Abstract
Background
Establishment of dedicated Stroke Centers has shown to be effective on the outcome of patients with acute ischemic stroke, as well as mechanical thrombectomy (MTE) in acute large vessel occlusion. The cost-effectiveness of this treatment has also been proven in several countries, but so far not in Switzerland.
Methods
We compare the pathways and economic impact of patients with acute large vessel occlusions causing acute ischemic stroke before the establishment of the stroke center and MTE in 2016 with the time afterwards in the years 2016–2020. Local data from the Swiss Stroke Registry and hospital accounting as well as economic data from a healthcare insurance company was used for evaluation in an economic model. Both payer and societal perspectives were considered, and probabilistic sensitivity analysis was undertaken to explore uncertainty.
Results
Establishment of a new Stroke Center in Central Switzerland increased the absolute number of thrombectomies from 0 in 2015 to 55 in 2016 to 83 in 2020, as well as the percentage of MTE in large vessel occlusions (LVO) from 50.9% in 2016 to 58.2% in 2020. Over a 15-year horizon, predicted average additional costs of CHF 7,978 were associated with the establishment of a new stroke center, as well as 0.60 quality-adjusted life-years (QALY) per patient and an additional survival of 0.59 years per patient. The calculated incremental cost-effectiveness ratio was therefore CHF 13,297 per QALY gained. When societal costs were included, the new stroke care model was predicted to dominate the old care model. Robustness of model results was confirmed via probabilistic sensitivity analysis.
Limitations
The results rely on data from a single stroke center and, therefore, cannot be generalized.
Conclusions
Establishment of a new Stroke Center can be cost-effective and provide better outcomes in terms of functional independence as well as quality-adjusted life-years.
Introduction
Worldwide, stroke is the second most common cause of death and the third most common cause of death and disability combinedCitation1,Citation2. Every year, around 16,000 strokes occur in Switzerland, of which around 3,600 are fatalCitation3. Stroke is the third most common cause of death in Switzerland and the main reason for long-term disabilityCitation4. This long-term disability represents a substantial burden for patients and relatives, but also places a significant burden on the health system. Depending on the study, the costs in the first year after a stroke amount to between CHF 31,115Citation5 and CHF 62,139Citation6 in Switzerland.
Most cerebral strokes (approximately 85%) result from a thromboembolic occlusion of a cerebral arteryCitation1. Until 2015, intravenous thrombolysis was considered the treatment of choice and was the only therapy with a curative aspect in the treatment of acute ischemic stroke. More recently, five large-scale randomized controlled trials (RCTs) were published which established the positive effect of endovascular therapy in acute stroke with a large vessel occlusion (LVO)Citation7–11. A meta-analysis of these five trials was subsequently published showing a reduction in disability in acute occlusion of the proximal anterior circulation compared with intravenous thrombolysis. Since these publications, MTE has become the established treatment of choice for LVOs and is part of the current guidelines for stroke care (European Stroke OrganisationCitation12, American Heart Association (AHA) Stroke GuidelinesCitation13–15, Swiss Stroke SocietyCitation16). In the case of intra-arterial LVO, mechanical removal of the thrombus via an endovascular approach has been shown to be safe and effective up to 24 h from symptom onsetCitation12–14. However, acute ischemic stroke (AIS) is a very time-critical condition and every 30-minute delay in reperfusion reduces the likelihood of functional independence by 10–15%Citation17.
Regardless of the treatment modality used for acute ischemic stroke, many stroke survivors remain impaired and suffer a reduction in their quality-of-life after the eventCitation18,Citation19. Accordingly, stroke survivors are in greater need of social support and are more dependent on the health care system, which has an impact on health care costs and upon society more broadly via work absenteeismCitation20. MTE has been demonstrated to be a cost-effective use of resources in several countriesCitation21–25 and studies focusing on the cost-effectiveness of specialist stroke centers have also shown them to represent value for moneyCitation26–28.
According to the current status (spring 2023), 10 stroke centers and 15 stroke units provide therapeutic care for stroke patients in SwitzerlandCitation29. Back in 2015, only nine stroke centers and 13 stroke units provided care to patients with LVO in Switzerland. Central Switzerland has a catchment area of approximately 850,000 people. Affected LVO patients could not be treated on site but had to be transported to one of the specialized surrounding centers for MTE. Until 2016, only intravenous lytic treatment with recombinant tissue plasminogen activator (rtPA) and medical management was available for treatment of acute ischemic stroke in central Switzerland. Although there may have been isolated cases in which patients were transferred to neighbouring stroke centers, these case numbers were not documented and, to our knowledge, do not exceed the frequency of two cases per month. The establishment of a new (tenth) stroke center in central Switzerland (at the Lucerne Cantonal Hospital [LUKS]) in 2016 enabled highly specialized treatment (including MTE) to be provided closer to home and thus more quickly, offering round-the-clock care for AIS patients in this region. The hospital was certified as a maximum care stroke center (per national criteria) in 2016. Since all stroke centers in Switzerland are obliged to record their key data in a national stroke registry, high-quality data on the case load, development of treatment times and treatment outcomes are available and allow an analysis of the procedural and economic effects of the establishment of a new stroke center.
With this analysis we aim to understand and quantify the costs and health outcomes (quality-adjusted life-years and life-years) associated with the establishment of a new stroke center at LUKS, compared with the previous approach to stroke management, and to estimate its cost-effectiveness. We use treatment-specific data from the Swiss Stroke Registry from 2015 to represent short-term patient outcomes prior to the center’s establishment and compare against equivalent data from the period 2016–2020 (during which the treatment mix changed). In both cases, we use modelling methods to extrapolate these short-term outcomes to predict longer-term survival, quality-of-life, and cost. We consider the alterations of patient pathways and costs (to the health service and to society), comparing against expected outcomes in the absence of the LUKS stroke center to demonstrate the value of specialized care for patients with AIS due to LVO.
