshold, 160,000 THB (US$5,042), the probability that Thailand’s national smoking cessation services were cost-effective was 96.5% ().
Discussion
This is the first study evaluating the cost-effectiveness of Thailand’s national smoking cessation services (FAH-SAI clinics) among Thai COPD patients. Our findings suggest that FAH-SAI clinics are cost-effective among COPD patients compared to the usual care from a societal perspective, with an ICER of 37,675 THB (US$1,187) per QALY gained. Our study results were robust to the model’s assumptions and input parameters. The ICER was most sensitive to the effectiveness of FAH-SAI clinics, the relative risk of mortality in males, and the cost of medicines. Nevertheless, FAH-SAI clinics remained a cost-effective option compared to usual care in all scenarios. In addition, at the willingness-to-pay threshold of 160,000 THB (US$5,042) per QALY gained, PSA results suggested that FAH-SAI clinics are cost-effective compared to usual care, with a probability of approximately 96.5%. The results of this study support the consideration of Thailand’s national smoking cessation services as part of the benefit packages for Universal Health Coverage Schemes.
Our findings align with those of a previous cost-effectiveness analysis (CEA) study on FAH-SAI clinics in cardiovascular disease (CVD) patientsCitation16, demonstrating that FAH-SAI clinics resulted in greater gains in QALYs compared to usual care. Also, results from our study are consistent with a systematic review study by Hoogendoorn et al.Citation39, which included nine randomized controlled trial studies on smoking cessation interventions in patients with COPD and suggested that smoking cessation interventions, either intensive counseling or counseling with pharmacologic interventions, were cost-effective compared to the usual care among COPD patients. Moreover, our results are also consistent with a cost-effectiveness study on smoking cessation in COPD patients in CanadaCitation40 which showed that the smoking cessation provided by multidisciplinary care teams was cost-effective compared to the usual care among patients with COPD. Our study showed a larger QALY gain (0.11 vs 0.06); this difference can be attributed to our model consisting of patients across the spectrum from mild to very severe COPD, whereas the Canadian study included only moderate to very severe COPD patients, and less severe COPD cases could lead to higher QALY gains. In the one-way sensitivity analysis, our findings align with those of a CEA conducted on a community pharmacist-based smoking cessation program in ThailandCitation7. The key influential parameters to the results were the effectiveness of the services and the relative risk of mortality. Despite the variations in populations and interventions between this study and our study, the results were comparable, possibly owing to the resemblance between a structured community pharmacist-based smoking cessation approach and Thailand’s national smoking cessation services, with both studies utilizing usual care as a comparator.
Evidence has shown that smoking cessation interventions can improve COPD patients’ quality-of-life, slow COPD progression, and reduce COPD-related mortalityCitation5–8,Citation40–43. These reasons may explain our findings because an increase in quality-of-life and slower progression to more severe COPD stages would improve the cost-effectiveness of the national smoking cessation services.
Limitations of our study should be noted. Due to limited Thailand- and COPD-specific data, certain input data used in this study were based on studies conducted in other countries or non-COPD populations. For example, the age-specific mortality for the general Thai populationCitation44 was adjusted with the relative risk of death due to COPD obtained from a Dutch studyCitation27. The utility weights associated with individual disease severity were derived from a study evaluating the association between health-related quality-of-life and lung function in SwedenCitation30. The transition probabilities and the relapse rates used in our study were also adopted or calculated from studies conducted in other countriesCitation25–27. We acknowledged that using information from other countries might not be accurate estimates for the Thai population, potentially influencing the model’s outcomes. This could result in a shift from a conclusion of cost-effectiveness to one of non-cost-effectiveness. However, based on the limited available data, we believe that all the input parameters used in our analyses were derived from the best available sources. To address these uncertainties and assess our model’s robustness and the input parameters, we performed sensitivity analyses, and the results showed that the conclusion remains unchanged. Additionally, our model initially simulated a single quit attempt within the first year. This was influenced by the interest of Thailand’s NHSO in assessing the cost-effectiveness of FAH-SAI clinics. Typically, this service is anticipated to be offered as a one-time intervention. However, in Chen et al.’sCitation45 study on the cost-effectiveness of nicotine replacement therapy, scenario analyses were conducted, which considered the effects of a second quit attempt. The results from these scenarios indicated an increase in the QALYs gained, rising from 0.008 in the base case analysis to 0.013 and 0.015 in the scenario analyses. Consequently, it is likely that our findings, which simulated only a single quit attempt, have underestimated the QALYs gained in comparison to scenarios involving multiple attempts. This suggests that the ICER for multiple quit attempts is expected to decrease. This expectation is based on the assumption that QALYs gained will be higher, and costs will be lower in the context of multiple quit attempts. It is important for readers to recognize these limitations when interpreting the results.
