Abstract
Aims
Assess the relationship between New York Heart Association (NYHA) functional class and cardiovascular (CV) outcomes in obstructive hypertrophic cardiomyopathy (HCM).
Materials and methods
This retrospective cohort study used the Optum Market Clarity database with linked claims and electronic health records. Adults (aged ≥18 years) with obstructive HCM and ≥1 NYHA class assessment after first HCM diagnosis were eligible (selection period: 2007–2021). Thirteen outcomes were assessed following the index date (first documented NYHA class assessment after first HCM diagnosis in the study period): all-cause mortality; first occurrences of all-cause hospitalization; CV-related hospitalization; primary ischemic stroke or transient ischemic attack (TIA); myocardial infarction (MI); deep vein thrombosis (DVT) or pulmonary embolism (PE); and major adverse CV event (MACE); as well as first incident events of atrial fibrillation or flutter; primary ischemic stroke or TIA; heart failure; acute MI; DVT/PE; and a composite endpoint of pacemaker and cardiac resynchronization therapy. Their associations with the index NYHA class were described using the Kaplan–Meier method (mortality) or cumulative incidence functions (other outcomes). Hazard ratios between NYHA class over time and outcomes were evaluated using time-varying Cox models, adjusting for age at first observed HCM diagnosis, sex, and race.
Results
Among 4,631 eligible patients, the mean age was 59 years at the first observed HCM diagnosis (female, 47%; White, 77%). The risks of all outcomes increased with worse (higher) index NYHA class and worsening NYHA class over time. Deterioration in the NYHA class from the index date was associated with increased risks of outcomes.
Limitations
The study population may not be representative of all patients with obstructive HCM in the real world. Documented NYHA classes may not fully reflect the longitudinal variation of NYHA class for each patient.
Conclusions
Worsening NYHA class was associated with increased risks of all-cause mortality and CV outcomes in obstructive HCM.
PLAIN LANGUAGE SUMMARY
The New York Heart Association (NYHA) class is a simple way for doctors to measure how bad a patient’s heart failure is by how it affects a person’s ability to do everyday activities. It is a 4-point scale from 1, indicating no limitations on activity and no shortness of breath, to 4, at which patients have symptoms even at rest and any activity leaves people struggling to catch their breath. NYHA class is also used to assess patients with obstructive hypertrophic cardiomyopathy (HCM), a disease that causes thickening of the heart muscle. While doctors know that as obstructive HCM becomes worse, patients are at greater risk of having to go to the hospital, getting other conditions (like atrial fibrillation or heart failure), having to have more treatments (like surgery), or even death, doctors and researchers do not know how much risk the patient has and how it changes as the disease changes over time. Although there have been some smaller studies that have estimated this risk, we studied a large, national database and found that patients with worse (higher) NYHA class over time had an increased risk of dying, having to go to the hospital for heart-related care, and developing other heart-related conditions. This finding suggests that it is important for doctors to follow up patients with obstructive HCM carefully and to adjust treatments in order to help patients to stay at lower NYHA classes to improve long-term outcomes.
Introduction
The New York Heart Association (NYHA) functional classification, or NYHA class, was developed for physicians to assess the effects of symptoms on daily activities among patients with cardiac diseaseCitation1. It includes four categories (classes I, II, III, and IV) that are based on limitations of physical activity and symptoms (specifically fatigue, palpitations, dyspnea, and/or anginal pain) with ordinary physical activity and at rest, with higher classes indicative of more functional limitations and increasingly severe symptoms. NYHA class has been validated as a measure of patients’ functional status and disease severityCitation2.
NYHA class has been associated with several cardiovascular (CV) outcomes among patients with cardiac diseases and has been widely applied in disease monitoring and management of such patients. In patients with heart failure, many studies have shown the linkage between a worse (higher) NYHA class and increased risks of death and hospitalizationCitation3–11. Similar associations have also been observed among patients with hypertrophic cardiomyopathy (HCM)Citation12–19. For instance, a meta-analysis by Liu et al. showed that, in patients with HCM, NYHA class III/IV was a risk factor for CV deathCitation18. Maron et al. also observed that individuals with NYHA classes II, III, or IV had a significantly increased risk of all-cause mortality, HCM-related mortality, and disease progression from heart failure or stroke compared with individuals with NYHA class I in HCMCitation13.
The associations between NYHA class and outcomes, however, have not been well established in patients with obstructive HCM, a subgroup with left ventricular outflow tract (LVOT) obstruction. Given the differences in the risk of long-term adverse outcomes and disease management strategies for patients with obstructive vs nonobstructive HCMCitation13, a stratified analysis focusing on patients with obstructive HCM is warranted to minimize the potential heterogeneities within the patient population with HCM. To date, only a few studies have reported data in this population, each focusing on only a limited number of outcomes (particularly mortality). For example, Lakdawala et al. showed that worse NYHA class was correlated with increased all-cause mortality by using data from an international HCM registryCitation20; Elliott et al. also showed a significant relationship between 5-year survival and NYHA classCitation21; and Desai et al. found that a worse NYHA class was associated with an increased risk of a composite of sudden death and appropriate implantable cardioverter defibrillator dischargeCitation22. However, the associations of the NYHA class with clinically relevant outcomes beyond mortality remained unclear. Such associations may help to bridge the potential pathways between NYHA class and mortality, improving the understanding of how symptoms and functional limitations may alter the risk of death. Furthermore, none of these studies accounted for longitudinal changes in NYHA class over time that may reflect disease progression, as previous studies examined the association of outcomes with baseline NYHA class only. We hypothesized that the risk of long-term outcomes may be associated with NYHA class not only assessed at baseline but also as it changes over time. If such a hypothesis is true, it may imply the need of monitoring the temporal variations in NYHA class for patients with obstructive HCM and identifying treatment strategies to improve NYHA class over time to reduce the risk of long-term adverse outcomes. Such results may also be used to assess the long-term clinical value of innovative therapies for obstructive HCM that can improve NYHA class.