Methods
Overview
We used an existing economic model of stroke careCitation27 and adapted it to the setting of Central Switzerland to reflect the establishment of the stroke center in Lucerne in 2016. In particular, the analysis compared an “old stroke care model” (in which the only treatment options in Lucerne were intravenous lytic treatment with rtPA or medical management) with the “new stroke care model” in which MTE (with or without rtPA) was available for patients with acute ischemic stroke due to large vessel occlusion. Data on patient numbers and short-term health outcomes were taken from the Swiss Stroke Registry and combined with adverse event rates, local unit cost data, plus other published data on long-term outcomes (including mortality and recurrent stroke), to predict total costs and outcomes (life-years and quality-adjusted life-years [QALYs]) for patients with an LVO between 2016 and 2020.
The local ethics committee clarified that this research project does not fall under the scope of the Human Research Act and an authorization from the ethics committee is therefore not required.
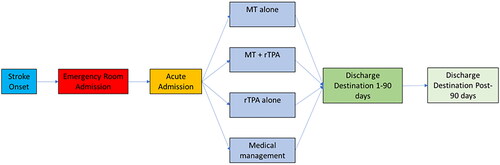
We extrapolated the model to 15 years (i.e. up to the year 2030) to capture long-term differences in cost and outcomes under the two treatment models. We then used the cost and QALY results to calculate the incremental cost-effectiveness ratio (ICER) to compare the old and new care models. The basic model structure is shown in .
Treatment data
We used data from each year from 2016 to 2020 from the Swiss Stroke Registry (SSR) to represent the number of LVO patients treated each year and applied these patient numbers to both the “old” and “new” stroke care models. This ranged from 55 patients in 2016 to 83 patients in 2020. The SSR is a pseudo-anonymized web-registry for medical studies based on the “Secutrial” platform (www.secutrial.com). All certified Swiss Stroke Centers (and stroke units) are required to enter the clinical data of all of their stroke patients into this register. This is done continuously by either a dedicated stroke nurse from the Neurocenter – or in times of unavailability of the stroke nurse by neurology residents working for the stroke center. Source data is supplied via the clinical information system of the hospital (EPIC; www.epic.com). The proportion of cases with complete data for admission, hospitalization, and follow-up phase for iv-thrombolysis and HSM (highly specialized medicine) cases is monitored by Secutrial and lies between 93% (2018) and 98% (2020). The consistency and quality of the data entered is ensured by standardized input masks and also by integrated explanations, for example, on scores, which have to be entered. The registry did not classify patients explicitly according to stroke type/severity/location (e.g. M1, M2), and we therefore used the National Institutes of Health Stroke Scale (NIHSS) to identify probable LVOs, filtering for patients with NIHSS score of at least 10 based on existing evidence concerning the appropriate cut-off scoreCitation30–32. The treatment mix for patients under the “old” model was based on data from the SSR from 2015 (the year immediately prior to the establishment of the stroke center), with treatment split between rtPA (63.6%) and medical management (36.4%) from a sample of 44 patients with LVO treated at LUKS. This treatment mix was then applied consistently over the period 2016 to 2020 to estimate how patients would have been treated in the absence of a stroke center. We thus assumed that this treatment mix would not change over time, nor would any new treatment options enter the mix for the “old” care model. However, the publication of the ECASS-4 trial in 2019Citation15 showed that patients could benefit from an extended time window for systemic rtPA therapy and the guideline was adopted to allow for intravenous rtPA treatment until 9 h after stroke onset. In addition, the team of treating physicians was expanded to include another specialized neurointerventionalist in July 2019 and the infrastructure for MTE was upgraded from a monoplane neuroangiography unit to a modern biplane neuroangiography unit in April 2020. Under the “new” stroke care model, the treatment mix varied year by year and was based on actual treatment patterns at LUKS over the period 2016–2020. shows the treatment mix over time for the “new” stroke care model.
Table 1. Treatment mix for the “new” stroke care model.
Short-term health outcomes
Short-term patient outcomes were established by classifying patients according to the modified Rankin Scale (mRS) which assesses level of disability following a stroke, ranging from 0 (no symptoms) to 6 (death from stroke) and having become the primary outcome measurement methods in stroke studiesCitation33. Data from the SSR provided the mRS distribution at 90 days post-stroke by treatment received (again using NIHSS score of at least 10 as a proxy for LVO), with year-by-year data from 2016 to 2020 available to use for the “new” stroke care model. For the “old” stroke care model, we applied mRS outcomes aggregated over the period 2016–2020 for patients receiving rtPA or medical management, again using data from the SSR. This approach was taken to avoid basing stroke outcomes on data from a small cohort of patients prior to the establishment of the stroke center. shows the mRS distribution, by treatment received, for each year from 2016 to 2020.
Table 2. Modified Rankin Scale outcomes at 90 days by treatment received.
Long-term health outcomes
Long-term health outcomes were then modelled as a function of patients’ mRS at 90 days. In the period between 90 days and 1-year post-stroke (“rehabilitation phase”), the effect of rehabilitation was incorporated by allowing patients in each mRS category to deteriorate (worsening by one mRS category), remain stable (staying in the same mRS category), or improve (by one mRS category) using data from a study of evolution of disability over time amongst stroke patientsCitation34. During this same period, a recurrent stroke rate of 4.91% was applied to survivorsCitation35, with patients suffering a second stroke assumed to be treated with medical management and have outcomes as per the data in . Beyond 1 year, annual mortality probabilities were applied using Swiss life tablesCitation36, adjusted for the mean age of patients in the cohort (70.1 years), the gender split of the Swiss population patients (which is age-specific), and after applying a relative risk of mortality according to the patient’s mRS at 1 year post-stroke (such that patients with a better mRS score live longer than those with a worse mRS score)Citation37. Furthermore, an ongoing risk of recurrent stroke of 2.01% was applied every year thereafterCitation35. QALYs were calculated by applying mRS-specific utility weights (ranging from 0.959 for mRS 0 to −0.02 for mRS 5) to the health state populations over the model horizon. A published function to map mRS scores to the domain scores of the “EuroQol 5-dimension” (EQ-5D) questionnaire was used to derive a utility weight for each mRSCitation38, and EQ-5D preference weights from GermanyCitation39, ItalyCitation40, and FranceCitation41 were averaged in the absence of a Swiss value set. One thousand patients were sampled, and their domain scores mapped according to the mRS functionsCitation38. Preference weights were then applied from the three countries in turn, from which a country-specific utility weight was calculated for each mRS score. A simple average across the three countries was then taken to calculate a mean Swiss utility by mRS.
