Abstract
Aims
To identify and synthesize evidence regarding how coronavirus disease 2019 (COVID-19) interventions, including vaccines and outpatient treatments, have impacted healthcare resource use (HCRU) and costs in the United States (US) during the Omicron era.
Materials and methods
A systematic literature review (SLR) was performed to identify articles published between 1 January 2021 and 10 March 2023 that assessed the impact of vaccination and outpatient treatment on costs and HCRU outcomes associated with COVID-19. Screening was performed by two independent researchers using predefined inclusion/exclusion criteria.
Results
Fifty-eight unique studies were included in the SLR, of which all reported HCRU outcomes, and one reported costs. Overall, there was a significant reduction in the risk of COVID-19-related hospitalization for patients who received an original monovalent primary series vaccine plus booster dose vs. no vaccination. Moreover, receipt of a booster vaccine was associated with a lower risk of hospitalization vs. primary series vaccination. Evidence also indicated a significantly reduced risk of hospitalizations among recipients of nirmatrelvir/ritonavir (NMV/r), remdesivir, sotrovimab, and molnupiravir compared to non-recipients. Treated and/or vaccinated patients also experienced reductions in intensive care unit (ICU) admissions, length of stay, and emergency department (ED)/urgent care clinic encounters.
Limitations
The identified studies may not represent unique patient populations as many utilized the same regional/national data sources. Synthesis of the evidence was also limited by differences in populations, outcome definitions, and varying duration of follow-up across studies. Additionally, significant gaps, including HCRU associated with long COVID and various high-risk populations and cost data, were observed.
Conclusions
Despite evidence gaps, findings from the SLR highlight the significant positive impact that vaccination and outpatient treatment have had on HCRU in the US, including periods of Omicron predominance. Continued research is needed to inform clinical and policy decision-making in the US as COVID-19 continues to evolve as an endemic disease.
Introduction
The coronavirus (COVID-19) pandemic has caused more than 6.2 million hospitalizations and more than 1.1 million deaths in the United States (US) as of 24 August 2023Citation1. COVID-19 was the fourth leading cause of death in the US in 2022 after heart disease, cancer, and unintentional injuryCitation2, and weekly new hospital admissions due to COVID-19 in the US remained among the highest compared to other countries globally as of 10 August 2023Citation3.
The pandemic has resulted in a vast economic burden for both adults and children, particularly in patients with severe COVID-19 who require hospitalization, intensive care unit (ICU) admission, and mechanical ventilationCitation4–6. Long COVID (also known as post-COVID conditions [PCC]), which can continue for months after initial severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection, has been reported to impact approximately 15% of patientsCitation7. Long COVID is also associated with considerable economic burden as a result of outpatient visits and unemploymentCitation7–10. Furthermore, the redirection of healthcare resources towards COVID-19 has delayed healthcare for patients with other conditions such as cancer, communicable diseases, and mental disordersCitation11.
Many studies have been published on the costs and healthcare resource utilization (HCRU) associated with COVID-19 in the US early on in the pandemic during 2020 and 2021 when the Alpha and Delta variants were predominantCitation4,Citation9,Citation12–15. However, evidence gaps remain in terms of more recent data on costs and HCRU associated with the Omicron variantCitation16. Understanding the costs and HCRU during the Omicron predominant era (February 2022 through the presentCitation16,Citation17) is also complicated by multiple variables such as vaccine uptake (including of the updated bivalent vaccines), heterogeneity in the level and duration of protection elicited by vaccination or prior infection, and the availability and uptake of anti-SARS-CoV-2 treatments.
Although COVID-19 is no longer considered a public health emergency in the US, responding to the spread of SARS-CoV-2 remains a public health priority for the Department of Health and Human ServicesCitation18. The World Health Organization has also emphasized the importance of continuing to limit SARS-CoV-2 transmission and treating patients with COVID-19 to reduce mortality and morbidityCitation19. Quantifying the ongoing economic burden of COVID-19 and understanding the impact of vaccines and treatments can help inform policy decisions aimed at ensuring efficient operation of healthcare systems and uptake of medical countermeasures or interventions for COVID-19 management. The aim of this systematic literature review (SLR) was to identify and synthesize the evidence regarding how COVID-19 interventions, including vaccines and outpatient treatments, have influenced the economic burden (in terms of HCRU and costs) of COVID-19 in the US during the Omicron era.
Methods
An SLR was conducted following the standards set out in the Cochrane Handbook for Systematic Reviews of Interventions and the Centre for Review and Dissemination (CRD) Guidance developed by the University of YorkCitation20. The protocol for the review was registered on the PROSPERO International prospective register of systematic reviews database (registration number: CRD42022370145).
Identification and Selection of studies
Structured search strategies were developed and executed in Embase, MEDLINE (and MEDLINE In-progress), and EconLit via OvidSP. The searches included free-text terms and controlled vocabulary terms for COVID-19, outcomes and study designs of interest, and US geographies (Table S1). The searches were run on 10 March 2023 and included studies published since 1 January 2021. Studies were not limited by language. Preprint articles were excluded if they were not published prior to the development of this manuscript (i.e. 10 July 2023). Citations and corresponding data for preprint articles captured by the SLR were updated to reflect the accepted peer-reviewed articles prior to development of this manuscript. Supplemental searching of the bibliography lists of relevant SLRs/meta-analyses identified by the database searches were also carried out to ensure that no relevant articles were missed.
Predefined inclusion/exclusion criteria following population, intervention, comparator, outcomes, and study (PICOS) design criteria () were used to evaluate all records identified from the searches. Given the interest in understanding how the economic outcomes may be impacted by variant, age group, level of risk for severe COVID-19 outcomes, and vaccination/treatment status, subgroup data based on these factors were captured by the SLR. Two researchers independently performed all title/abstract and full-text screening, with any discrepancies resolved by a third, independent researcher.
Table 1. Study Selection criteria.
Data extraction and synthesis
Data of interest were extracted by a single, independent researcher, and fully validated by a second researcher to ensure quality and accuracy. Any disagreements were resolved by conferring with a third reviewer. Data extraction templates were developed in Microsoft Excel® to capture and present key evidence from each study identified for inclusion. During data extraction, related publications and data sources were linked via reference identification numbers to ensure that overlapping data were identified and reported appropriately.
The results were summarized using narrative synthesis and forest plots for reported vaccine effectiveness (VE) or treatment effectiveness against COVID-19 hospitalization. When VE (or treatment effectiveness) was not reported, reported hazard ratios (HRs) or rate ratios (RRs) were used to calculate VE using the following formula: VE = (1 – R) × 100, where R could be a HR or RR. Given the variability of the data identified by the SLR, no quantitative synthesis (i.e. meta-analysis) of the data was feasible.