For future research, we recommend the collection of data specific to Thailand’s context, particularly parameters like utility weights and transition probabilities, to enhance the accuracy of future analyses and the collection of long-term data to observe success rates across time and multiple quit attempts.
Our study has certain strengths. First, unlike other cost-effectiveness studies of smoking cessation in Thailand, the effectiveness of Thailand’s national smoking cessation services in our analyses was obtained from a recent real-world studyCitation12, which can represent the impact of the smoking cessation services on the Thai population in actual practice. Second, the costs used in this model are representative of the Thai population as we derived the costs from a recent study that examined COPD costs using COPD registry data in ThailandCitation22. Third, as the inclusion of exacerbations has an impact on cost-effectivenessCitation46,Citation47, we have incorporated the costs and the utilities of exacerbations into our model. Relapse rates were also taken into account.
Despite clear evidence of the value for money of smoking cessation services demonstrated by several studies, especially in the COPD populationCitation6,Citation7,Citation17, Thai smokers have limited access to certain smoking cessation treatments and services. Upon the introduction of Thailand’s national smoking cessation services, positive outcomes were observed. The six-month Continuous Abstinence Rates (CAR) stood at 8.33% for general smokers, 13.81% for individuals with cardiovascular disease, and 17.31% for those with COPD, as reported by Chaisai et al.Citation12. In the period between 2020 and 2022, data sourced from the registry indicated utilization of the services by a total of 10,366 smokers. Notably, around 10% of these service users were individuals diagnosed with COPD. Limited use of the services could have been due to the lack of reimbursement as a health package, resulting in relatively limited budgets for pharmaceuticals and staff compensation. The limitation in budget contributes to a reduced number of referrals from healthcare providers, potentially leading to instances where some healthcare providers may not actively recommend smokers to engage with the cessation services. Providing smoking cessation free of charge to all Thai citizens may improve access to FAHSAI clinics as it could remove financial strain, one of the important barriers to quitting smokingCitation48–50.
Our findings demonstrated the cost-effectiveness of the national smoking cessation services in Thailand. In terms of policy implication, these can be used as additional evidence to support the decision to include smoking cessation services along with pharmaceutical products in the public health insurance schemes and to consistently implement the MPOWER package in Thailand smoking cessation services in order to decrease the number of Thai smokers and improve COPD patients’ quality-of-life. Furthermore, our results may help policymakers in determining potential reimbursement models, such as considering reimbursement based on successful quitters.
Conclusion
Compared with usual care, Thailand’s national smoking cessation services were cost-effective from a societal perspective. Our results could assist health policymakers in allocating resources to support smoking cessation services in Thailand. For future research, we suggest exploring a budget impact analysis for financial assessment, investigating subgroup variations linked to deprivation and rurality, expanding the analysis to extended cost-effectiveness assessments to help evaluate sustainability and address equity concerns, and collecting further data from the Thai context to improve the accuracy of the estimation. Also, primary studies are needed to collect the utility weights, transition probabilities, and out-of-pocket health expenditures, which will be used to redefine our model and future economic studies such as budget impact analysis and extended cost-effectiveness analysis or distributional cost-effectiveness analysis.
Transparency
Declaration of funding
This work was supported by National Alliance for Tobacco Free Thailand.
Declaration of financial/other relationships
No potential conflict of interest was reported by the author.
Author contributions
RP, KT, and NC contributed to the study concept and design, data acquisition, data analysis, data interpretation, and manuscript drafting. All authors (RP, KT, CP, SW, SR, AT, and NC) critically revised and approved the final version of the manuscript. RP: Methodology, Software, Data Curation, Formal analysis, Original draft, Visualization. KT: Conceptualization, Methodology, Validation, Review and Editing, Supervision. CP: Methodology, Validation, Data curation, Review and Editing. SW: Resources, Review and Editing. SR: Resources, Review and Editing. AT: Resources, Review and Editing. NC: Conceptualization, Methodology, Validation, Review and Editing, Supervision, Funding acquisition.