Cost analysis
Cost analysis covered several areas of stroke care: acute treatment costs (including materials, staff and tests); acute care costs up to 90 days post-stroke (including time on specialist stroke unit, neurology ward time, and neurorehabilitation); costs from 90 days to 12 months post-stroke; annual costs beyond 12 months; and societal costs associated with work absenteeism. In each case, average per-patient costs were derived for each mRS category. Three-quarters of patients were assumed to be treated in non-specialized settings, with the remaining 25% attending an acute stroke unit. The acute therapy costs as well as the post-acute in-hospital costs were generated from the hospital database at LUKS. For this purpose, the average of the cost incurred by a sample of ten patients was calculated for each of the four treatment modalities.
The subsequent out-of-hospital (post-discharge) costs were generated with the help of a local health insurance company using the DRG (diagnosis-related groups) codes produced by the main diagnosis of ischemic stroke. This analysis included all stroke patients treated at LUKS in the period from 1 January 2016 to 31 December 2020 and who had basic insurance with the respective health insurance company (n = 440). The patient population was divided into four categories based on the care received (no care, only outpatient care, inpatient care, or death within the first 30 days), with separate analyses undertaken for three time periods of interest (within 90 days; 90 days to 1 year; annually beyond 1 year). Since costs could not be extracted directly for each mRS category, the four cost categories were assigned to mRS stages as follows: “no care” corresponded to mRS 0–1; “only outpatient care” to mRS 2–3; “inpatient care” to mRS 4–5; and death to mRS 6. These costs were then applied to each mRS category for the three time periods mentioned above, allowing long-term costs to be calculated for each treatment group.
Societal costs were incorporated by combining data on Swiss wagesCitation42, age- and gender-specific labor force participation ratesCitation43, and unemployment ratesCitation44, with published data used on the proportion of patients able to return to work following acute ischemic stroke (stratified by mRS)Citation45. A full list of unit costs used in the model is given in Supplementary Table S1, and all costs are reported in Swiss francs (CHF).
Analyses performed
The main model results are presented through a deterministic analysis – that is, with all model input parameters fixed at their mean values, and with the total costs, QALYs, and life-years calculated for the “old” and “new” stroke models over a 15-year period. These results were then used to calculate the incremental cost-effectiveness ratio under both payer and societal perspectives. To explore the effect of uncertainty in the model inputs, probabilistic sensitivity analysis was undertaken by assigning a statistical distribution to each input parameter, repeatedly sampling from each distribution and re-calculating the model results for each set of sampled inputs. Appropriate distribution types were applied to ensure plausible sampled values and correlation between inputs where necessary. Thus, Dirichlet distributions were used to reflect uncertainty in treatment mix over time and for short-term mRS outcomes as well as the proportion of patients in each mRS stage who improve, deteriorate, or remain at the same level between 90 days and 1 year. Uncertain in cost data was represented using gamma distributions (to ensure non-negative values and to reflect potential skew in cost data), which were also applied for relative risk parameters (such as the relative risk of mortality by mRS stage). Beta distributions were used for utility and probability parameters to ensure sampled values within the range 0–1, and a Normal distribution was applied for the mean patient age at the start of the model. The probabilistic analysis consisted of model 1000 replications, allowing uncertainty to be quantified for cost and QALY results for the “old” and “new” stroke care set-ups.
Model assumptions
A half-cycle correction was applied, and future costs and health outcomes were discounted at 3% per year. A number of other assumptions were required to extrapolate the results to a 15-year horizon. Firstly, no further improvement or deterioration in a patient’s mRS stage was possible beyond the first 12 months (except in the case of a second stroke). Secondly, the risk of recurrent stroke was assumed to be higher in the first year, with a lower annual risk applied thereafter. The distribution of mRS outcomes following a recurrent stroke was also applied in the same way over time, with such strokes being at least as severe as the index stroke. Any recurrent stroke was assumed to be treated with non-thrombolytic (medical) management, regardless of the intervention received for the index stroke. Outcomes from recurrent strokes were limited to mRS stages at least as severe as the index stroke. Mortality risk increases with age and according to mRS stage, meaning that a patient with mRS 2 or above would remain at a higher risk of death than someone with mRS 0 or 1 for the remainder of their life. Finally, no new interventions were added to the treatment mix over time – thus, the treatment options for new patients remained limited to MTE (with or without rtPA), rtPA, or non-thrombolytic management.
Results
The establishment of a new stroke center in central Switzerland made it possible to provide rapid, comprehensive treatment for the population close to home. This changed the patient pathways, and patients with suspected acute stroke were increasingly brought primarily to the new stroke center for care. In 2016–2020, there were 352 patients with acute ischemic stroke who were initially assessed with an NIHSS of ≥10 points. Of these, 58.2% (n = 205) received mechanical thrombectomy, 18.5% received systemic lysis exclusively, and 23.3% received medical management alone.
shows the predicted total costs, life-years, and QALYs associated with the “old” and “new” treatment models, covering all patients (n = 352) treated over the period 2016–2020 and extrapolating outcomes and costs for these patients to the year 2030. Results are shown as discounted and for the payer perspective (i.e. excluding costs of work absenteeism); mean values per patient are given in parentheses.
Table 3. Key cost and health outcome results (discounted) – payer perspective.