Results
Summary of included studies
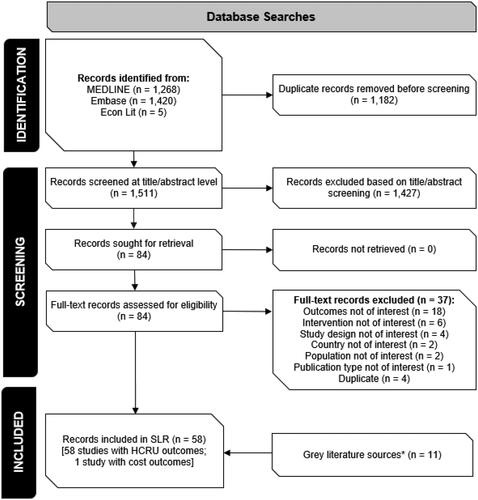
The study selection process is summarized in . A total of 2,693 articles, published between 1 January 2021 and 10 March 2023, were identified by the database searches. Following removal of duplicates and title/abstract screening, 84 articles were screened at the full text level and 58 studies met the PICOS criteria for inclusion in the SLR, representing over 126 million patients. All included studies reported HCRU outcomes and one study reported cost data.
Study characteristics
The majority of included studies were retrospective cohort studies (n = 43; 74.1%); there were also six prospective cohort studies (10.3%), seven retrospective case-control studies (12.1%), and two cross-sectional studies (3.4%). HCRU data were obtained from hospital (n = 17; 29.3%), regional (n = 17; 29.3%), and national (n = 24; 41.4%) databases. Most studies were multicenter (n = 53; 91.4%), with the most common data sources being the US Department of Veterans Affairs healthcare system records (n = 10; 17.2%), electronic health records for Kaiser Permanente members in Southern California (n = 6; 10.3%), and the Centers for Disease Control and Prevention (CDC) Surveillance Networks (COVID-NET and Overcoming COVID; n = 3; 5.2%). Data collection was predominantly conducted in inpatient settings (n = 54; 93.1%), while four studies included outpatient encounters (6.9%). The most common reported duration of follow-up was 30 days post-confirmed infection (n = 7), with the shortest duration of follow-up reported being >28 days and the longest, 285.5 days post-infection. Over half of the included studies did not report follow-up duration (n = 38; 65.5%), and only one study assessed reinfection rates (90 days after initial infection, or 45 to 89 days after a symptomatic second episode).
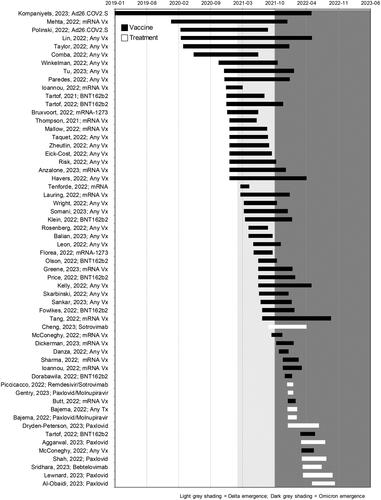
The most common interventions compared within the included studies were primary series vaccination vs. no vaccination (n = 35; 60.3%), booster vaccination vs. primary series vaccination (n = 11; 19.0%) of original monovalent vaccines, and treatment vs. no treatment (n = 11; 19.0%). BNT162b2 (Pfizer Inc. and BioNTech) was the most frequently assessed vaccine and nirmatrelvir/ritonavir (NMV/r) (Pfizer Inc.) was the most frequently assessed antiviral treatment. All studies assessing outcomes related to outpatient treatments were conducted during periods of Omicron variant emergence and/or predominance, while only a small portion of vaccine studies were conducted solely during the Omicron era (n = 7). Many of the vaccine studies had study periods that spanned Omicron variant emergence and/or predominance (n = 18), but few reported outcomes of interest stratified by variant including Omicron (n = 8). presents an overview of intervention assessed characteristics by study period.
Figure 2. Timeline of data collection period of included studies.
Light grey shading represents time periods of Delta predominance in the US, dark grey shading represents Omicron predominance. Prior to Delta emergence, Alpha was the predominant strain in the US. The y-axis presents the first author, publication year, and summary of the main interventions assessed for each study. Black bars correspond to studies assessing the impact of vaccines and white bars represent studies assessing the impact of treatments.
Abbreviations. mRNA, messenger ribonucleic acid; NMV/r, nirmatrelvir/ritonavir; Vx, vaccine.
Population characteristics
The characteristics of all studies are summarized in Table S2.
Most studies assessing vaccines focused on the adult population aged ≥18 years (n = 29), of which two studies included adults age ≥50 years. Eleven studies included mixed age populations (i.e. age <18 and ≥18 years) and six focused only on children (age ≤18 years). Most vaccine studies reported high-risk characteristics among patients at baseline (n = 26), which were highly variable. Forty studies confirmed SARS-CoV-2 detection with a nucleic acid amplification or antigen test while six studies did not specify methods for detecting SARS-CoV-2.
Most studies assessing outpatient treatments focused on adult populations (n = 9; two studies including age ≥50 and ≥65 years). The remaining three studies included both children and adults. Seven treatment studies specifically enrolled patients at high risk of progression to severe COVID-19 and five studies recruited patients treated in the outpatient setting regardless of risk. Ten studies reported high-risk characteristics of patients at baseline, which were highly variable. All studies confirmed COVID-19 diagnosis with a nucleic acid amplification or antigen test.
Impact of booster vaccination
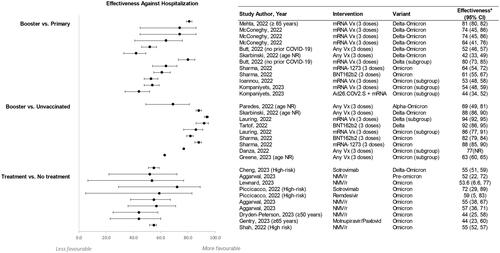
All booster studies were conducted during periods of Omicron and/or Delta predominance and assessed the original monovalent vaccines. Studies evaluated the VE of a booster dose (eight studies) or a primary series without a booster dose (12 studies), and the most commonly reported outcome was COVID-19-related hospitalization ().
Figure 3. Effectiveness against hospitalization for booster vs. primary series vaccination (panel a), booster vs. no vaccination (panel B), and treatment vs. no treatment (panel C).
*Effectiveness is described as reported or when not reported, was calculated from the reported hazard ratios or rate ratios using the following formula: Effectiveness = (1 – R) × 100, where R could be a hazard ratio or rate ratio.
Abbreviations. CI, confidence interval; mRNA, messenger ribonucleic acid; NMV/r, nirmatrelvir/Ritonavir; NR, not reported; Vx, vaccine.
Booster compared to no vaccination
Among the eight studies that assessed the impact of the original monovalent booster vaccination compared with no vaccination, five studies were conducted in adults, three were conducted in mixed populations (all ≥12 years), and two were conducted in children (aged 6 to 17 years). Overall, these studies found a significant reduction in the risk of COVID-related hospitalization for patients who received an original monovalent primary series plus booster dose compared with no vaccination. One study conducted in California in mixed age populations estimated the risk of COVID-related hospitalization in unvaccinated individuals to be more than eight times greater than that of individuals who had received a booster dose (of any vaccine type) during periods of Delta and Omicron predominance (adjusted hazard ratio [aHR] 8.34 [95% confidence interval (CI): 7.25, 9.69])Citation21. Across other studies reporting the risk of hospitalization, the VE of a booster compared with no vaccination ranged from 63% (95% CI: 60%, 65%) to 94% (95% CI: 92%, 95%) ()Citation22,Citation23.