To address these gaps, the present study used a large, national US database to assess the associations between NYHA class and multiple CV outcomes among patients with obstructive HCM, while accounting for potential changes in NYHA class over time.
Methods
Data source
This was a retrospective cohort study performed using data from the Optum Market Clarity database. The Optum Market Clarity database integrates the Optum electronic health records (EHR) for patients who received treatments in various integrated delivery networks or outpatients settings with Optum Clinformatics Data Mart claims and third-party medical and pharmacy claims in the US, capturing patients from diverse geographic locations and diverse payers (e.g. commercial payers, including but not limited to Optum, as well as Medicare and Medicaid)Citation23. The structured EHR and claims data were both used in the present study to identify diagnoses, procedures, and healthcare activities, such as hospitalizations. Besides the structured data, natural language processing was applied to extract relevant clinical variables recorded in provider notes, including NYHA class and LVOT peak gradient. Additionally, all-cause mortality was identified by linking the database with the United States Social Security Death Index, which was further augmented by the death information recorded within the EHR. Institutional review board approval was not needed because the study was a secondary analysis of de-identified data.
Study population
The patient selection period was from January 1, 2007, to December 31, 2021. While the patient selection period covers a total of 15 years, there were no major changes in the treatment paradigm for obstructive HCM, with the mainstay of the treatments being beta-blockers, calcium channel blockers, disopyramide, and septal reduction therapy (SRT). Thus, patients selected during such a long period may not differ substantially due to the therapies received. However, the 15-year patient selection period helped to improve the length of the follow-up for some patients, increasing the likelihood of observing outcomes that would only occur in the long term. The study population included adults (aged ≥ 18 years) with obstructive HCM and at least one assessment of NYHA class. Specifically, eligible patients were required to meet one of the following four criteria as evidence for obstructive HCM: (a) at least two HCM diagnoses that were at least 30 d apart, with at least one being an obstructive HCM diagnosis (International Classification of Diseases, 9th Revision, Clinical Modification [ICD-9-CM]: 425.11; International Classification of Diseases, 10th Revision, Clinical Modification [ICD-10-CM]: I42.1); (b) at least one HCM diagnosis and at least one SRT procedure; (c) at least one HCM diagnosis and at least one LVOT peak gradient of 30 mm Hg or more; or (d) at least one obstructive HCM diagnosis in the inpatient setting. In addition, patients were required to have had at least one NYHA class assessment after the first HCM diagnosis, and at least one recorded healthcare activity in either the EHR or claims data that occurred at least 365 days before the date of the first NYHA class assessment in the database. Patients were also required to have had a last active date in either the EHR or claims data and, when applicable, a death date (assigned as the 15th day of the month, using the year and month of death) on or after the date of the first eligible NYHA class assessment.
For the purposes of analysis, the date of the first NYHA class assessment after obstructive HCM diagnosis was termed the index date. The time from the first recorded healthcare activity observed to the index date was termed the baseline period. The time from the index date +1 day until the end of the patient selection period or death, whichever occurred first, was termed the follow-up period.
Outcomes
The study examined 13 outcomes in total during the follow-up period: all-cause mortality; first occurrences during follow-up of all-cause hospitalization, CV-related hospitalization, primary ischemic stroke or transient ischemic attack (TIA), myocardial infarction (MI), deep vein thrombosis (DVT)/pulmonary embolism (PE) and major adverse CV events (MACE, defined as the occurrence of MI, stroke, or all-cause mortality); as well as first incident events of atrial fibrillation (AF) or flutter, primary ischemic stroke or TIA, heart failure, acute MI, DVT/PE; and a composite endpoint of pacemaker and cardiac resynchronization therapy (CRT). The six incident outcomes were assessed among patients without evidence of the corresponding conditions at baseline, and the rest were assessed during the follow-up period among all patients regardless of whether the patient had evidence of the event during the baseline period. For all-cause mortality, patients without a record of death were censored at the end of the follow-up period. For other outcomes, patients who did not develop the outcome of interest were censored at the last healthcare activity or death.
NYHA class
Because the NYHA class for each patient may vary over time, the present study considered data for NYHA class (result being I, II, III, or IV) on the index date as well as during the follow-up period to capture potential worsening or improvement over time. Taken together, these assessments constituted the “time-varying” NYHA class (i.e. NYHA class over time), and they were utilized in the time-varying models described in the “Statistical analyses” section.