Supplemental Material
Download MS Word (24.3 KB)Supplemental Material
Download MS Word (70.3 KB)Acknowledgements
We thank Rosarin Sruamsiri for her advice on model development. We also thank the National Alliance for Tobacco Free Thailand for providing a grant. In addition, we gratefully thank Chia Jie Tan for the editorial support with the manuscript. We thank the National Alliance for Tobacco Free Thailand for the financial support and valuable input as a knowledge user in this project. Their involvement aided in refining our research and improving the clarity of our work.
Data availability statement
Data supporting the findings of this study are available from the corresponding authors upon request.
References
- Institute for Health Metrics and Evaluation (IHME). Data from: Global Tobacco Control and Smoking Prevalence Scenarios 2017. Seattle, WA; 2020. doi: 10.6069/QAZ7-6505.
- World Health Organization. Tobacco. 2021. Available from: https://www.who.int/news-room/fact-sheets/detail/tobacco
- Böckerman P, Hyytinen A, Kaprio J. Smoking and long-term labour market outcomes. Tob Control 2015;24(4):348–353. doi: 10.1136/tobaccocontrol-2013-051303.
- World Health Organization. MPOWER: a policy package to reverse the tobacco epidemic. World Health Organization; 2008.
- Ranney L, Melvin C, Lux L, McClain E, Lohr KN. Systematic review: smoking cessation intervention strategies for adults and adults in special populations. Ann Intern Med. 2006;145(11):845–856. doi: 10.7326/0003-4819-145-11-200612050-00142.
- Tosanguan J, Chaiyakunapruk N. Cost-effectiveness analysis of clinical smoking cessation interventions in Thailand. Addiction. 2016;111(2):340–350. doi: 10.1111/add.13166.
- Thavorn K, Chaiyakunapruk N. A cost-effectiveness analysis of a community pharmacist-based smoking cessation programme in Thailand. Tob Control. 2008;17(3):177–182. doi: 10.1136/tc.2007.022368.
- Tashkin DP. Smoking cessation in chronic obstructive pulmonary disease. Semin Respir Crit Care Med. 2015;36(4):491–507. doi: 10.1055/s-0035-1555610.
- World Health Organization. Framework convention on tobacco control. Parties to the WHO FCTC. 2021. Available from: https://fctc.who.int/who-fctc/overview/parties
- Levy DT, Yuan Z, Luo Y, Mays D. Seven years of progress in tobacco control: an evaluation of the effect of nations meeting the highest level MPOWER measures between 2007 and 2014. Tob Control. 2018;27(1):50–57. doi: 10.1136/tobaccocontrol-2016-053381.
- Kaur J, Rinkoo A, Gouda H, Prasad V, Pendse R. Implementation of MPOWER package in the South-East Asia Region: evidence from the WHO report on the global tobacco epidemic (2009-2021). Asian Pac J Cancer Prev. 2021;22(S2):71–80. doi: 10.31557/APJCP.2021.22.S2.71.
- Chaisai C, Thavorn K, Wattanasirichaigoon S, et al. The impact of Thai multidisciplinary smoking cessation program on clinical outcomes: a multicentre prospective observational study. Front Public Health. 2022;10:965020. doi: 10.3389/fpubh.2022.965020.
- Burden of Disease Thailand. Thailand burden of diseases attributable to risk factor 2014. International Health Policy Program. Nonthaburi, Thailand; 2017.
- National Statistical Office. The 2021 Health Behavior of Population Survey. 2021, p. 222. Available from: http://www.nso.go.th/sites/2014en/Survey/social/health/health_behavior/2021/new_fullreport_64.pdf
- Komonpaisarn T. Economic cost of tobacco smoking and secondhand smoke exposure at home in Thailand. Tob Control 2022;31(6):714–722. doi: 10.1136/tobaccocontrol-2020-056147.
- Grant A, Tan CJ, Wattanasirichaigoon S, et al. Cost-effectiveness analysis of the SMART quit clinic program in smokers with cardiovascular disease in Thailand.Tob Induc Dis. 2023;21:47–49. doi: 10.18332/tid/161024.