The VE range for those who received a booster compared to no vaccination showed some correlation with SARS-CoV-2 variant. Hospitalization risk was lower during Omicron predominance compared with Delta predominance (). Despite this reduction in VE associated with Omicron, unvaccinated individuals were reported in one study to have 10 to 11 times higher risk of Omicron-related hospitalization (January to April 2022) compared with individuals who had received a booster doseCitation24.
The reduction in risk for severe COVID-19 following booster vaccination was reflected not only in hospitalization rates but also in the requirements for ICU admission, oxygen support, and invasive mechanical ventilation (IMV)Citation21. During the period January 2021 through April 2022, 21.7% of unvaccinated adults infected with SARS-CoV-2 (1,961/8,575) required admission to an ICU, compared with 19.5% of adults (505/2,552) who had been vaccinated with a primary series with or without a booster dose (p = 0.13)Citation24. In a second study, unvaccinated individuals were at greater risk of needing low-flow oxygen support (aHR 5.68 [95% CI: 4.19, 7.69]), high-flow oxygen support (aHR 5.39 [95% CI: 3.45, 8.4]) or IMV (aHR 5.51 [95% CI: 2.44, 12.4]) during the Delta predominant period compared with booster dose recipientsCitation21. Overall, Omicron infection was associated with a lower risk of severe outcomes, with fewer patients requiring low-flow or high-flow oxygen support, or IMV during the Omicron vs. Delta predominant period (low-flow oxygen: 1.6% vs. 6.4%; high-flow oxygen: 0.6% vs. 2.8%; IMV: 0.1% vs. 0.7%)Citation21. Despite this, unvaccinated individuals remained at greater risk of severe outcomes during Omicron predominance compared with booster dose recipients, with increased likelihood of needing low-flow oxygen support (aHR 11.95 [95% CI: 9.58, 14.92]), high-flow oxygen support (aHR 21.8 [95% CI: 14.8, 32.11]), or IMV (aHR 14.16 [95% CI: 6.93, 28.93])Citation21.
Data on VE among children and adolescents were available in two of the studies identified in the SLR. Using an age-matched unvaccinated population as a reference, one study estimated the VE of a booster dose in preventing emergency department (ED)/urgent care visits among 16- to 17-year-olds to be 86% (95% CI: 73%, 95%)Citation25. A second study estimated that VE in preventing ED or urgent care encounters related to acute respiratory failure caused by Omicron was 87% (95% CI: 72%, 94%) among 12- to 17-year-olds who had received a booster dose a median of 19 days earlierCitation26.
Booster compared to primary series
The VE of a booster dose compared with primary series alone in preventing hospitalizations was evaluated in 12 studies (seven studies in adults, three studies with mixed populations, and two studies in elderly populations [≥70 years old]). Overall, receipt of a booster vaccine was associated with a lower risk of hospitalization compared to receipt of only the primary series ()Citation21,Citation27. A study conducted between January and April 2022 found that individuals who received an original monovalent primary series but no booster dose were up to three times more likely to be hospitalized with COVID-19 compared with individuals who received a booster doseCitation24.
The VE of booster vaccination relative to an original monovalent primary series vaccination alone in the prevention of COVID-19-related hospitalization was estimated to range from 42% (95% CI: 33%, 49%) among individuals aged 0 to >80 years, to 81% (95% CI: 80%, 82%) in a study of individuals aged ≥65 yearsCitation21,Citation28. Despite this range, no obvious trends were observed according to variant or vaccine type (). A study of 18.9 million adults found no significant differences in VE across various age groups or when comparing various combinations of primary and booster vaccinesCitation28,Citation29.
The reduction in the risk of requiring low- or high-flow oxygen support or IMV among booster dose recipients compared with primary series-only recipients has also been demonstratedCitation21. During Omicron predominance, individuals vaccinated with the primary series were found to be at increased risk of needing low-flow oxygen support (aHR 2.62 [95% CI: 2.09, 3.28]), high-flow oxygen support (aHR 2.47 [95% CI: 1.63, 3.76]), or IMV (aHR 1.38 [95% CI: 0.61, 3.12])Citation21.
Impact of primary vaccination
Thirty-eight studies reported outcomes of interest for primary series vaccines, spanning periods of Alpha, Delta, and/or Omicron predominance.
A total of 20 studies compared the risk of hospitalization among recipients of primary series vaccination with that of unvaccinated individuals (Figure S1). Compared to no vaccination, the VE of primary series vaccination in the prevention of hospitalization ranged from 23% (95% CI: 18%, 28%)Citation22 to 96% (95% CI: 92%, 98%)Citation30. VE estimates were higher during Delta predominance (ranging from 42% [95% CI: 38%, 45%]Citation31 to 96% [95% CI: 92%, 98%])Citation30, and lower during Omicron predominance (ranging from 23% [95% CI: 18%, 28%]Citation22 to 85% [95% CI: 82%, 88%])Citation23. Studies evaluating the impact of prior SARS-CoV-2 infection on VE of the primary series were inconclusiveCitation31–34. There were also no clear trends in VE according to age groupCitation23,Citation35–38.
Primary series vaccination not only provided a reduction in hospitalization risk, but also reduced the risk of ICU admission and the IMV requirement, compared with no vaccinationCitation22,Citation24,Citation34. A study of adults hospitalized with COVID-19 in the US between April 2021 and January 2022, found the length of hospital stay to be significantly greater among unvaccinated vs. vaccinated patients (8.92 [±9.80] days vs. 6.47 [±4.76] days; p = 0.03)Citation39. Unvaccinated patients were also more likely to require ICU admission (p = 0.006) and IMV use (p = 0.0004) than vaccinated patients, and the mean duration of IMV was significantly longer among the unvaccinated compared with vaccinated patients (17.81 [±14.00] days vs. 3.01 [±2.75] days; p = 0.003)Citation39. Unadjusted hospitalization data showed that costs were nominally higher for the unvaccinated vs. vaccinated group ($191,146 [± $328,233] vs. $119, 630 [± $78,833]; p = 0.006), with the cost of hospitalization per day being significantly higher in the former ($29,245 vs. $13,845; p < 0.0001)Citation39. Correspondingly, following an adjustment for comorbidities and age, hospitalization costs were significantly lower for vaccinated compared with unvaccinated individuals (Δ 26%, p = 0.004)Citation39.
Primary series vaccination was also associated with reduction in HCRU among the pediatric population. In one study of individuals aged five through 17 years, conducted between April 2021 and January 2022, 71.6% of ED or urgent care encounters were accounted for by unvaccinated individuals (n = 28,084/39,217), while 19.9% (n = 7,821/39,217) and 8.3% (n = 3,238/39,217) were accounted for by individuals who had received a second vaccine dose 14 to 149 days or ≥150 days earlier, respectivelyCitation25. In a multicenter study of individuals aged 12 through 18 years, across 23 US states, ICU admissions and IMV requirements occurred in 42% (178/427) and 11% (48/425) of unvaccinated individuals, respectively, compared with 11.1% (n = 2/18) and 5.6% (n = 1/18) of vaccinated individuals, respectivelyCitation40. A third study of individuals five to 15 years conducted between July 2021 and February 2022 found unvaccinated individuals missed a mean average of 26.2 h of school (standard error 17.5 h) as a result of COVID-19, approximately 11 h more than that missed by vaccinated individuals (OR 11.1 [95% CI: 4.6, 17.6]; p = 0.01)Citation41. The number of days spent sick in bed with COVID-19 was also greater among unvaccinated vs. vaccinated individuals (p = 0.016). Despite this, the proportion of pediatric patients who sought medical care due to COVID-19 was not significantly different between the unvaccinated and vaccinated population (p = 0.949)Citation41.