Statistical analyses
The time to each of the outcomes was described by the index NYHA class. Specifically, time to all-cause mortality by index NYHA class was described using the Kaplan–Meier method, and time to all other outcomes were described by index NYHA class using cumulative incidence functions (the “%CIF” macro in SAS), treating death as the competing risk. The probabilities of developing each outcome over time were derived based on the Kaplan–Meier estimates or cumulative incidence functions.
Hazard ratios (HRs) between time-varying NYHA class (i.e. NYHA class assessed on the index date and over time during the follow-up period) and each outcome were estimated using time-varying Cox proportional hazards models (using “proc PHREG” in SAS with Andersen-Gill data format), adjusting for age at the first observed obstructive HCM diagnosis, sex (female patients vs male patients), and race (White vs non-White). Specifically, three models were developed to estimate various HRs of interest. In model 1, time-varying NYHA classes II, III, and IV were compared with class I. Model 2 was a modified version from model 1, in which NYHA classes III and IV were bundled into a single category following other studies in patients with HCM partly due to the small sample sizes for patients in NYHA class IVCitation18,Citation19,Citation24. Model 3 was an alternative model specification, in which time-varying Cox proportional hazards models were fitted to estimate the HRs associated with a change in NYHA class after the index date by including an ordinal variable of change in NYHA class (range, −3 to 3, with negative values indicating improvement and positive values indicating deterioration relative to the index NYHA class), further controlling for the index NYHA class. Specifically, worsening in the NYHA class reflects disease progression. Additionally, given that death is a competing risk to other CV outcomes, the cause-specific Cox model was applied in the analysis of CV outcomes other than all-cause mortality.
All statistical analyses were conducted using Statistical Analysis Software 9.4 (SAS Institute Inc., Cary, NC, USA). Statistical significance was determined based on a p value < .05 or a 95% confidence interval (CI) excluding the null.
Results
Study population
In total, 4,631 eligible patients were included in the study population, which had a median follow-up time of 3.48 years (range, 0.00–14.47 years). The sample sizes for the analysis of incident outcomes were 2,492 for incident AF or flutter, 1,781 for incident heart failure, 3,804 for incident primary ischemic stroke or TIA, 3,230 for incident acute MI, 3,063 for incident composite endpoint of pacemaker or CRT, and 4,045 for incident DVT/PE. The flow of patient selection is also described in Supplementary Figure S1. On the index date, 24% of eligible patients were classified as NYHA class I, 39% as NYHA class II, 32% as NYHA class III, and 5% as NYHA class IV. The change in the distribution of NYHA classes over time in the study is shown in Supplementary Table S1. The median follow-up time decreased from NYHA class I to NYHA class IV (). The mean number of NYHA class assessments for each patient (including the index NYHA class) was 4.4 (median, 2; 25th–75th percentile, 1–5).
Table 1. Sample sizes and median follow-up years by NYHA class on the index date.
The study population comprised 47.1% women and included diverse races and ethnicities (77.1% White, 13.4% Black, 2.7% Hispanic, 2.1% Asian, and 4.8% other or unknown race) (). The mean (standard deviation) age at first observed HCM diagnosis was 58.5 (15.3) years. The mean age at the earliest date of an HCM diagnosis code in the study period ranged from 53.5 years to 63.2 years across subgroups based on baseline NYHA class. The number of years from the first observed HCM diagnosis to the index date was similar and was 2.51, 2.53, 2.49, and 2.39 years for baseline NYHA class I, II, III, and IV, respectively. Furthermore, patients with a higher NYHA class had more comorbidities and more frequent use of implantable cardioverter defibrillator and pacemaker or CRT than those with a lower NYHA class (). The characteristics of the subgroups eligible for the analysis of each incident outcome are provided in Supplementary Table S2. Such populations generally had lower percentages of patients with comorbidities or medical device use during the baseline period than the overall cohort.
Table 2. Baseline patient characteristics for the overall population and by NYHA classes I, II, III, and IV.
Risk of outcomes during the follow-up period
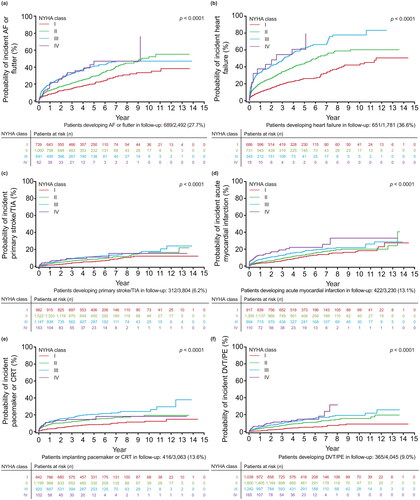
The risk of all-cause mortality increased with a worse index NYHA class, as shown with the clear separation of the Kaplan–Meier curves for each NYHA class (). The risks of all other outcomes were also generally lowest for patients with index NYHA class I and generally highest for patients with index NYHA classes III and IV, including time-to-event outcomes () as well as incident outcomes (i.e. outcomes for which the patient had no previous history) (). For some outcomes, patients had progressively greater risk for each successively higher NYHA class, such as MACE and DVT/PE (), as shown by the clear separation of the cumulative incidence function curves for each NYHA class. For other outcomes, patients had increasing risk with higher NYHA classes but less differentiation in risk between NYHA classes III and IV, such as primary ischemic stroke or TIA (), or less differentiation of NYHA classes III and IV in later years, such as all-cause hospitalization, CV-related hospitalization, or acute MI (, respectively). Similarly, some incident outcomes (i.e. those for which the patient had no previous history) showed greater risk with higher NYHA classes but less differentiation between NYHA class III and IV, such as AF or flutter, heart failure, primary ischemic stroke/TIA, or DVT/PE ( respectively). Although the risk of a new acute MI was greater with worsening NYHA class I–IV (), the difference in risk between NYHA classes was not as large as for the risk of newly-diagnosed heart failure between NYHA classes I and III/IV (). As expected, the risk of a pacemaker or CRT device was greater with each increasing NYHA class from I to III, while the risk for NYHA class IV was similar to NYHA class III in earlier years and NYHA class II in later years because implantation of pacing devices is less common in NYHA class IV patients ().