Thus, the model predicted slightly higher overall costs under the “new” stroke care model, equivalent to CHF 7,978 per patient, but with a predicted mean gain of 0.59 life-years and 0.60 QALYs per patient. Treatment and acute costs up to 90 days post-stroke were higher under the “new” care model due to the cost of mechanical thrombectomy (CHF 101,197 per patient for the “new” care model, versus CHF 93,069 for the “old” care model). Across all treated patients, the model predicted an additional cost of CHF 2.8 m over the 15-year period. The calculated ICER was CHF 13,297 per QALY gained, which is within the unofficial willingness-to-pay threshold in Switzerland. Under the societal perspective, the new stroke care model was predicted to save CHF 4,669 per patient with the same gain of 0.60 QALYs per patient (Supplementary Table S2). Thus, the new stroke model was predicted to dominate the old care model.
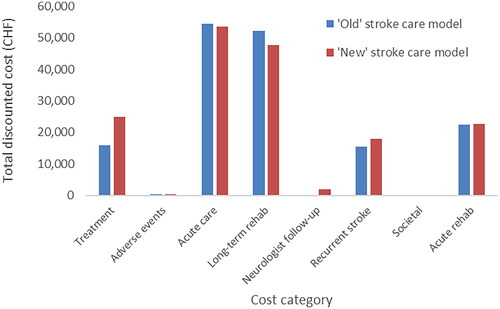
The breakdown of the total cost (for the payer perspective) is shown in (on a per-patient basis for ease of interpretation).
Acute treatment costs
The mean costs for the in-house non-thrombolytic (medical) management of stroke patients (in the “new” and “old” models) were CHF 11,157 average (including costs for the emergency room, lab tests and treatment until the patient is transferred to the ward/ICU/stroke unit). Costs for tests (including CT-imaging) were CHF 513, and CHF 613 for materials; however, the majority of the cost was due to medical staff (CHF 10,031) which accounted for 89.9% of total costs in the non-thrombolytic management group.
If an intravenous rtPA treatment was added (in the “old”, as well as in the “new” model), the costs were raised by an average of CHF 8,108, which included: medication (rtPA) (CHF 959); additional tests (CHF 370); additional materials (CHF 561); and additional medical staff time (CHF 6,218). In this treatment category, staff time accounted for 84.3% of total acute treatment cost.
If a mechanical thrombectomy was added (only under the “new” model), average costs of CHF 20,835 were added to either only the costs of medical management or the costs for medical management plus intravenous rtPA (“bridging therapy”). These costs include the following: materials costing (CHF 9,831; for example, stent retriever, catheters, wires); additional tests (CHF 682; lab values and imaging); and medical staff costs (CHF 10,322). In the case of “MTE only”, the percentage of staff costs was 63.6%, versus 91.3% for “bridging” cases.
Personnel costs account for the largest share in all treatment categories. The percentage of personnel costs for MTE without additional bridging is the lowest, as the costs for the catheter materials used are more significant.
One hospital day on the stroke unit created costs of CHF 3,883; 1 day on the general neurology ward created costs of CHF 1,650; and 1 day on the neurorehabilitation ward incurred costs of CHF 970.
These results () indicate that the majority of the cost comes from the acute care phase and long-term rehabilitation requirements for stroke survivors. Treatment costs were higher under the “new” stroke care model because of the cost of the MTE equipment and procedure, but these were largely offset by savings in acute care and long-term rehabilitation. Recurrent stroke costs were higher under the “new” model since patients treated with MTE have, on average, better survival outcomes and, thus, are at risk of recurrent stroke for an extended period (). When a societal perspective was adopted on cost, societal costs were the largest contributor to the total mean cost per patient (see Supplementary Figure S1).
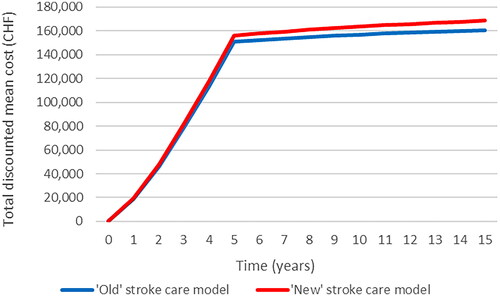
The mean cost per patient remains relatively consistent between the two service models over the period in which new patients are treated (years 1–5), after which there is some divergence owing to improved life expectancy for patients treated under the “new” model, leading to higher costs overall. Note that the gradient of the line reduces after year 5 because, from that point forward, the costs relate only to the patients already in follow-up (i.e. no new strokes occur beyond year 5). Under a societal perspective, a similar accumulation of costs was observed over time, with the “new” stroke service becoming less costly after year 5 as a result of reduced work absenteeism (Supplementary Figure S2).
A final metric with which to measure the changes made to stroke services in Central Switzerland is the mean mRS score at 90 days post-stroke, for which the economic model predicted values of 2.84 (“old” stroke service model) and 2.45 (“new” stroke service model). The predicted proportion of functionally independent patients (that is, the proportion with mRS ≤ 2 at 90 days) was 42.9% (151/352) for the “old” model, compared with 50% (176/352) for the “new” model.
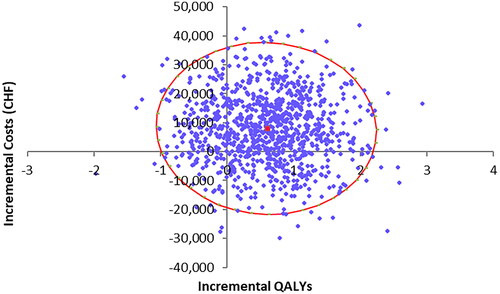
The results of the probabilistic sensitivity analysis are shown via the scatter plot depicted in . The incremental costs and QALYs for each of the 1,000 probabilistic model runs are shown in blue, with the red dot and circle representing the deterministic result and 95% cost-effectiveness ellipse, respectively.
The spread of the points shows some uncertainty in the results, which can largely be explained by the relatively small number of patients modeled year-on-year and the corresponding variability in health outcomes for the different treatment mix modeled. Uncertainty in costs is also driven by the use of relatively small patient samples to inform acute and post-acute costs for each treatment modality. The model predicted cost savings for the “new” stroke model in 25.8% of model replications, and QALY gains in 81.7% of cases. This supports the deterministic result that the new stroke service provides health benefits at minimal additional cost.