Impact of treatment
Overall, results from the 12 treatment publications identified in the SLR suggest there is a significantly reduced risk of hospitalizations among recipients of NMV/r, remdesivir, sotrovimab, and molnupiravir compared to non-recipients ()Citation42–47. Between 1 January and 17 July 2022, patients treated with NMV/r in the outpatient setting were significantly less likely to be hospitalized than patients who received no treatment (adjusted risk ratio: 0.60 [95% CI: 0.44, 0.81]; p = NR])Citation42. One study found that the effectiveness of NMV/r at preventing hospitalization was comparable before (OR 0.48 [95% CI: 0.28, 0.78]) and during (OR 0.43 [95% CI: 0.29, 0.64]) predominance of Omicron BA.4/BA.5 subvariantsCitation48. NMV/r also reduced the odds of ED visits compared to no treatment (OR 0.74 [95% CI: 0.63, 0.87]; p = 0·0002)Citation48. In two additional studies that specifically enrolled patients at high risk of progression to severe COVID-19 during periods of Omicron predominance in 2022, NMV/r was associated with lower rates of hospitalization, mortality, and ED visits than no treatmentCitation43,Citation47.
The odds of hospitalization or ED visits within 29 days from symptom onset were lower in patients treated with remdesivir (OR: 0.41 [95% CI: 0.17,0.95]) and sotrovimab (OR: 0.28 [95% CI: 0.11, 0.71]) than in patients without treatment between 27 December 2021 and 4 February 2022Citation44. Sotrovimab also reduced the risk of 30-day hospitalization by 55% among high-risk patients diagnosed with COVID-19 between 1 September 2021 and 30 April 2022, compared to no monoclonal antibody treatmentCitation45. While molnupiravir was not evaluated separately in any of the studies identified in the SLR, one study conducted in adults aged ≥65 years between 1 January and 6 February 2022 reported reduced odds of hospitalization or death in patients treated with molnupiravir or NMV/r compared to untreated patientsCitation46. However, bebtelovimab did not significantly decrease the risk of 30-day all-cause hospitalization and/or mortality in high-risk patients infected with Omicron subvariants (mainly BA.2, BA.2.12.1, and BA.5) compared with no treatmentCitation49.
Age ≥50 years is the strongest risk factor for severe COVID-19 outcomes, including hospitalization and death, based on February 2020 to July 2022 data from the US National Center for Health StatisticsCitation50. Patients who were unvaccinated or received fewer than the recommended number of vaccinations are also at higher risk of severe COVID-19 outcomesCitation50. Results from two studies found patients receiving treatment for COVID-19 were more likely to be older than untreated patientsCitation45,Citation51. Between 1 September 2021 and 30 April 2022, the median age of patients treated with sotrovimab was 55 years compared to 48 years for patients not treated with a monoclonal antibody (p < 0.0001)Citation45. Similarly, patients treated with NMV/r, molnupiravir, remdesivir, or sotrovimab between 1 January and 28 February 2022 were more likely to be older (65 through 74 and ≥65 years vs. 50 through 64 years) than untreated patientsCitation51. Patients aged 50 through 64 years were also more likely to receive treatment than patients aged 18 through 49 yearsCitation51. Among high-risk patients, NMV/r reduced the risk of hospitalization in patients aged 18 through 49 years (aHR 0.59 [95% CI: 0.48, 0.71]), 50 through 64 years (aHR 0.40 [95% CI: 0.34, 0.48]), and ≥65 years (aHR 0.53 [95% CI: 0.48, 0.58]) between 1 April 2022 and 31 August 2022Citation43.
NMV/r was associated with reduced odds of hospitalization among patients who were unvaccinated (aOR 0.46 [95% CI: 0.27, 0.77]), vaccinated with one to two doses (aOR 0.40 [95% CI: 0.20, 0.79]), and vaccinated with three or more doses (aOR 0.47 [95% CI: 0.29, 0.74]) compared to no treatment during periods of Omicron BA.4/BA.5 predominanceCitation48. A second study reported similar reduction in hospitalizations among those who had received primary series or booster mRNA vaccines (50% risk reduction for both) compared to the overall population of patients treated with NMV/r (51% risk reduction)Citation43. Likewise, sotrovimab reduced hospitalizations by 57% in vaccinated patients vs. 55% in all treated patients compared to no monoclonal antibodyCitation45, and oral antiviral treatments (NMV/r and molnupiravir) were associated with significantly lower death rates vs. no oral antivirals regardless of vaccination statusCitation46. However, two studies also reported greater oral antiviral treatment effect in unvaccinated or partially vaccinated patients compared to fully vaccinated patientsCitation42,Citation46.
Discussion
After screening more than 2,500 articles published as of March 10, 2023, 58 studies reporting on the impact of COVID-19 vaccination and outpatient treatment on the economic burden (in terms of HCRU and costs) of the disease in the US were included in this SLR. All included studies reported HCRU outcomes stratified by treatment and/or vaccination status and one reported cost data stratified by vaccination status. Overall, the introduction of COVID-19 vaccines and outpatient treatments have had a significant positive impact on HCRU in the US. Most of the included studies reported a reduction in the risk of COVID-related hospitalizations among treated patients and/or patients who received an original monovalent primary series vaccine plus booster dose compared with no treatment or vaccination. Receipt of a booster vaccine was associated with as much as an 81% reduction in the risk of COVID-related hospitalization compared to primary series vaccination, while receipt of an outpatient treatment was associated with as much as 72% reduction in risk compared to no treatment. Other evidence also supported reduced rates of ICU admission among hospitalized patients, as well as reductions in other HCRU outcomes (e.g. length of stay, ED and urgent care clinic encounters), in treated and/or vaccinated patients.
Specifically, receipt of a booster vaccine was associated with a reduced risk of hospitalization among adults and a reduced risk of ED and urgent care clinic visits among adolescents compared with no vaccination across studies. Administration of a booster vaccine was also associated with reduced rates of hospitalization and ICU admission compared with administration of only the primary vaccine series in adults. Several studies including both periods of Delta and Omicron predominance reported that booster VE against hospitalization was lower during Omicron than DeltaCitation23,Citation52, and one study found that there was a lower risk of hospitalization overall (regardless of vaccination status) following Omicron infection vs. DeltaCitation53. Hospitalization rates and VE against hospitalization did not differ significantly by age groupCitation28,Citation29,Citation54, although data stratified by age groups for outcomes of interest were very limited.
