Abstract
Background
VAD therapy has revolutionized the treatment of end-stage heart failure, but infections remain an important complication. The objective of this study was to characterize the clinical and economic impacts of VAD-specific infections.
Methods
A retrospective analysis of a United States claims database identified members ≥ 18 years with a claim for a VAD implant procedure, at least 6 months of pre-implant baseline data, and 12 months of follow-up between 1 June 2016 and 31 December 2019. Cumulative incidence of infection was calculated. Infection and non-infection cohorts were compared regarding mortality, healthcare utilization, and total cost. Regression models were used to identify risk factors associated with infections and mortality.
Results
A total of 2,259 patients with a VAD implant were included, with 369 experiencing infection (12-month cumulative incidence 16.1%). Patients with infection were 2.1 times more likely to die (p < 0.001, 95% CI [1.5–2.9]). The mean 12-month total cost per US patient was $354,339 for the non-infection cohort and $397,546 for the infection cohort, a difference of $43,207 (p < 0.0001).
Conclusions
VAD infections were associated with higher mortality, more healthcare utilization, and higher total cost. Strategies to minimize VAD-specific infections could lead to improved clinical and economic outcomes.
Keywords:
Introduction
Mechanical circulatory support has become an important management option for patients with end-stage heart failure. The prevalence of end-stage heart failure has increased globally, and despite cardiac transplantation being a highly effective intervention, it is limited by donor heart availability and patient eligibility. Ventricular assist devices (VADs) were originally developed as a bridge to heart transplantation but are now used for longer periods of time, either in patients enduring longer wait times on transplant lists or in patients that are ineligible for heart transplantationCitation1,Citation2. While the evolution and improvements of VADs have revolutionized the treatment of end-stage heart failure, infection remains an important complication that can lead to substantial morbidity and mortalityCitation3.
Contemporary continuous flow VADs require a driveline to connect the implanted pump to an external power source, and despite ongoing research to replace drivelines with transcutaneous energy transfer systems, this technology is years away. Consequently, the driveline remains a vulnerable component for infectionCitation4. Driveline infection (DLI) rates in VAD patients are estimated at around 13% − 17% at 1-year post-implantCitation5,Citation6 and between 24% − 34% longer-termCitation7, and are among the leading cause of hospital readmission for VAD patients costing approximately $12K-14K per infectionCitation6,Citation8. Moreover, DLIs can lead to other more invasive infections such as bacteremia, endocarditis and pump infection, and may indirectly contribute to other VAD complications, such as ischemic and hemorrhagic strokesCitation9, and increase the priority for a heart transplant.
Data evaluating the risk factors, complications, and costs of VAD-specific and VAD-related infections come from VAD clinical trials and single-center experiences, which could be strengthened by corroboration in a real-world setting. This analysis aimed to characterize the real-world clinical and economic impact of durable VAD infections in the U.S. by evaluating incidence, risk factors, and cost burden in a U.S. healthcare claims dataset.
Methods
This retrospective analysis utilized the U.S. Medicare Fee-for-Service 100% Standard Analytical Files (SAFs) inpatient and outpatient claims database from 1 June 2016 – 31 December 2019. Medicare is available for people aged 65 years or older, younger people with disabilities and people with end-stage renal disease. The SAFs do not include claims from skilled nursing or long-term care facilities. This study was designed to be consistent with the STROBE guidelinesCitation10 and the ISPOR recommendations for retrospective studiesCitation11. Pursuant to the Health Insurance Portability and Accountability Act (HIPAA), the use of de-identified data does not require institutional review board (IRB) approval or a waiver of authorization.