Figure 1. Risk of outcomes over time by index NYHA class for all-cause mortality (N = 4,631). Abbreviation. NYHA, New York Heart Association.
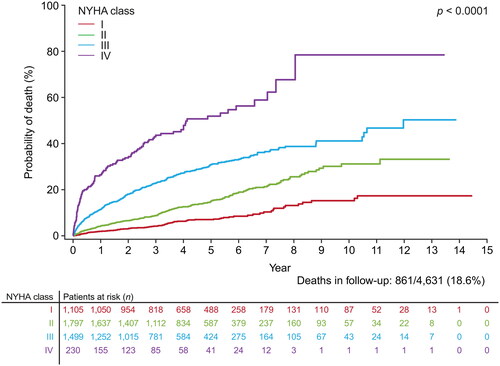
Figure 2. Probability of outcomes over time by index NYHA class, accounting for death as a competing risk for (a) MACE (N = 4631); (b) all-cause hospitalization (N = 4631); (c) CV-related hospitalization (N = 4631); (d) primary ischemic stroke or TIA (N = 4631); (e) MI (N = 4631); and (f) DVT/PE (N = 4631). Abbreviations. CV, cardiovascular; DVT, deep vein thrombosis; MACE, major adverse cardiovascular event; MI, myocardial infarction; NYHA, New York Heart Association; PE, pulmonary embolism; TIA, transient ischemic attack.
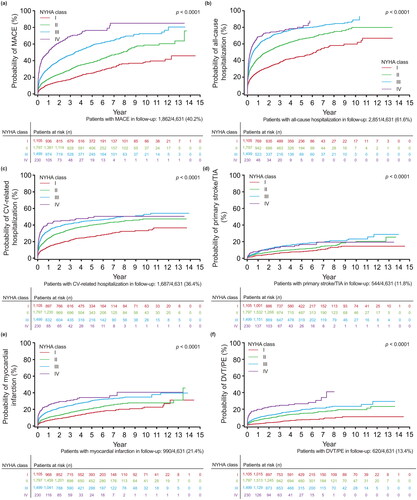
Figure 3. Probability of outcomes over time by index NYHA class, accounting for death as a competing risk, for (a) incident AF or flutter (N = 2,492); (b) incident heart failure (N = 1,781); (c) incident primary ischemic stroke or TIA (N = 3,804); (d) incident acute MI (N = 3,230); (e) incident composite of pacemaker and CRT (N = 3,063); and (f) DVT/PE (N = 4,045). Abbreviations. AF, atrial fibrillation; CRT, cardiac resynchronization therapy; DVT, deep vein thrombosis; MI, myocardial infarction; NYHA, New York Heart Association; PE, pulmonary embolism; TIA, transient ischemic attack.
Associations between time-varying NYHA class and outcomes
The Cox models 1 and 2 showed that worse (higher) NYHA classes (II, III, or IV) were associated with significantly higher risks of all long-term outcomes, relative to NYHA class I (, with 95% CIs for NYHA class II, III, and IV vs I excluding the null). For example, in model 1 (NYHA classes I, II, III, and IV modeled separately), compared with patients with NYHA class I, the risks (95% CI) for patients with NYHA classes II, III, and IV were, respectively, 1.800 (1.399–2.317), 4.119 (3.235–5.245), and 10.904 (8.284–14.352) times higher for all-cause mortality. This trend of increasing risk with worsening NYHA class was similar across all outcomes, including those applicable to the total population () as well as incident outcomes (). Additionally, the results based on model 2 (NYHA classes III and IV combined) were consistent with those based on model 1 (). Based on models 1 and 2, there was little difference in the risk of outcomes by sex, with the exception of all-cause mortality, any acute MI, or MACE; for these three outcomes male patients had a greater risk than female patients in both models 1 and 2. Non-White patients had a significantly greater risk (with 95% CIs for race excluding the null) than White patients for all-cause mortality, any primary ischemic stroke/TIA, any or new acute MI, any or new DVT/PE, and MACE; no differences existed for all-cause or CV-related hospitalizations, new primary ischemic stroke/TIA, or new heart failure.