Discussion
This study sought to quantify the economic consequences and health outcomes of establishing a comprehensive stroke center in Central Switzerland (with a catchment population of ∼ 850,000 people) in 2015, using patient data from the period 2016–2020 to reflect changing treatment patterns toward novel technologies such as mechanical thrombectomy, and using real-world 90-day outcome data for these patients to predict long-term costs and health outcomes. Our results suggest that this change led to substantial survival (0.59 years) and quality-adjusted survival (0.60 QALYs) gains at minimal additional cost (CHF 7,978 per patient over 15 years), despite higher treatment costs in the acute phase due to the implementation of MTE. The incremental cost-effectiveness ratio of CHF 13,230 per QALY gained is below the unofficial Swiss threshold of CHF 100,000 per QALY gained. When the societal costs of work absenteeism were incorporated, the “new” stroke care model was predicted to save money versus the “old” model. The results of the analysis are consistent with previous studies that have shown mechanical thrombectomy to be a cost-effective use of health care budgetsCitation21–23, and with those which have looked at specific elements of modernizing stroke careCitation26–28. Probabilistic sensitivity analysis has indicated that the overall model conclusions are consistent with the base-case analysis, despite the relatively small patient numbers modelled. With all economic models, there is uncertainty regarding long-term costs and outcomes and therefore overall cost-effectiveness of therapies or changes to service provision. The results of our analysis are consistent with those of other studies which have concluded that the use of MTE improves health outcomes (QALYs and life-years) at acceptable additional costCitation23,Citation46–48. While previous models have used data from meta-analyses to inform short-term treatment-specific mRS outcomes, our approach used local data from the Swiss Stroke Registry to ensure replication of real-world outcomes at the center of interest. Although some differences were observed in mRS distribution between the Swiss data and a published meta-analysis (such as a smaller proportion of patients in mRS 0–2 at 90 days)Citation49, the overall trend toward better outcomes for MTE was consistent. Nevertheless, the effect is smaller than expected in several respects: the development of thrombectomy numbers remained below the anticipated case numbers, which we estimated to be around 160 cases per year based on the catchment area and on epidemiological data. Likewise, considering publications on the cost-effectiveness of MTE from other European countries, a larger effect on QALY and cost savings over the long-term was expected.
Possible explanations are the fact that the Central Switzerland Stroke Center was certified relatively late in 2016 (as the last of the 10 Swiss stroke centers so far) and at that time patient pathways (with transport to surrounding stroke centers) had already been established for this clinical picture – thus, modification of these practices takes some time. From 2016 to 2019, thrombectomies were performed by four physicians, only one of whom was a fully trained neurointerventionalist. The other three physicians were experienced interventional radiologists with training in cerebral thrombectomies but without dedicated neurointerventional training and “neuro-mindset”. For quality improvement reasons, two more fully-trained neurointerventionalists have been recruited since 2019 and the interventional background service was separated into a general radiology background service and a neurointerventional service in early 2022. Since then, thrombectomy numbers have increased to 130 (in 2022), which is not reflected in our analysis, since this data is not yet available from the SSR.
Of the costs of acute treatment, personnel costs account for the largest share (of up to 91%). On the one hand, this means that the greatest leverage exists here to save costs of treatment, but our data, as well as published data, show precisely the benefit of specialized treatment for affected patients and the societal costs. This leads to the question as to which conditions have to be fulfilled to optimize the benefit of specialized stroke treatment in terms of medical outcome and costs for health insurances and the general public. The size of the catchment area is certainly an important factor, as only beyond an expected minimum threshold of case numbers can the required reserve services be justified (for example, stroke unit and intensive care unit, 24/7 on-call service of neurology, anesthesia, neurosurgery, vascular surgery, interventional neuroradiology). Consideration of an existing infrastructure (e.g. emergency, intensive care unit, angiography facilities) as well as a culture of cooperation between clinical partners (such as in the context of a neurocenter) is also relevant, as is the distance (and thus transport times of stroke patients) from existing stroke centers. Achieving a minimum number of cases (as already demanded by the HSM in Switzerland with at least 50 MTE cases per year) is certainly reasonable, as are defined criteria on infrastructure, personnel, and processes.
If these factors are taken into account, our data show that it is possible to develop a new stroke center and thereby create a “win–win–win” situation for patients (better care and outcomes), service providers (attractiveness as an employer, cost-covering medical offer, reputation), payers (better care with lower costs), and society (lower costs, less need for care, and loss of work).
Our study has numerous strengths. Firstly, this is the first analysis of its kind in Switzerland and provides local insight into the costs and health outcomes associated with developing a centralized stroke service with the ability to provide the latest technology and care. Secondly, the model used relatively high-quality “real-life” local data from the Swiss National Stroke Registry covering the periods before and since the establishment of the stroke center, allowing the true treatment mix and 90-day outcome data to be modeled for both time periods and extrapolated to estimate long-term cost and outcomes. A further relative strength is that it was possible to obtain long-term data from a national health insurance company that has a high market share in central Switzerland and thus to analyse cost data that go beyond the acute treatment period.