Study design
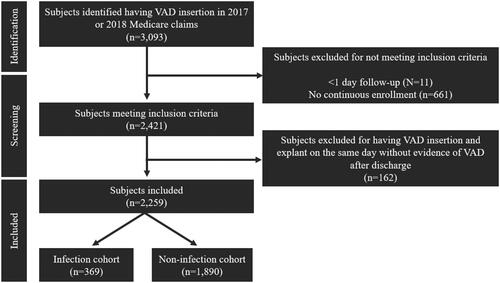
Patients ≥ 18 years old were identified with at least one inpatient record of a VAD insertion ICD-10-PCS code 02HA0QZ. The date of this claim was considered the index VAD insertion date. Patients were required to have at least 6 months of continuous health plan enrollment prior to their index VAD insertion date to characterize baseline characteristics, and continuous health plan enrollment after the index VAD insertion date to death or 12 months, whichever occurred first. Patients did not meet continuous enrollment measures if they were enrolled in Medicare Part A only or Medicare Part B only or indicated health management organization (HMO) coverage. The follow-up period began the day after the index VAD insertion and continued for 12 months. VAD insertion years were limited to 2017 and 2018 to ensure the availability of 6 months of continuous enrollment prior to and 12 months of follow-up post-VAD insertion during the ICD-10 coding era. If a patient had a VAD explant code ICD-10-PCS 02PA0QZ (heart assist system removal) on the same claim as their index VAD insertion code, the patient was included in the analysis only if there was evidence that a VAD was present after hospital discharge (Supplement 1) (). A 12-month follow-up was chosen to be consistent with prior reports on implantable device infection analysesCitation12 and to have consistent data for analysis (2016 is the beginning of ICD-10 coding implementation and the end of 2019 represents data unaffected by COVID-19).
Infections
VAD infections were defined as VAD-specific infections aligned with the ISHLT monographCitation13 for mechanical circulatory support, which includes driveline, pump, pump pocket and cannula infections. VAD infections were defined by at least one claim with ICD-10-CM diagnostic code of T82.72XXA in any diagnostic position. VAD infections that occurred at VAD insertion were not considered as an outcome for patients with evidence of a VAD prior to this hospitalization date as a pre-existing VAD infection could not be ruled out. Evidence that a VAD was present prior to the index VAD insertion date (durable VAD at baseline) was indicated by a miscellaneous VAD supply code and/or a VAD interrogation code (Supplement 2). The infection code (T82.72XXA) is a general cardiac device infection code, so all other general cardiac devices, implants and grafts associated with this code (e.g. cardiac implantable electronic device, hemodialysis catheter, graft) were defined as other possible sources of infection (see ). Since some patients had one or more of these devices in addition to a VAD, this was adjusted for in the analyses.
Table 1. Baseline characteristics.
Primary outcomes
The primary outcomes for this study were cumulative incidence of first VAD infection, the association between VAD infection and mortality, and cost. The average cost of healthcare utilization (HCU) during follow-up was calculated by summing all costs divided by the total number of patients. The average total 12-month costs for the non-infection cohort were subtracted from the average total 12-month costs of the VAD infection cohort to calculate the difference in costs. Costs are from the hospital perspective and represent U.S. 2021-dollars. Costs were adjusted using U.S. city average consumer price index for medical care services for the respective yearCitation14.
Secondary outcomes
Secondary outcomes were risk factors associated with VAD infection and mortality, HCU, and occurrence of stroke and pump thrombus. Baseline risk factors (Supplement 2) were used to determine association with the rate of VAD infection. HCU was characterized through frequency of HCUs stratified by patients with and without a VAD infection. Stroke and pump thrombus were also characterized by the first occurrence of their respective codes at any point during the 12-month follow-up period. The timing of stroke and/or pump thrombus was categorized as pre- or post-index VAD infection.