Table 3. Hazard ratios (95% CI) for the associations between time-varying NYHA class and outcomes (models 1 and 2).
In the alternative model specification (model 3: ordinal variable for change in NYHA class), the HRs (95% CI) for each unit of worsening of NYHA class following the index date (i.e. disease progression) showed an increased risk for all outcomes (). Interestingly, the risk for all-cause mortality still increased for each index NYHA class from II to IV compared with NYHA class I after controlling for disease progression. For example, NYHA class IV patients had an HR (95% CI) of 11.587 (8.578–15.652) in model 3 when controlling for disease progression (), compared with 10.904 (8.284–14.352) in model 1 ().
Table 4. Hazard ratios (95% CI) for the associations between change in NYHA class and outcomes (model 3).
Discussion
The present study demonstrated the prognostic value of NYHA class over time in patients with obstructive HCM. Specifically, patients with worse NYHA classes had increased risks of a wide variety of CV outcomes (i.e. all 13 outcomes) assessed in our study: all-cause mortality, all-cause hospitalization, CV-related hospitalization, primary ischemic stroke/TIA, MI, DVT/PE and MACE; as well as first incident events of AF or flutter, primary ischemic stroke/TIA, heart failure, acute MI, DVT/PE and a composite endpoint of pacemaker and CRT. The associations between worse NYHA class and increased risk of adverse outcomes were consistently observed in this study using different methodologies. First, descriptive analyses using the Kaplan–Meier method and cumulative incidence functions suggested that higher NYHA classes at the index date were associated with increased risks of outcomes. Second, Cox models with time-varying NYHA classes (model 1 and model 2) showed that patients who presented with symptoms and functional limitations at the index date or during the follow-up period (i.e. NYHA classes II–IV) had significantly elevated risks of developing all outcomes (with 95% CIs for NYHA class II, III, and IV vs I excluding the null) compared with those without symptoms or functional limitations (i.e. NYHA class I). Modeling the NYHA class as a time-varying covariate, compared with considering the index NYHA class only, better incorporated potential worsening or improvement in disease over time when assessing its associations with the outcomes. For most outcomes except incident composite endpoint of pacemaker and CRT, the HRs increased from NYHA class II to classes III and IV, suggesting an association between disease severity and risks of outcomes. Third, the alternative Cox model specification (model 3) demonstrated the importance of considering disease progression, as measured by worsening NYHA class, in predicting the risks of long-term outcomes. Disease progression during the follow-up period (i.e. worsening in NYHA class over time) was associated with significantly increased risks of all outcomes (with 95% CIs excluding the null for the term representing deterioration by one NYHA class), except for incident composite endpoint of pacemaker and CRT. These HRs also suggest that patients with improvements in NYHA class may experience benefits in long-term outcomes. Moreover, the trend of greater risk of outcomes for patients with NYHA classes II–IV than for those with NYHA class I still existed after controlling for disease progression and was greater for all-cause mortality.
The finding that a worse NYHA class is associated with an increased risk of mortality is consistent with other studies in the literature for HCM and obstructive HCMCitation12–22,Citation24. By comparison, the mortality risks observed in the present study for each NYHA class were generally higher than or comparable to those reported in other obstructive HCM cohorts in the literature. Lakdawala et al. reported that the 1-year probabilities of all-cause mortality were 0.4% for patients with NYHA class I at the index date, 0.9% for NYHA class II, and 3.6% for NYHA classes III and IV using the Sarcomeric Human Cardiomyopathy Registry (SHaRe), an international registry representing patients from ten HCM specialty centersCitation20. The 1-year probabilities of mortality in the present study were higher: 1.9%, 4.2%, 11.4%, and 26.4% for NYHA classes I, II, III, and IV, respectively. The difference in mortality rates between the two studies may be attributable to differences in factors such as patient demographics and clinical characteristics. The present study may be more representative of the overall obstructive HCM population compared with a registry study of HCM specialty centers only. For example, this present study had a lower percentage of patients in NYHA class I (24% vs 38%), more female patients (47% vs 42%), and fewer White patients (77% vs 89%) than the study in patients with obstructive HCM from SHaRe. In a separate study, Elliott et al. observed the following 5-year mortality probabilities in patients with obstructive HCM: 9.0%, 16.7%, and 17.4% for NYHA classes I, II, and III/IV, respectivelyCitation21. These values were comparable to the 5-year probability of death for NYHA classes I and II in the present study (7.0% and 14.9% for NYHA class I and II, respectively), but were lower than the ones for NYHA classes III and IV (30.9% and 51.9% for NYHA class III and IV, respectively).