Limitations
A drawback of our analysis is the limited data available regarding treatment patterns and health outcomes prior to the stroke center being established, meaning that the treatment mix for the “old” stroke care model was based solely on 44 admissions from 2015 with an NIHSS of 10 or above. Nevertheless, the probabilistic sensitivity analysis led to similar conclusions as the deterministic (base-case) analysis, via the use of appropriate statistical distributions for each parameter type to ensure a robust assessment of overall uncertainty. There were also some incomplete data in the hospital database, including the absence of a formal flag to identify patients with large vessel occlusion. For this reason, we extracted outcomes data from patients with NIHSS ≥ 10, which has historically been used as a strong indicator of LVOCitation30–32. During the observation period, the dedicated stroke study nurse resigned, and clinical data were temporarily entered into the stroke register by various residents (responsible for each patient). In a minority of cases (<7%), the clinical data were incompletely transferred to the SSR or there were inconsistencies that could not be clearly clarified afterward. Long-term cost data were also not available specific to each mRS category, and we assumed that the annual costs for each mRS category were constant over time. This is consistent with previous studies of long-term post-stroke management costsCitation23,Citation48. The annual percentage of work loss per population and mRS was assumed according to published dataCitation45 and combined this with local data on the population distribution by age categoryCitation50 and age-specific labor force participation ratesCitation51 plus Swiss wage dataCitation50,Citation51 to determine the costs of work absenteeism. During the observation period, the treating neurointerventionalists may have experienced a learning curve and advances in catheter material and stents may have occurred. Likewise, the trend in MTE was to carry out the procedures under general anesthesia at a low level. These limitations affect the accuracy of our analyses and the effect upon costs can not only be attributed to MTE, but also other cofounding factors. Most limitations are due to the use of retrospective data. In order to improve data quality, it would be advantageous to examine the effects of establishing a new stroke center prospectively rather than retrospectively in a future study. Because of these limitations, and due to the comparison of overall stroke services rather than interventions, we have not presented the results as a standard cost-effectiveness analysis.
Our results show that, under the given conditions, it was possible to establish better medical care with proven benefits for patients, almost cost-neutrally for the service payers and cost-saving for society. We are convinced that further optimization of the treatment process can increase these effects even further.
We see highly specialized stroke care as an opportunity to create a win–win situation for patients, health insurance companies, and society. In our opinion, promising measures for optimization include specialization in high-quality neurointerventional- and stroke treatment, rapid implementation of proven effective new treatment methods (e.g. extending treatment windows up to 24 h after AIS), and the exploitation of synergy effects through increasing case numbers.
The cost-effectiveness of these measures should be monitored prospectively. If a cost-neutral – or even cost-saving – benefit is confirmed, healthcare policy should quickly provide the resources and remove hurdles to ensure that the benefits are shared across the board by all stakeholders in the healthcare system. The approach taken in this study is somewhat different from conventional cost-effectiveness studies, which would typically compare two or more interventions for a single cohort of patients. Our approach involves five separate cohorts of patients and models changes to the treatment mix over time, rather than directly comparing specific interventions against one another. Nevertheless, the calculation of both costs and QALYs lends itself to a calculation of the ICER and allows interpretation of the results within a general cost-effectiveness framework.
Measures that have already been introduced (but not yet taken into account in this analysis) include specialization in high-quality endovascular treatment by dedicated neurointerventionalists, rapid implementation of proven effective treatment methods such as extending the treatment window up to 24 h after AIS, and synergy effects through increasing case numbers.
Conclusions
In conclusion, this study supports the establishment of comprehensive stroke services, with a localized analysis drawing on real-world treatment patterns and patient outcomes in Central Switzerland to determine the costs and health outcomes associated with a change in stroke care patterns. Improving health outcomes at limited expenditure further highlights the cost-effectiveness of such an approach and provides a framework for other countries or regions wishing to undertake a similar analysis. It also provides a broader approach to retrospectively assessing the benefits of implementing changes to health care services. Ongoing prospective collection of health outcomes data and more broadly applicable cost data collection (especially with regard to long-term costs by mRS category), such as via registries and micro-costing studies, will allow increasingly robust analyses to be undertaken and validate the results presented here.
The establishment of a new stroke center can be cost-effective in Switzerland and provide better outcomes in terms of functional independence as well as quality-adjusted life years.
Transparency
Declaration of funding
There was no sponsoring of this study.
Declaration of financial/other relationships
AVH is a consultant for Rapid Medical. SE and VSR are employees of Medtronic, which manufactures a mechanical thrombectomy technology. SE holds stock in Medtronic. The Section of Neuroradiology received educational grants from Rapid Medical in the consecutive years 2020–2023. Neither is related to this work. MB received an advisory fee (Astra Zeneca, Sandoz) and travel grant (Novonordisk) that are not related to this work. GK received advisory board fees (Bayer AG) that are not related to this work. MS and TRDC have no conflict of interest.
Author contributions
AVH: Study design, data collection, writing; MS: Study design, data collection, writing; VR: important intellectual content, data analysis, critical revising; TR: important intellectual content, data collection, critical revising; MB: important intellectual content, data collection, critical revising; JR: Study design, important intellectual content, critical revising; GK: important intellectual content, data collection, critical revising; SE: Study design, data analysis, writing; All authors agree to be accountable for all aspects of the work.
Reviewer disclosures
Peer reviewers on this manuscript have no relevant financial or other relationships to disclose.
Supplemental Material
Download MS Word (72 KB)Supplemental Material
Download PNG Image (18.4 KB)Supplemental Material
Download PNG Image (13.3 KB)Acknowledgements
We would like to thank CSS Insurance for the provision of long-term cost data. There are no further acknowledgments.
References
- Feigin VL, Brainin M, Norrving B, et al. World Stroke Organization (WSO): Global Stroke Fact Sheet 2022. Int J Stroke. 2022;17(1):18–29. doi: 10.1177/17474930211065917.
- Feigin VL, Stark BA, Johnson CO, et al. Global, regional, and national burden of stroke and its risk factors, 1990-2019: a systematic analysis for the Global Burden of Disease Study 2019. Lancet Neurol. 2021;20(10):1–26. doi: 10.1016/S1474-4422(21)00252-0.
- Meyer K, Simmet A, Arnold M, et al. Stroke events and case fatalities in Switzerland based on hospital statistics and cause of death statistics. Swiss Med Wkly. 2009;139(5-6):65–69. doi: 10.4414/smw.2009.12448.
- Schweizerische Hirnschlaggesellschaft. Schlaganfall und seine Ursachen. Available from: https://www.neurovasc.ch/betroffene/
- Mahler MP, Züger K, Kaspar K, et al. A cost analysis of the first year after stroke—early triage and inpatient rehabilitation may reduce long term costs. Swiss Med Wkly. 2008;138(31-32):459–465. doi: 10.4414/smw.2008.11845.