Outpatient treatment with NMV/r, remdesivir, sotrovimab, and molnupiravir generally reduced the risk of HCRU outcomes, such as hospitalizations and ED visits, compared to no treatmentCitation42–46. Most studies of outpatient treatments evaluated HCRU outcomes in a population of patients at high risk for progressing to severe COVID-19, including hospitalization or death (in line with Food and Drug Administration [FDA] approval/authorization language for the treatment of mild-to-moderate COVID-19)Citation43–45,Citation47,Citation49,Citation51,Citation55. Studies without inclusion criteria restricting analyses to high-risk patients potentially included a mix of patients at high-risk and standard-risk for progressing to severe COVID-19; therefore, these studies are not representative of patients at standard-risk of severe COVID-19 and should not be used to draw conclusions for risk of HCRU outcomes among this populationCitation21,Citation42,Citation46,Citation48,Citation56. The impact of age on treatment patterns and outcomes was assessed in several studies however. Treatments tended to be effective regardless of ageCitation43,Citation48. Treated patients were more likely to be older than untreated patientsCitation45,Citation51, and one study found age to be an independent risk factor for severe COVID-19 outcomesCitation21. Few studies specifically evaluated the impact of SARS-CoV-2 variants on HCRU outcomes with COVID-19 outpatient treatment; one study found no difference in the impact of NMV/r vs. no treatment on the risk of hospitalization during Omicron BA.4/BA.5 predominance compared with prior to the emergence of these subvariantsCitation48.
Findings were inconclusive in terms of the impact of vaccination status on HCRU outcomes with outpatient treatment, with some studies demonstrating increased protection against hospital admission among treated, vaccinated patients (compared with unvaccinated treated patients)Citation42,Citation45,Citation46 and others demonstrating similar treatment effects regardless of vaccination statusCitation43,Citation48.
The COVID-19 treatment landscape continues to evolve as the virus mutates. Although four monoclonal antibodies (bebtelovimab, bamlanivimab + etesevimab, casirivimab + imdevimab, and sotrovimab) have previously received emergency use authorization from the FDA for the treatment of outpatients with mild to moderate COVID-19, these treatments are no longer authorized in the US or recommended by the NIH as of April 2022Citation57 because the predominant Omicron subvariants are not expected to be susceptible to these treatmentsCitation58. For example, a study of bebtelovimab identified by this SLR found no benefit with treatment in terms of 30-day all-cause hospitalization and/or mortality among patients infected with SARS-CoV-2 Omicron subvariants (mainly BA.2, BA.2.12.1, and BA.5)Citation49. Studies of sotrovimab identified by this SLR did report a treatment benefitCitation44,Citation45; however, these findings were largely based on patients treated prior to the emergence of the BA.2 subvariant and the subsequent revision of the emergency use authorization by the FDA in April 2022Citation59.
There is a lack of data available on the cost impact of COVID-19 vaccination and outpatient treatment on economic burden in the US, representing a substantial gap in evidence. The one cost study identified by this SLR reported the adjusted cost of hospitalization per day to be on average $15,400 more for unvaccinated patients compared to vaccinated patients, which would equate to an additional $37,730 per patient when considering the additional mean length of stay estimated for unvaccinated patients of 2.45 daysCitation39. In addition to increased length of stay, higher rates of ICU admission and IMV were significant drivers of increased costs for unvaccinated vs. vaccinated patientsCitation39. These findings are consistent with findings of a retrospective claims study of ∼1.2 million Medicare fee for service beneficiaries that was conducted between April and December 2020 (prior to the availability of COVID-19 vaccines in the US), which found the mean cost of hospitalization per patient to be $27,689 higher for patients requiring IMV compared to those hospitalized without IMVCitation6. In this study patients over 75 years had a higher rate of hospitalization, but were associated with a lower hospitalization cost compared to younger patientsCitation6.
Our SLR found significant gaps in data on costs or HCRU stratified by age groups and/or level of risk for severe COVID-19 outcomes and therefore, no clear trends regarding the impact of these factors on vaccine and/or treatment effectiveness could be deduced. The CDC has defined age as the strongest risk factor for severe COVID-19 outcomes, along with the presence of specific underlying medical conditions, and vaccination statusCitation50. It is therefore of key interest to understand how economic outcomes are impacted by these factors, not only independently, but also in light of newer adapted COVID-19 vaccines in the US.
In addition to data gaps, this review encountered some other specific limitations. Data for the same patients may be represented in multiple studies given that most of the identified studies were conducted during periods of Omicron variant emergence and/or predominance and that many of the studies utilized the same regional or national data sources (such as Kaiser Permanente Southern California [KPSC], HealthVerity, the VISION network and Veterans Affairs [VA] databases). Additionally, synthesis of the evidence identified by the SLR was limited by differences in outcome definitions across studies, as well as whether adjustment for covariates was undertaken. Varying duration of follow-up also impacts the findings and the ability to compare across studies.
COVID-19 is expected to become endemic with potential peaks during fall and winter seasonsCitation19. It is difficult to predict when COVID-19 can be considered endemic given uncertainty around the strength and duration of immune protection from vaccination, the transmissibility of the virus, and the development of mutations resulting in new variants. With the ending of the COVID-19 Public Health Emergency (PHE) declaration in the US on 11 May 2023, vaccines and treatments will remain available, and the US Department of Health and Human Services have noted that access to vaccines and certain treatments (including NMV/r and molnupiravir) will generally not be affectedCitation18; however, the national reporting of COVID-19 data by the CDC has changed towards an approach more typical of endemic disease monitoring, focusing on measures of severe illness and healthcare system capacity rather than virus transmissionCitation60.
Thus, evaluation of HCRU and cost outcomes remain of high priority as COVID-19 shifts to a new phase, and published literature on these topics continue to evolve. Since the searches for this SLR were updated in March 2023, additional studies demonstrating the positive impact of booster vaccines and outpatient treatments on HCRU outcomes, including hospitalizations, ICU admissions, and ventilation use, during periods of Omicron predominance have been publishedCitation61–64. In particular, while this SLR only identified studies of original monovalent vaccines, relevant studies on the HCRU impact of bivalent vaccines are now emergingCitation65–68; these studies suggest that mRNA based bivalent booster doses provide additional protection against hospitalization, ICU visits and ED/urgent care clinic encounters, though with waning effectiveness over timeCitation65–68. In terms of recently published outpatient treatment studies, two emulations of randomized target trials based on VA databases reported that treatment with NMV/r or molnupiravir reduced the risk of hospital admission/death at 30 days vs. no treatment regardless of the predominant variant being Omicron BA.1/BA.2 or BA.5 and regardless of vaccination statusCitation63,Citation64. Additionally, while the studies identified by the SLR generally evaluated outcomes associated with acute COVID-19, a study reporting on post-acute hospitalization has now been published; treatment with NMV/r was associated with a reduced risk of post-acute hospitalization vs. no treatment at 180 days among patients who had at least one risk factor for progression to severe illnessCitation69.
To our knowledge, this is the first SLR conducted with a focus on how COVID-19 vaccines and outpatient treatments have impacted HCRU and costs in the US. More general global SLRs of the economic impact of COVID-19 itself have been conducted, generally without specifically assessing the influence of treatment or vaccinationCitation70,Citation71. One global SLR of remdesivir studies found that COVID-19 treatment with remdesivir (largely among hospitalized patients) was associated with decreases in hospital and ICU bed occupancyCitation72.
Conclusions
This comprehensive SLR identified and synthesized the available evidence demonstrating the impact of COVID-19 interventions (specifically vaccines and outpatient treatments) on the economic burden of COVID-19 in the US. The identified studies were heterogenous in terms of study design, outcome measures, and patient populations. Overall, these studies demonstrated a significant positive impact of COVID-19 vaccination and targeted outpatient treatment administration on HCRU in the US, including during periods of Omicron predominance. However, significant gaps were identified in terms of the following: cost data; HCRU associated with long COVID; and data for patients infected with the most recently circulating Omicron variants (such as XBB and its sub-lineages). These are important areas of continued research to inform clinical and policy decision-making in the US as we move beyond the pandemic into a new phase of COVID-19.