Besides all-cause mortality, this study also provided valuable data for the associations of NYHA class with other important long-term clinical outcomes in obstructive HCM. Among these outcomes, AF has been considered to be a common sequela of HCM in the literature. For comparison, Olivotto et al. and Guttmann et al. also observed that NYHA class was highly predictive of developing AF in patients with HCMCitation25,Citation26. Further, Olivotto et al. suggested that the development of AF in patients with HCM may represent a clinical turning point and could be a key determinant of subsequent HCM-related mortality (e.g. heart failure- and stroke-related mortality)Citation25. Similarly, Siontis et al. and Fauchier et al. found that AF was significantly associated with increased all-cause mortality as well as ischemic stroke in patients with HCMCitation27,Citation28. A meta-analysis reported that patients with HCM with AF had 2.49-times higher odds of death and 2.80-times higher odds of cardiac death than those without AFCitation29. A systematic review and meta-analysis published in 2022 estimated that AF was significantly associated with an increased risk of thromboembolic events and total strokeCitation30. This study further strengthens the association between AF and HCM, specifically obstructive HCM, and shows an increasing risk of AF with worsening NYHA class.
Further, Guttmann et al. identified NYHA classes III/IV, compared with class I, as significant predictors of thromboembolic risks in patients with HCMCitation31. Specifically, the study reported HRs of 1.25 (p = .21) for NYHA class II and 2.07 (p < .001) for NYHA classes III–IV relative to NYHA class I in a multivariable prediction model of thromboembolism for an HCM population; these were lower in magnitude than the results of the present study for primary stroke/TIA and DVT/PE. One potential reason for this difference is the Guttmann et al. study included all patients with HCM, whereas the present study included only patients with obstructive HCM. It is clinically plausible that the risk of thromboembolic events is greater in patients with obstructive HCM than in patients with any HCM. Moreover, Maron et al. found that patients with NYHA class II had a 3.4-times higher risk of the composite outcome of progression to NYHA class III–IV or death from heart failure or stroke, relative to patients with NYHA class ICitation13. Although the present study did not have a comparable composite outcome, this study builds the evidence supporting an increased risk of thromboembolic events such as stroke, TIA, and DVT/PE with worsening NYHA class.
Previously, there has been little evidence linking NYHA classes with MI in patients with HCM. The present study bridged the data gap, identifying significant associations between NYHA classes and MI in the overall population, as well as incident acute MI in patients without a history of previous MI at baseline. Lastly, the results for MACE are consistent with the results for its individual components: MI, stroke, and all-cause mortality.
The present study has multiple notable strengths. First, although the associations between NYHA class and some CV outcomes had been assessed for obstructive HCM, to our knowledge, this is the first large study that demonstrated the associations and used multiple methods to show consistency in results. Second, the present study was the first to evaluate disease progression as a predictor of long-term CV outcomes with a longitudinal perspective, which further strengthened the linkage between NYHA class and the outcomes. Third, the study used a large, national database with linked claims data and EHR, including a large number of patients from diverse demographic groups and different geographic regions across the USA. The large sample size also ensured sufficient statistical power for evaluating the associations and enabled the inclusion of NYHA class IV separately in a model rather than combined with NYHA class III. Compared with SHaRe, which recruited patients from top medical centers specialized in HCM, the Optum Market Clarity database included patients who received care beyond just HCM care at such centers of excellence; this may make the results of this study more generalizable to the broader obstructive HCM population in the real world.
This study is also subject to several limitations. First, although this database had significantly more documented NYHA class data than other administrative data sets, NYHA class may be more likely to be assessed among patients with heart failure. As such, the results in the study population may not be generalizable to all patients with obstructive HCM in the real world. Second, the present study used all NYHA class assessments documented in the EHR for each patient at and after the index date to assess the impact of time-varying NYHA class on the outcomes. However, the time-varying NYHA class variable used in this study may not have captured the potential changes in NYHA class when an assessment did not take place or was not documented in the EHR, leading to potential misclassification of NYHA class. Such exposure misclassifications may potentially bias the associations observed in the present study towards the null, as it is likely that an NYHA class assessed a longer time ago may become less associated with the present risk of developing adverse CV outcomes. Third, the Optum Market Clarity database or the study time period may not have captured all healthcare activities and disease history of each patient. Some patients may have been misclassified as not having certain outcomes at baseline and were included into the analyses of incident outcomes. The initial HCM diagnosis for some patients may have been earlier than the one first documented in the database; not capturing NYHA class assessments and clinical events before the first observed HCM diagnosis in the database could underestimate changes that occur earlier in the disease just after diagnosis. Additionally, the natural language processing algorithm to abstract NYHA class from charts has not been validated for its accuracy and may not comprehensively or always correctly extract NYHA class assessments documented in medical notes. Fourth, the present study relied on a combination of diagnosis code, procedural code, and documented LVOT peak gradient to identify patients with obstructive HCM. However, the database does not contain sufficient clinical detail to ascertain whether obstructive HCM was a correct diagnosis as coded for each patient. For example, the assessment for mitral insufficiency by systolic anterior motion of the anterior mitral valve leaflet is unavailable in the data set for further ascertainment of HCM. Future studies may be useful to validate the eligibility criteria for identifying patients with obstructive HCM applied here. Fifth, the associations between NYHA class and outcomes may not be causal due to potential residual or unobserved confounding factors. Lastly, the present study was focused on assessing the predictive ability of NYHA class. Future studies may consider assessing other clinically meaningful measurements for obstructive HCM, such as LVOT peak gradient or biomarkers, and their associations with CV outcomes.