- Snozzi P, Blank PR, Szucs TD. Stroke in Switzerland: social determinants of treatment access and cost of illness. J Stroke Cerebrovasc Dis. 2014;23(5):926–932. doi: 10.1016/j.jstrokecerebrovasdis.2013.07.042.
- Jovin TG, Chamorro A, Cobo E, et al. Thrombectomy within 8 hours after symptom onset in ischemic stroke. N Engl J Med. 2015;372(24):2296–2306. doi: 10.1056/nejmoa1503780.
- Goyal M, Demchuk AM, Menon BK, et al. Randomized assessment of rapid endovascular treatment of ischemic stroke. N Engl J Med. 2015;372(11):1019–1030. doi: 10.1056/nejmoa1414905.
- Campbell BCV, Mitchell PJ, Kleinig TJ, et al. Endovascular therapy for ischemic stroke with perfusion-imaging selection. N Engl J Med. 2015;372(11):1009–1018. doi: 10.1056/nejmoa1414792.
- Berkhemer OA, Fransen PSS, Beumer D, et al. A randomized trial of intraarterial treatment for acute ischemic stroke. N Engl J Med. 2015;372(1):11–20. doi: 10.1056/nejmoa1411587.
- Saver JL, Goyal M, Bonafe A, et al. Stent-retriever thrombectomy after intravenous t-PA vs. t-PA alone in stroke. N Engl J Med. 2015;372(24):2285–2295. doi: 10.1056/nejmoa1415061.
- Turc G, Bhogal P, Fischer U, et al. European Stroke Organisation (ESO)—European Society for Minimally Invasive Neurological Therapy (ESMINT) guidelines on mechanical thrombectomy in acute ischaemic stroke. Endorsed by Stroke Alliance for Europe (SAFE). Eur Stroke J. 2019;4(1):6–12. doi: 10.1177/2396987319832140.
- Goyal M, Menon BK, van Zwam WH, et al. Endovascular thrombectomy after large-vessel ischaemic stroke: a meta-analysis of individual patient data from five randomised trials. Lancet. 2016;387(10029):1723–1731. doi: 10.1016/S0140-6736(16)00163-X.
- Powers WJ, Rabinstein AA, Ackerson T, et al. Guidelines for the early management of patients with acute ischemic stroke: 2019 update to the 2018 guidelines for the early management of acute ischemic stroke: a guideline for healthcare professionals from the American Heart Association/American Stroke Association. Stroke. 2019;50(12):e344–e418. doi: 10.1161/STR.0000000000000211.
- Campbell BV, Ma H, Ringleb PA, et al. Extending thrombolysis to 4·5-9 h and wake-up stroke using perfusion imaging: a systematic review and meta-analysis of individual patient data. Lancet. 2019;394(10193):139–147. doi: 10.1016/S0140-6736(19)31053-0.
- Hirnschlaggesellschaft S. Stroke units und stroke centers in der Schweiz: Richtlinien und Anforderungsprofil. EMPFEHLUNGEN Schweiz Med Forum. 2012;12(47):918–922.
- Ribo M, Molina CA, Cobo E, et al. Association between time to reperfusion and outcome is primarily driven by the time from imaging to reperfusion. Stroke. 2016;47(4):999–1004. doi: 10.1161/STROKEAHA.115.011721.
- Luengo-Fernandez R, Gray AM, Bull L, et al. Quality of life after TIA and stroke: ten-year results of the oxford vascular study. Neurology. 2013;81(18):1588–1595. doi: 10.1212/WNL.0b013e3182a9f45f.
- Christensen H. Long-term disability after transient ischaemic attack or minor stroke. Lancet Neurol. 2022;21(10):859–860. doi: 10.1016/S1474-4422(22)00342-8.
- Kolominsky-Rabas PL, Heuschmann PU, Marschall D, et al. Lifetime cost of ischemic stroke in Germany: results and national projections from a population-based stroke registry—The Erlangen Stroke Project. Stroke. 2006;37(5):1179–1183. doi: 10.1161/01.STR.0000217450.21310.90.
- Khunte M, Wu X, Payabvash S, et al. Cost-effectiveness of endovascular thrombectomy in patients with acute stroke and M2 occlusion. J Neurointerv Surg. 2021;13(9):784–789. doi: 10.1136/neurintsurg-2020-016765.
- Kunz WG, Hunink MG, Dimitriadis K, et al. Cost-effectiveness of endovascular therapy for acute ischemic stroke: a systematic review of the impact of patient age. Radiology. 2018;288(2):518–526. doi: 10.1148/radiol.2018172886.
- Lobotesis K, Veltkamp R, Carpenter IH, et al. Cost-effectiveness of stent-retriever thrombectomy in combination with IV t-PA compared with IV t-PA alone for acute ischemic stroke in the UK. J Med Econ. 2016;19(8):785–794. doi: 10.1080/13696998.2016.1174868.
- Koto PS, Hu SX, Virani K, et al. From the Research Methods Unit, Nova Scotia Health Authority, Halifax, NS, Canada (PSK); Queen Elizabeth II Health Sciences Centre (QEII) and Nova Scotia Health Authority. Can J Neurol Sci. 2020;47(1):50–60. doi: 10.1017/cjn.2019.308.
- de Andrés-Nogales F, Álvarez M, de Miquel MÁ, et al. Cost-effectiveness of mechanical thrombectomy using stent retriever after intravenous tissue plasminogen activator compared with intravenous tissue plasminogen activator alone in the treatment of acute ischaemic stroke due to large vessel occlusion in Spa. Eur Stroke J. 2017;2(3):272–284. doi: 10.1177/2396987317721865.
- Guzauskas GF, Boudreau DM, Villa KF, et al. The cost-effectiveness of primary stroke centers for acute stroke care. Stroke. 2012;43(6):1617–1623. doi: 10.1161/STROKEAHA.111.648238/-/DC1.
- Al-Senani F, Al-Johani M, Salawati M, et al. An epidemiological model for first stroke in Saudi Arabia. J Stroke Cerebrovasc Dis. 2020;29(1):104465. doi: 10.1016/j.jstrokecerebrovasdis.2019.104465.