Transparency
Declaration of funding
Funding for this study was provided by Pfizer, Inc. Employees of Pfizer were involved with the study design, interpretation of data, writing of the manuscript, and decision to submit the paper for publication.
Declaration of financial/other interests
FD, MDF, JY, SL, MMM, JN, ACS, MG, and AY are employees of Pfizer Inc. and may hold stock or stock options. VP, SNG, JK, PA, NS, and HB are employed by Evidera, a part of Thermo Fisher Scientific that provides consulting and other research services to pharmaceutical, medical device, and related organizations. In their salaried positions, VP, SNG, JK, PA, NS, and HB work with a variety of companies and are precluded from receiving payment or honoraria directly from these organizations for services rendered. Evidera received payment from Pfizer Inc. for the conduct of this study.
Author contributions
Conception and design: VP, FD, MDF, JY, MMM, JN, MG, HB; Analysis and interpretation of the data: VP, FD, MDF, JY, SNG, JK, SL, MMM, JN, PA, ACS, MG, AY, NS, HB.
Drafting of the paper: VP, NS, HB; Revising the paper critically for intellectual content: VP, FD, MDF, JY, SNG, JK, SL, MMM, JN, PA, ACS, MG, AY, NS, HB; Final approval of the version to be published: VP, FD, MDF, JY, SNG, JK, SL, MMM, JN, PA, ACS, MG, AY, NS, HB.
Reviewer disclosures
Peer reviewers on this manuscript have no relevant financial or other relationships to disclose.
Previous presentations
None.
Supplemental Material
Download MS Word (293.7 KB)Acknowledgements
The authors would like to acknowledge Denise So and Jennifer Knight for their editorial support.
Data availability
This article is based on published literature and therefore does not contain any applicable data sets.
Correction Statement
This article was originally published with errors, which have now been corrected in the online version. Please see Correction (http://dx.doi.org/10.1080/13696998.2024.2304418)
References
- Centers for Disease Control and Prevention. COVID Data Tracker 2023; [cited 2023 Aug 9]. Available from: https://covid.cdc.gov/covid-data-tracker/#datatracker-home.
- Ahmad FB, Cisewski JA, Xu J, et al. Provisional mortality data – United States, 2022. MMWR Morb Mortal Wkly Rep. 2023;72(18):488–492. doi:10.15585/mmwr.mm7218a3.
- Our World in Data. Coronavirus (COVID-19) Hospitalizations 2023; [cited 2023 Aug 14]. Available from: https://ourworldindata.org/covid-hospitalizations.
- Glaser GE, Lara OD, Pothuri B, et al. Clinical outcomes in patients with COVID-19 and gynecologic cancer: a society of gynecologic oncology COVID-19 and gynecologic cancer registry study. Gynecol Oncol. 2022;166:s34. doi:10.1016/j.ygyno.2022.09.017.
- Di Fusco M, Vaghela S, Moran MM, et al. COVID-19-associated hospitalizations among children less than 12 years of age in the United States. J Med Econ. 2022;25(1):334–346. doi:10.1080/13696998.2022.2046401.
- Tsai Y, Vogt TM, Zhou F. Patient characteristics and costs associated with COVID-19-related medical care among medicare fee-for-Service beneficiaries. Ann Intern Med. 2021;174(8):1101–1109. www.acponline.org/authors/icmje/ConflictOfInterestForms.do?msNum=M21-1102.eng. doi:10.7326/m21-1102.
- Centers for Disease Control and Prevention. Long COVID - Household Pulse Survey. https://www.cdc.gov/nchs/covid19/pulse/long-covid.htm. 2023.
- Perlis RH, Lunz Trujillo K, Safarpour A, et al. Association of Post-COVID-19 condition symptoms and employment status. JAMA Netw Open. 2023;6(2):e2256152. doi:10.1001/jamanetworkopen.2022.56152.
- Hernandez-Romieu AC, Leung S, Mbanya A, et al. Health care utilization and clinical characteristics of nonhospitalized adults in an integrated health care system 28-180 days after COVID-19 Diagnosis – Georgia, may 2020-March 2021. MMWR Morb Mortal Wkly Rep. 2021;70(17):644–650. doi:10.15585/mmwr.mm7017e3.
- Bach K. New data shows long Covid is keeping as many as 4 million people out of work. https://www.brookings.edu/research/new-data-shows-long-covid-is-keeping-as-many-as-4-million-people-out-of-work/. 2022.
- World Health Organization. Fourth round of the global pulse survey on continuity of essential health services during the COVID-19 pandemic: November 2022–January 2023. 2023.
- Taylor CA, Whitaker M, Anglin O, et al. COVID-19-Associated hospitalizations among adults during SARS-CoV-2 Delta and omicron variant predominance, by race/ethnicity and vaccination Status - COVID-NET, 14 states, July 2021–January 2022. MMWR Morb Mortal Wkly Rep. 2022;71(12):466–473. doi:10.15585/mmwr.mm7112e2.
- Admon AJ, Iwashyna TJ, Kamphuis LA, et al. Assessment of symptom, disability, and financial trajectories in patients hospitalized for COVID-19 at 6 months. JAMA Netw Open. 2023;6(2):e2255795. doi:10.1001/jamanetworkopen.2022.55795.
- Gibbons DC, Marijam A, Symons JM, et al. Healthcare resource utilization and costs associated with COVID-19 among hospitalized patients in the United States – a population study. San Francisco: American Thoracic Society; 2022.
- Centers for Disease Control and Prevention. COVID-19 Pandemic Planning Scenarios. Available from: https://www.cdc.gov/coronavirus/2019-ncov/hcp/planning-scenarios.html. 2021.
- Centers for Disease Control and Prevention. COVID data tracker. 2023.
- World Health Organization. Statement on omicron sublineage BA.2. 2022.
- US Department of Health and Human Services. Fact sheet: end of the COVID-19 public health emergency. 2023.
- World Health Organization. From emergency response to long-term COVID-19 disease management: sustaining gains made during the COVID-19 pandemic. 2023.
- Centre for Reviews and Dissemination. Systematic reviews: CRD’s guidance for undertaking reviews in health care. New York: CRD, University of York; 2009.
- Skarbinski J, Wood MS, Chervo TC, et al. Risk of severe clinical outcomes among persons with SARS-CoV-2 infection with differing levels of vaccination during widespread Omicron (B.1.1.529) and Delta (B.1.617.2) variant circulation in Northern California: a retrospective cohort study. Lancet Reg Health Am. 2022;12:100297. doi:10.1016/j.lana.2022.100297.
- Greene SK, Levin-Rector A, Kyaw NTT, et al. Comparative hospitalization risk for SARS-CoV-2 Omicron and Delta variant infections, by variant predominance periods and patient-level sequencing results, New York City, August 2021–January 2022. Influenza Other Respir Viruses. 2023;17(1):e13062. doi:10.1111/irv.13062.