This study is important because, as new therapies targeted specifically for patients with obstructive HCM are developed, the prevalence of obstructive HCM limits the possibility of conducting large CV trials that directly target long-term CV outcomes as the primary or secondary endpoints (because such trials may require large sample sizes to ensure sufficient statistical power). Understanding the association of intermediate disease measures, such as NYHA class, with long-term CV outcomes is essential to bridging the evidence gap due to the lack of randomized controlled trials on such long-term CV outcomes. Additionally, the study demonstrated the importance of temporal variations in NYHA class in predicting the risk of long-term adverse outcomes, implying that it may be important for physicians to monitor and identify treatment strategies to help patients with obstructive HCM to improve in NYHA class over time; it also suggests a potential long-term value of treatments for obstructive HCM that can improve NYHA class.
Conclusions
Using multiple methods on a large, population-based cohort of patients, this study provides strong evidence that worsening NYHA class and disease progression were associated with increased risk of CV outcomes, especially all-cause mortality and MACE, in obstructive HCM.
Transparency
Author contributions
All authors made substantial contributions to the conception and design of the work and interpretation of the data. WG and JJ acquired and analyzed the data. YW drafted the manuscript. All authors revised the manuscript critically and approved the final version.
Reviewer disclosures
Peer reviewers on this manuscript have no relevant financial or other relationships to disclose.
Previous presentations
An earlier version of this analysis was presented at the 71st Annual Scientific Session & Expo of the American College of Cardiology (Washington, DC, USA, April 2–4, 2022).
Supplemental Material
Download ()Acknowledgements
The authors thank Yuanhui Zhang (Bristol Myers Squibb employee at the time the study was conducted) for the helpful discussions and thoughtful comments on the study, and Surya Prasanth Kuppuswamy Krishnamurthy from MuSigma for analytical support. Medical writing and editorial assistance were provided by (a) Shelley Batts, PhD, from Analysis Group, Inc., and (b) Kate Ward, from Oxford PharmaGenesis, Oxford, UK.
Declaration of funding
Funding for this study was provided by Bristol Myers Squibb. Medical writing assistance was funded by Bristol Myers Squibb.
Declaration of financial/other relationship
YW is an employee of Analysis Group, Inc., which received payment from Bristol Myers Squibb for participation in this research. WG, XH, JJ, BS, and XL are current employees of Bristol Myers Squibb and may own Bristol Myers Squibb stock or stock options. CZ is a contractor of Bristol Myers Squibb.
Data availability statement
Bristol Myers Squibb policy on data sharing may be found at: https://www.bms.com/researchers-and-partners/independent-research/data-sharing-request-process.html
References
- New York Heart Association. Nomenclature and criteria for diagnosis of diseases of the heart and great vessels. Boston: Little, Brown and Co; 1994.
- Bennett JA, Riegel B, Bittner V, et al. Validity and reliability of the NYHA classes for measuring research outcomes in patients with cardiac disease. Heart Lung. 2002;31(4):262–270. doi: 10.1067/mhl.2002.124554.
- Ahmed A, Aronow WS, Fleg JL. Higher New York Heart Association classes and increased mortality and hospitalization in heart failure patients with preserved left ventricular function. Am Heart J. 2006;151(2):444–450. doi: 10.1016/j.ahj.2005.03.066.
- Ahmed A. A propensity matched study of New York Heart Association class and natural history end points in heart failure. Am J Cardiol. 2007;99(4):549–553. doi: 10.1016/j.amjcard.2006.08.065.
- Muntwyler J, Abetel G, Gruner C, et al. One-year mortality among unselected outpatients with heart failure. Eur Heart J. 2002;23(23):1861–1866. doi: 10.1053/euhj.2002.3282.
- Lombardi C, Peveri G, Cani D, et al. In‐hospital and long‐term mortality for acute heart failure: analysis at the time of admission to the emergency department. ESC Heart Fail. 2020;7(5):2650–2661. doi: 10.1002/ehf2.12847.
- Pocock SJ, Wang D, Pfeffer MA, et al. Predictors of mortality and morbidity in patients with chronic heart failure. Eur Heart J. 2006;27(1):65–75. doi: 10.1093/eurheartj/ehi555.
- Bouvy ML, Heerdink E, Leufkens H, et al. Predicting mortality in patients with heart failure: a pragmatic approach. Heart. 2003;89(6):605–609. doi: 10.1136/heart.89.6.605.
- De Blois J, Simard S, Atar D, et al. COPD predicts mortality in HF: the Norwegian Heart Failure Registry. J Card Fail. 2010;16(3):225–229. doi: 10.1016/j.cardfail.2009.12.002.
- Störk S, Handrock R, Jacob J, et al. Epidemiology of heart failure in Germany: a retrospective database study. Clin Res Cardiol. 2017;106(11):913–922. doi: 10.1007/s00392-017-1137-7.
- Citu IM, Citu C, Gorun F, et al. Using the NYHA classification as forecasting tool for hospital readmission and mortality in heart failure patients with COVID-19. J Clin Med. 2022;11(5):1382. doi: 10.3390/jcm11051382.
- Ntusi NA, Shaboodien G, Badri M, et al. Clinical features, spectrum of causal genetic mutations and outcome of hypertrophic cardiomyopathy in South Africans. Cardiovasc J Afr. 2016;27(3):152–158. doi: 10.5830/CVJA-2015-075.