- Requena M, Seguel-Ravest V, Vilaseca-Jolonch A, et al. Evaluating the cost-utility of a direct transfer to angiosuite protocol within 6 h of symptom onset in suspected large vessel occlusion patients. J Med Econ. 2022;25(1):1076–1084. doi: 10.1080/13696998.2022.2113221.
- Stroke Center/Stroke Units. Available from: https://www.neurovasc.ch/portrait/stroke-center-stroke-units
- Nakajima M, Kimura K, Ogata T, et al. Relationships between angiographic findings and National Institutes of Health stroke scale score in cases of hyperacute carotid ischemic stroke. Am J Neuroradiol. 2004;25(2):238–241.
- Heldner MR, Zubler C, Mattle HP, et al. National Institutes of Health stroke scale score and vessel occlusion in 2152 patients with acute ischemic stroke. Stroke. 2013;44(4):1153–1157. doi: 10.1161/STROKEAHA.111.000604.
- intravenous and intra-arterial recanalization for acute ischemic stroke: the Interventional Management of Stroke Study. Stroke. 2004;35(4):904–911. doi: 10.1161/01.STR.0000121641.77121.98.
- van Swieten JC, Koudstaal PJ, Visser MC, et al. Interobserver agreement for the assessment of handicap in stroke patients. Stroke. 1988;19(5):604–607. doi: 10.1161/01.str.19.5.604.
- Ganesh A, Luengo-Fernandez R, Wharton RM, et al. Time course of evolution of disability and cause-specific mortality after ischemic stroke: implications for trial design. J Am Heart Assoc. 2017;6(6):e005788. doi: 10.1161/JAHA.117.005788.
- Mohan KM, Wolfe CDA, Rudd AG, et al. Risk and cumulative risk of stroke recurrence: a systematic review and meta-analysis. Stroke. 2011;42(5):1489–1494. doi: 10.1161/STROKEAHA.110.602615.
- Bundesamt für Statistik. Life tables for Switzerland 2008–2013. Available from: www.bds.admin.ch/news/de/2017-0316
- Slot KB, Berge E, Sandercock P, et al. Causes of death by level of dependency at 6 months after ischemic stroke in 3 large cohorts. Stroke. 2009;40(5):1585–1589. doi: 10.1161/STROKEAHA.108.531533.
- Rivero-Arias O, Ouellet M, Gray A, et al. Mapping the modified Rankin scale (mRS) measurement into the generic EuroQol (EQ-5D) health outcome. Med Decis Making. 2010;30(3):341–354. doi: 10.1177/0272989X09349961.
- Greiner W, Claes C, Busschbach JV, et al. Validating the EQ-5D with time trade off for the German population. Eur J Health Econ. 2005;6(2):124–130. doi: 10.1007/s10198-004-0264-z.
- Scalone L, Cortesi PA, Ciampichini R, et al. Italian population-based values of EQ-5D health states. Value Health. 2013;16(5):814–822. doi: 10.1016/j.jval.2013.04.008.
- Chevalier J, de Pouvourville G. Valuing EQ-5D using time trade-off in France. Eur J Health Econ. 2013;14(1):57–66. doi: 10.1007/s10198-011-0351-x.
- Gross monthly wage (median and quartile range) by age, professional position and gender—private and public sectors combined [T9_b]—2012, 2014, 2016, 2018, 2020 | Table | Federal Statistical Office. Available from: https://www.bfs.admin.ch/bfs/en/home/statistics/catalogues-databases/tables.assetdetail.21224918.html
- Taux d’activité en équivalents plein temps selon le sexe, la nationalité, les groupes d’âges, le type de famille. 1.4.1996-30.6.2020 | Tableau | Office fédéral de la statistique. Available from: https://www.bfs.admin.ch/bfs/fr/home/statistiques/travail-remuneration/enquetes/espa/publications-resultats.assetdetail.13227480.html
- Bundesamt für Statistik. Erwerbslose gemäss ILO, registrierte Arbeitslose und registrierte Stellensuchende—2000-2022 | Tabelle | Bundesamt für Statistik. Available from: https://www.bfs.admin.ch/bfs/de/home/statistiken/arbeit-erwerb/erwerbslosigkeit-unterbeschaeftigung.assetdetail.24065513.html
- Steen Carlsson K, Andsberg G, Petersson J, et al. Long-term cost-effectiveness of thrombectomy for acute ischaemic stroke in real life: an analysis based on data from the Swedish Stroke Register (Riksstroke). Int J Stroke. 2017;12(8):802–814. doi: 10.1177/1747493017701154.
- Candio P, Violato M, Leal J, et al. Cost-Effectiveness of mechanical thrombectomy for treatment of nonminor ischemic stroke across Europe. Stroke. 2021;52(2):664–673. doi: 10.1161/STROKEAHA.120.031027.
- Ruggeri M, Basile M, Zini A, et al. Cost-effectiveness analysis of mechanical thrombectomy with stent retriever in the treatment of acute ischemic stroke in Italy. J Med Econ. 2018;21(9):902–911. doi: 10.1080/13696998.2018.1484748.
- Al-Senani F, Al-Johani M, Salawati M, et al. A national economic and clinical model for ischemic stroke care development in Saudi Arabia: a call for change. Int J Stroke. 2019;14(8):835–842. doi: 10.1177/1747493019851284.
- Campbell BV, Hill MD, Rubiera M, et al. Safety and efficacy of solitaire stent thrombectomy: individual patient data meta-analysis of randomized trials. Stroke. 2016;47(3):798–806. doi: 10.1161/STROKEAHA.115.012360.
- Sektion Demografie und Migration. Federal Statistics Office 2019 data Switzerland. Available from: Pxweb.bfs.admin.ch/pxweb/de/
- Sektion Arbeit und Erwerbsleben. Federal Statistics Office 2019 data Switzerland. Available from: pxweb.bfs.admin.ch/pxweb/de/