- Lauring AS, Tenforde MW, Chappell JD, et al. Clinical severity of, and effectiveness of mRNA vaccines against, covid-19 from omicron, Delta, and alpha SARS-CoV-2 variants in the United States: prospective observational study. Bmj. 2022;376:e069761. doi:10.1136/bmj-2021-069761.
- Havers FP, Pham H, Taylor CA, et al. COVID-19-Associated hospitalizations among vaccinated and unvaccinated adults 18 years or older in 13 US states, January 2021 to April 2022. JAMA Intern Med. 2022;182(10):1071–1081. doi:10.1001/jamainternmed.2022.4299.
- Klein NP, Stockwell MS, Demarco M, et al. Effectiveness of COVID-19 Pfizer-BioNTech BNT162b2 mRNA vaccination in preventing COVID-19-Associated emergency department and urgent care encounters and hospitalizations among nonimmunocompromised children and adolescents aged 5–17 Years – VISION network, 10 States, April 2021–January 2022 [technical report]. MMWR Morb Mortal Wkly Rep. 2022;71(9):352–358. doi:10.15585/mmwr.mm7109e3.
- Tartof SY, Frankland TB, Slezak JM, et al. Effectiveness associated with BNT162b2 vaccine against emergency department and urgent care encounters for Delta and omicron SARS-CoV-2 infection among adolescents aged 12 to 17 years [research support, Non-U.S. Gov’t]. JAMA Netw Open. 2022;5(8):e2225162. doi:10.1001/jamanetworkopen.2022.25162.
- Butt AA, Talisa VB, Shaikh OS, et al. Relative vaccine effectiveness of a SARS-CoV-2 mRNA vaccine booster dose against the omicron variant. Clin Infect Dis. 2022;3:03. doi:10.1093/cid/ciac328.
- Mehta HB, Li S, Goodwin JS. Effectiveness of COVID-19 booster on the risk of hospitalization among medicare beneficiaries. Mayo Clin Proc. 2022;97(10):1780–1793. doi:10.1016/j.mayocp.2022.06.029.
- Kompaniyets L, Wiegand RE, Oyalowo AC, et al. Relative effectiveness of COVID-19 vaccination and booster dose combinations among 18.9 million vaccinated adults during the early SARS-CoV-2 omicron period – United States, 1 January 2022–31 March 2022. Clin Infect Dis. 2023;08:08. doi:10.1093/cid/ciad063.
- Bruxvoort KJ, Sy LS, Qian L, et al. Real-world effectiveness of the mRNA-1273 vaccine against COVID-19: interim results from a prospective observational cohort study. Lancet Reg Health Am. 2022;6:100134. doi:10.1016/j.lana.2021.100134.
- Anzalone AJ, Sun J, Vinson AJ, et al. Community risks for SARS-CoV-2 infection among fully vaccinated US adults by rurality: a retrospective cohort study from the national COVID cohort collaborative]. PLoS One. 2023;18(1):e0279968. doi:10.1371/journal.pone.0279968.
- Risk M, Shen C, Hayek SS, et al. Comparative effectiveness of coronavirus disease 2019 (COVID-19) vaccines against the Delta variant [comparative study]. Clin Infect Dis. 2022;75(1):e623–e629. doi:10.1093/cid/ciac106.
- Tang F, Hammel IS, Andrew MK, et al. COVID-19 mRNA vaccine effectiveness against hospitalisation and death in veterans according to frailty status during the SARS-CoV-2 Delta (B.1.617.2) variant surge in the USA: a retrospective cohort study. Lancet Healthy Longev. 2022;3(9):e589–e598. doi:10.1016/S2666-7568(22)00166-0.
- Lin DY, Gu Y, Xu Y, et al. Association of primary and booster vaccination and prior infection with SARS-CoV-2 infection and severe COVID-19 outcomes. Jama. 2022;328(14):1415–1426. doi:10.1001/jama.2022.17876.
- Tartof SY, Slezak JM, Puzniak L, et al. Effectiveness of a third dose of BNT162b2 mRNA COVID-19 vaccine in a large US health system: a retrospective cohort study. Lancet Reg Health Am. 2022;9:100198. doi:10.1016/j.lana.2022.100198.
- Winkelman TNA, Rai NK, Bodurtha PJ, et al. Trends in COVID-19 vaccine administration and effectiveness through october 2021 [research support, Non-U.S. JAMA Netw Open. 2022;5(3):e225018. doi:10.1001/jamanetworkopen.2022.5018.
- Tenforde MW, Patel MM, Ginde AA, et al. Effectiveness of severe acute respiratory syndrome coronavirus 2 messenger RNA vaccines for preventing coronavirus disease 2019 hospitalizations in the United States. Clin Infect Dis. 2022;74(9):1515–1524. doi:10.1093/cid/ciab687.
- Florea A, Sy LS, Luo Y, et al. Durability of mRNA-1273 against COVID-19 in the time of Delta: interim results from an observational cohort study. PLoS One. 2022;17(4):e0267824. doi:10.1371/journal.pone.0267824.
- Somani ST, Firestone RL, Donnelley MA, et al. Impact of vaccination on cost and course of hospitalization associated with COVID-19 infection. Antimicrob Steward Healthc Epidemiol. 2023;3(1):e19. doi:10.1017/ash.2022.364.
- Olson SM, Newhams MM, Halasa NB, et al. Effectiveness of BNT162b2 vaccine against critical covid-19 in adolescents. N Engl J Med. 2022;386(8):713–723. doi:10.1056/NEJMoa2117995.
- Fowlkes AL, Yoon SK, Lutrick K, et al. Effectiveness of 2-Dose BNT162b2 (pfizer BioNTech) mRNA vaccine in preventing SARS-CoV-2 infection among children aged 5-11 years and adolescents aged 12-15 Years - PROTECT cohort, July 2021–February 2022. MMWR Morb Mortal Wkly Rep. 2022;71(11):422–428. doi:10.15585/mmwr.mm7111e1.
- Dryden-Peterson S, Kim A, Kim AY, et al. Nirmatrelvir plus ritonavir for early COVID-19 in a large U.S. Health system: a population-based cohort study. Ann Intern Med. 2023;176(1):77–84. doi:10.7326/M22-2141.
- Shah MM, Joyce B, Plumb ID, et al. Paxlovid associated with decreased hospitalization rate among adults with COVID-19 – United States, April–September 2022. MMWR Morb Mortal Wkly Rep. 2022;71(48):1531–1537. doi:10.15585/mmwr.mm7148e2.
- Piccicacco N, Zeitler K, Ing A, et al. Real-world effectiveness of early remdesivir and sotrovimab in the highest-risk COVID-19 outpatients during the omicron surge. J Antimicrob Chemother. 2022;77(10):2693–2700. doi:10.1093/jac/dkac256.
- Cheng MM, Reyes C, Satram S, et al. Real-world effectiveness of sotrovimab for the early treatment of COVID-19 during SARS-CoV-2 Delta and Omicron waves in the USA. Infect Dis Ther. 2023;12(2):607–621. doi:10.1007/s40121-022-00755-0.
- Gentry CA, Nguyen P, Thind SK, et al. Characteristics and outcomes of US veterans at least 65 years of age at high risk of severe SARS-CoV-2 infection with or without receipt of oral antiviral agents. J Infect. 2023;86(3):248–255. doi:10.1016/j.jinf.2023.01.018.