- Maron MS, Olivotto I, Betocchi S, et al. Effect of left ventricular outflow tract obstruction on clinical outcome in hypertrophic cardiomyopathy. N Engl J Med. 2003;348(4):295–303. doi: 10.1056/NEJMoa021332.
- Spirito P, Autore C, Rapezzi C, et al. Syncope and risk of sudden death in hypertrophic cardiomyopathy. Circulation. 2009;119(13):1703–1710. doi: 10.1161/CIRCULATIONAHA.108.798314.
- Wang X-H, Qiu J-B, Ju Y, et al. Reduction of heart failure rehospitalization using a weight management education intervention. J Cardiovasc Nurs. 2014;29(6):528–534. doi: 10.1097/JCN.0000000000000092.
- Nasermoaddeli A, Miura K, Matsumori A, et al. Prognosis and prognostic factors in patients with hypertrophic cardiomyopathy in Japan: results from a nationwide study. Heart. 2007;93(6):711–715. doi: 10.1136/hrt.2006.095232.
- Cui H, Schaff HV, Nishimura RA, et al. Latent outflow tract obstruction in hypertrophic cardiomyopathy: clinical characteristics and outcomes of septal myectomy. J Thorac Cardiovasc Surg. 2020;164(6):1863.e1–1869.e1. doi: 10.1016/j.jtcvs.2020.12.016.
- Liu Q, Li D, Berger AE, et al. Survival and prognostic factors in hypertrophic cardiomyopathy: a meta-analysis. Sci Rep. 2017;7(1):11957. doi: 10.1038/s41598-017-12289-4.
- Geske JB, Ong KC, Siontis KC, et al. Women with hypertrophic cardiomyopathy have worse survival. Eur Heart J. 2017;38(46):3434–3440. doi: 10.1093/eurheartj/ehx527.
- Lakdawala N, Saberi S, Day S. New York Heart Association functional class and mortality in obstructive hypertrophic cardiomyopathy. Poster presented at: Heart Failure Society of America (HFSA). September 10–13, 2021, Denver, CO. 2021.
- Elliott PM, Gimeno JR, Tomé MT, et al. Left ventricular outflow tract obstruction and sudden death risk in patients with hypertrophic cardiomyopathy. Eur Heart J. 2006;27(16):1933–1941. doi: 10.1093/eurheartj/ehl041.
- Desai MY, Smedira NG, Bhonsale A, et al. Symptom assessment and exercise impairment in surgical decision making in hypertrophic obstructive cardiomyopathy: relationship to outcomes. J Thorac Cardiovasc Surg. 2015;150(4):928–935.e1. doi: 10.1016/j.jtcvs.2015.07.063.
- Optum.com. Market clarity: linked EHR and claims data. 2022 [cited 2022 Dec]. https://www.optum.com/business/life-sciences/real-world-data/market-clarity-data.html.
- Burghardt A, van Buuren F, Dimitriadis Z, et al. Risk marker profiles in patients treated with percutaneous septal ablation for symptomatic hypertrophic obstructive cardiomyopathy. Clin Res Cardiol. 2018;107(6):479–486. doi: 10.1007/s00392-018-1209-3.
- Olivotto I, Cecchi F, Casey SA, et al. Impact of atrial fibrillation on the clinical course of hypertrophic cardiomyopathy. Circulation. 2001;104(21):2517–2524. doi: 10.1161/hc4601.097997.
- Guttmann OP, Pavlou M, O'Mahony C, et al. Predictors of atrial fibrillation in hypertrophic cardiomyopathy. Heart. 2017;103(9):672–678. doi: 10.1136/heartjnl-2016-309672.
- Fauchier L, Bisson A, Bodin A, et al. Ischemic stroke in patients with hypertrophic cardiomyopathy according to presence or absence of atrial fibrillation. Stroke. 2022;53(2):497–504. doi: 10.1161/STROKEAHA.121.034213.
- Siontis KC, Geske JB, Ong K, et al. Atrial fibrillation in hypertrophic cardiomyopathy: prevalence, clinical correlations, and mortality in a large high‐risk population. J Am Heart Assoc. 2014;3(3):e001002.
- Masri A, Kanj M, Thamilarasan M, et al. Outcomes in hypertrophic cardiomyopathy patients with and without atrial fibrillation: a survival meta-analysis. Cardiovasc Diagn Ther. 2017;7(1):36–44. doi: 10.21037/cdt.2016.11.23.
- Ye TTST, Siah QZ, Tan BY, et al. Prevalence of adverse cerebrovascular events in hypertrophic cardiomyopathy patients with and without atrial fibrillation: a systematic review and meta-analysis. Stroke. 2022;53(Suppl_1):ATP208. doi: 10.1161/str.53.suppl_1.TP208.
- Guttmann OP, Pavlou M, O'Mahony C, et al. Prediction of thrombo‐embolic risk in patients with hypertrophic cardiomyopathy (HCM risk‐CVA). Eur J Heart Fail. 2015;17(8):837–845. doi: 10.1002/ejhf.316.