- Kongsakon R, Sruamsiri R. A cost-utility study of smoking cessation interventions for patients with chronic obstructive pulmonary disease in Thailand. J Med Assoc Thailand. 2019;102(4):463–471.
- Bundhamcharoen K, Aungkulanon S, Makka N, Shibuya K. Economic burden from smoking-related diseases in Thailand. Tob Control. 2016;25(5):532–537. doi: 10.1136/tobaccocontrol-2015-052319.
- World Bank Group. Prevalence of current tobacco use (% of adults)—Thailand, Malaysia, Vietnam, Philippines; 2023. Available from: https://data.worldbank.org/indicator/SH.PRV.SMOK?end=2020&locations=TH-MY-VN-PH&start=2020&view=bar
- Husereau D, Drummond M, Augustovski F, et al. Consolidated Health Economic Evaluation Reporting Standards (CHEERS) 2022 explanation and elaboration: a report of the ISPOR CHEERS II Good Practices Task Force. Value Health. 2022;25(1):10–31. doi: 10.1016/j.jval.2021.10.008.
- Singh D, Agusti A, Anzueto A, et al. Global strategy for the diagnosis, management, and prevention of chronic obstructive lung disease: the GOLD science committee report 2019. Eur Respir J. 2019;53(5):1900164. doi: 10.1183/13993003.00164-2019.
- Rodthong W, Rattanachotpanit T, Limwattanano S, Limwattananon C, Lertsinudom S, Boonsawat W. Cost of illness for chronic obstructive pulmonary disease. Isan J Pharm Sci. 2015;11(1):151–158.
- World Health Organization. Life tables: Thailand. 2016. Available from: https://apps.who.int/gho/data/node.country.country-THA?lang-en
- Anthonisen NR, Skeans MA, Wise RA, Manfreda J, Kanner RE, Connett JE, The effects of a smoking cessation intervention on 14.5-year mortality: a randomized clinical trial. Ann Intern Med. 2005;142(4):233–239. doi: 10.7326/0003-4819-142-4-200502150-00005.
- Wetter DW, Cofta-Gunn L, Fouladi RT, Cinciripini PM, Sui D, Gritz ER. Late relapse/sustained abstinence among former smokers: a longitudinal study. Prev Med. 2004;39(6):1156–1163. doi: 10.1016/j.ypmed.2004.04.028.
- Krall EA, Garvey AJ, Garcia RI. Smoking relapse after 2 years of abstinence: findings from the VA Normative Aging Study. Nicotine Tob Res. 2002;4(1):95–100. doi: 10.1080/14622200110098428.
- Hoogendoorn M, Rutten-van Molken MP, Hoogenveen RT, et al. A dynamic population model of disease progression in COPD. Eur Respir J. 2005;26(2):223–233. doi: 10.1183/09031936.05.00122004.
- Parimon T, Chien JW, Bryson CL, McDonell MB, Udris EM, Au DH. Inhaled corticosteroids and risk of lung cancer among patients with chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2007;175(7):712–719. doi: 10.1164/rccm.200608-1125OC.
- Phunmanee A, Wirasorn K, Thavornpitak Y, Sookprasert A, Chindaprasirt J. Lung cancer in hospitalized patients of Thailand. J Med Assoc Thai. 2012;95(Suppl 7):S201–S205.
- Ståhl E, Lindberg A, Jansson S-A, et al. Health-related quality of life is related to COPD disease severity. Health Qual Life Outcomes. 2005;3(1):56. doi: 10.1186/1477-7525-3-56.
- Howard P, Knight C, Boler A, Baker C. Cost-utility analysis of varenicline versus existing smoking cessation strategies using the BENESCO simulation model: application to a population of US adult smokers. Pharmacoeconomics. 2008;26(6):497–511. doi: 10.2165/00019053-200826060-00004.
- Paterson C, Langan CE, McKaig GA, et al. Assessing patient outcomes in acute exacerbations of chronic bronchitis: the measure your medical outcome profile (MYMOP), medical outcomes study 6-item general health survey (MOS-6A) and EuroQol (EQ-5D).Qual Life Res. 2000;9(5):521–527. doi: 10.1023/a:1008930521566.
- Spencer M, Briggs AH, Grossman RF, Rance L. Development of an economic model to assess the cost effectiveness of treatment interventions for chronic obstructive pulmonary disease. Pharmacoeconomics. 2005;23(6):619–637. doi: 10.2165/00019053-200523060-00008.