- Al-Obaidi MM, Gungor AB, Murugapandian S, et al. The impact of Nirmatrelvir-Ritonavir in reducing hospitalizations among high-risk patients with SARS-CoV-2 during the omicron predominant era. Am J Med. 2023;136(6):577–584. doi:10.1016/j.amjmed.2023.02.022.
- Aggarwal NR, Molina KC, Beaty LE, et al. Real-world use of nirmatrelvir-ritonavir in outpatients with COVID-19 during the era of omicron variants including BA.4 and BA.5 in Colorado, USA: a retrospective cohort study. Lancet Infectious Diseases. 2023;10:10. doi:10.1016/S1473-3099(23)00011-7.
- Sridhara S, Gungor AB, Erol HK, et al. Lack of effectiveness of bebtelovimab monoclonal antibody among high-risk patients with SARS-Cov-2 Omicron during BA.2, BA.2.12.1 and BA.5 subvariants dominated era [preprint]. medRxiv. 2022;07.doi:https://doi.org/10.1101/2022.12.06.22283183.
- Centers for Disease Control and Prevention. Underlying Medical Conditions Associated with Higher Risk for Severe COVID-19: information for Healthcare Professionals. https://www.cdc.gov/coronavirus/2019-ncov/hcp/clinical-care/underlyingconditions.html. 2023.
- Bajema KL, Wang XQ, Hynes DM, et al. Early adoption of anti-SARS-CoV-2 pharmacotherapies among US veterans with mild to moderate COVID-19, January and February 2022. JAMA Netw Open. 2022;5(11):e2241434. doi:10.1001/jamanetworkopen.2022.41434.
- Danza P, Koo TH, Haddix M, et al. SARS-CoV-2 infection and hospitalization among adults aged > =18 years, by vaccination status, before and during SARS-CoV-2 B.1.1.529 (omicron) Variant Predominance – Los angeles county, California, 7 November 2021–8 January 2022. MMWR Morb Mortal Wkly Rep. 2022;71(5):177–181. doi:10.15585/mmwr.mm7105e1.
- Paredes MI, Lunn SM, Famulare M, et al. Associations between severe acute respiratory syndrome Coronavirus 2 (SARS-CoV-2) variants and risk of coronavirus disease 2019 (COVID-19) hospitalization among confirmed cases in Washington State: a retrospective cohort study. Clin Infect Dis. 2022;75(1):e536–e544. doi:10.1093/cid/ciac279.
- Ioannou GN, Bohnert ASB, O’Hare AM, et al. Effectiveness of mRNA COVID-19 vaccine boosters against infection, hospitalization, and death: a target trial emulation in the omicron (B.1.1.529) Variant era. Ann Intern Med. 2022;175(12):1693–1706. doi:10.7326/M22-1856.
- Ioannou GN, Berry K, Rajeevan N, et al. Effectiveness of COVID-19 treatment with Nirmatrelvir-Ritonavir or molnupiravir among U.S. Veterans: target trial emulation studies with one-month and six-month outcomes. Ann Intern Med. 2023;176(6):807–816. www.acponline.org/authors/icmje/ConflictOfInterestForms.do?msNum=M22-3565. eng. doi:10.7326/m22-3565.
- Lewnard JA, McLaughlin JM, Malden D, et al. Effectiveness of nirmatrelvir-ritonavir in preventing hospital admissions and deaths in people with COVID-19: a cohort study in a large US health-care system. Lancet Infect Dis. 2023;23(7):806–815. doi:10.1016/s1473-3099(23)00118-4.
- National Institutes of Health. Coronavirus disease 2019 (COVID-19) treatment guidelines. 2022.
- National Institutes of Health. Coronavirus disease 2019 (COVID-19) treatment guidelines. 2023.
- Food and Drug Administration. FDA updates sotrovimab emergency use authorization. 2022.
- Centers for Disease Control and Prevention. End of the federal COVID-19 public health emergency (PHE) declaration. 2023.
- Lin DY, Xu Y, Gu Y, et al. Effects of COVID-19 vaccination and previous SARS-CoV-2 infection on omicron infection and severe outcomes in children under 12 years of age in the USA: an observational cohort study. Lancet Infect Dis. 2023;23(11):1257–1265. doi:10.1016/s1473-3099(23)00272-4.
- Bohnert AS, Kumbier K, Rowneki M, et al. Adverse outcomes of SARS-CoV-2 infection with Delta and omicron variants in vaccinated versus unvaccinated US veterans: retrospective cohort study. BMJ. 2023;381:e074521. doi:10.1136/bmj-2022-074521.
- Xie Y, Choi T, Al-Aly Z. Molnupiravir and risk of hospital admission or death in adults with Covid-19: emulation of a randomized target trial using electronic health records. BMJ. 2023;381:e072705. doi:10.1136/bmj-2022-072705.
- Xie Y, Bowe B, Al-Aly Z. Nirmatrelvir and risk of hospital admission or death in adults with covid-19: emulation of a randomized target trial using electronic health records. BMJ. 2023;381:e073312. doi:10.1136/bmj-2022-073312.
- Lin D-Y, Xu Y, Gu Y, et al. Durability of bivalent boosters against Omicron subvariants. N Engl J Med. 2023;388(19):1818–1820. doi:10.1056/NEJMc2302462.
- Link-Gelles R, Weber ZA, Reese SE, et al. Estimates of bivalent mRNA vaccine durability in preventing COVID-19-associated hospitalization and critical illness among adults with and without immunocompromising Conditions – VISION network, September 2022–April 2023. MMWR Morb Mortal Wkly Rep. 2023;72(21):579–588. doi:10.15585/mmwr.mm7221a3.
- Tenforde MW, Weber ZA, Natarajan K, et al. Early estimates of bivalent mRNA vaccine effectiveness in preventing COVID-19-Associated emergency department or urgent care encounters and hospitalizations among immunocompetent Adults – VISION network, nine states, September–November 2022. MMWR Morb Mortal Wkly Rep. 2023;71(53):1637–1646. doi:10.15585/mmwr.mm7153a1.
- Tseng H, Ackerson B, SyLS, et al. Effectiveness of mRNA-1273 bivalent (original and omicron BA.4/BA.5) COVID-19 vaccine in preventing hospitalizations for COVID-19, medically attended SARS-CoV-2 infections, and hospital death in the United States. medRxiv. 2023:2023.05.25.23290456. doi:10.1101/2023.05.25.23290456.
- Xie Y, Choi T, Al-Aly Z. Association of treatment with nirmatrelvir and the risk of Post-COVID-19 condition. JAMA Intern Med. 2023;183(6):554–564. doi:10.1001/jamainternmed.2023.0743.
- Rathnayaka IW, Khanam R, Rahman MM. The economics of COVID-19: a systematic literature review. J Eco Stud. 2023;50(1):49–72. doi:10.1108/JES-05-2022-0257.
- Richards F, Kodjamanova P, Chen X, et al. Economic burden of COVID-19: a systematic review. Clinicoecon Outcomes Res. 2022;14:293–307. doi:10.2147/ceor.S338225.
- Murton M, Drane E, Jarrett J, et al. Remdesivir-related cost-effectiveness and cost and resource use evidence in COVID-19: a systematic review. Infection. 2023;51(2):285–303. doi:10.1007/s15010-022-01930-8.