- Chaikledkaew U, Kittrongsiri K. Guidelines for health technology assessment in Thailand (second edition)-the development process. J Med Assoc Thailand. 2014;97(SUPPL. 5):S4–S9.
- Guideline Development Working Group. Thailand’s Health Technology Assessment (HTA) guideline. 2nd ed. Nonthaburi (Thailand): Watcharin P.P. printing; 2013.
- Riewpaiboon A. Standard cost lists for health economic evaluation in Thailand. J Med Assoc Thailand. 2014;97(SUPPL. 5):S127–S134.
- Bureau of Trade and Economic Indices. Consumer Price Index (CPI) of Thailand. Bureau of Trade and Economic Indices, Ministry of Commerce. 2017. Available from: http://www.price.moc.go.th/price/cpi/index_new_all.asp
- Bank of Thailand. Historical foreign exchange rates. Bank of Thailand. 2017. Available from: https://www.bot.or.th/App/BTWS_STAT/statistics/ReportPage.aspx?reportID=123&language=eng
- Hoogendoorn M, Feenstra TL, Hoogenveen RT, Rutten-van Mölken MPMH. Long-term effectiveness and cost-effectiveness of smoking cessation interventions in patients with COPD. Thorax 2010;65(8):711–718. doi: 10.1136/THX.2009.131631.
- Chandra K, Blackhouse G, McCurdy BR, et al. Cost-effectiveness of interventions for chronic obstructive pulmonary disease (COPD) using an Ontario policy model. Ont Health Technol Assess Ser. 2012;12:1–61. http://www.hqontario.ca/en/mas/mas_ohtas_mn.html. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3384363/pdf/ohtas-12-61.pdf
- Faulkner MA, Lenz TL, Stading JA. Cost-effectiveness of smoking cessation and the implications for COPD. Int J COPD. 2006;1(3):279–287. doi: 10.2147/copd.2006.1.3.279.
- Menn P, Leidl R, Holle R. A lifetime Markov model for the economic evaluation of chronic obstructive pulmonary disease. Pharmacoeconomics 2012;30(9):825–840. doi: 10.2165/11591340-000000000-00000.
- Atsou K, Chouaid C, Hejblum G. Simulation-based estimates of effectiveness and cost-effectiveness of smoking cessation in patients with chronic obstructive pulmonary disease. PLoS One. 2011;6(9):e24870. doi: 10.1371/journal.pone.0024870.
- Ministry of Public H. Public Health Statistics A.D.2017. 2018. Available from: https://bps.moph.go.th/new_bps/sites/default/files/stratistics60.pdf
- Chen B, Silvestri GA, Dahne J, Lee K, Carpenter MJ. The cost-effectiveness of nicotine replacement therapy sampling in primary care: a Markov cohort simulation model. J Gen Intern Med. 2022;37(14):3681–3691. doi: 10.1007/s11606-021-07335-x.
- Kirsch F. A systematic review of quality and cost-effectiveness derived from Markov models evaluating smoking cessation interventions in patients with chronic obstructive pulmonary disease. Expert Rev Pharmacoecon Outcomes Res. 2015;15(2):301–316. doi: 10.1586/14737167.2015.1001976.
- Hoogendoorn M, Feenstra TL, Asukai Y, et al. Cost-effectiveness models for chronic obstructive pulmonary disease: cross-model comparison of hypothetical treatment scenarios. Value Health. 2014;17(5):525–536. doi: 10.1016/j.jval.2014.03.1721.
- Kendzor DE, Businelle MS, Costello TJ, et al. Financial strain and smoking cessation among racially/ethnically diverse smokers. Am J Public Health. 2010;100(4):702–706. doi: 10.2105/AJPH.2009.172676.
- Reitzel LR, Langdon KJ, Nguyen NT, Zvolensky MJ. Financial strain and smoking cessation among men and women within a self-guided quit attempt. Addict Behav. 2015;47:66–69. doi: 10.1016/j.addbeh.2015.03.026.
- Siahpush M, Carlin JB. Financial stress, smoking cessation and relapse: results from a prospective study of an Australian national sample. Addiction. 2006;101(1):121–127. doi: 10.1111/j.1360-0443.2005.01292.x.