Statistical analysis
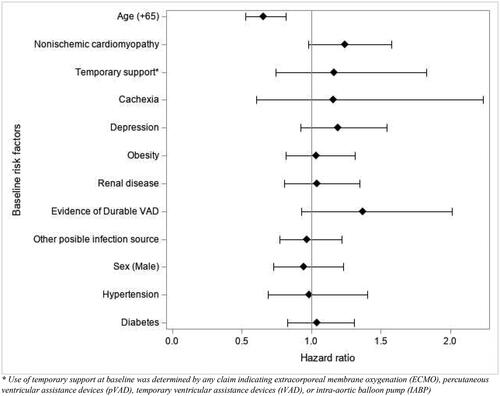
A p value of </= 0.05 was considered significant for all statistical comparisons. The time variable of interest was time from VAD insertion to first VAD infection. This date was right censored if any of the following occurred: death, VAD explant, heart transplant, or end of follow-up. Cumulative incidence of first VAD infection during follow-up was analyzed using a Fine Grey Model with death as a competing risk factor. The effect of baseline risk factors on VAD infection probability were analyzed using an adjusted Fine-Grey Model with death as a competing risk factor and adjusted for age, diabetes, sex, non-ischemic cardiomyopathy, cachexia, depression, renal disease, having other possible infection sources, evidence of durable VAD at baseline, use of temporary support, and hypertension.
All-cause mortality was analyzed using the Kaplan-Meier survival method. Hazard ratios and p-values for covariates associated with death were analyzed using a Cox Proportional Hazards Model and adjusted for the same variables listed above. Due to the first VAD infection variable being time varying, a time-dependent Cox Proportional Hazards Model was also adjusted for the variables listed above to analyze the hazard ratio and p-value of first VAD infection association with mortality. In this model, the time dependent variable was date of first VAD infection and was categorized in 30-day time intervals. Patients began contributing to the infection cohort at the time interval in which their first VAD infection claim appeared and continued throughout the follow up period. A 30-day time interval was used to allow for variance as ICD-10-CM diagnosis codes are categorized as the patient’s discharge date. A pooled variance t-test was used to determine significance between the means of the costs in the infection versus non-infection cohort. Mean costs are selected for the analysis rather than median costs as they include the impact of rare high-cost events that have a meaningful impact.
Results
Between 1 January 2017, and 31 December 2018, 2,259 patients who underwent VAD implantation were included in the analysis (). The baseline characteristics are shown in . The majority of patients were ≥ 65 years, white, male, with ischemic cardiomyopathy (51%); diabetes (48%), and renal disease (70%) were common.
Infection
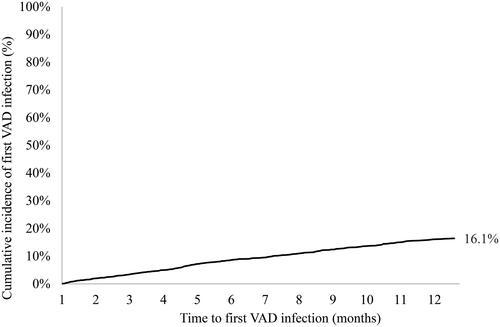
A total of 369 patients (16.1%) experienced at least one VAD infection (). After adjusting for confounders, age (65+) was the only covariate significantly associated with VAD infection (hazard ratio [HR], 0.66 [95% CI, 0.53–0.82]; p < 0.0002), mea-ning that those aged 65 and older were less likely to have a VAD infection when controlling for the competing risk of death (). Sensitivity analyses are included in Supplement 5.
Mortality
The 12-month Kaplan-Meier estimate of all-cause mortality post-index VAD insertion was estimated at 18.5% (N = 418) independent of infection status. Using a time-varying Cox Proportional Hazards model, patients who experienced an infection were 2.1 times more likely to die than patients who did not experience an infection (p < 0.001,95% CI [1.5–2.9]). Unadjusted results for this model were not meaningfully different than the adjusted results (Supplement 3).
After adjusting for confounders, the baseline risk factors associated with an increased rate of death were age (≥ 65yrs) (HR 1.8 [95% CI, 1.47–2.2]; p < 0.0001), obesity (HR 1.3 [95% CI, 1.1–1.6]; p < 0.01), and a history of prior temporary support (HR 1.6 [95% CI, 1.2–2.2]; p < 0.006). Use of temporary mechanical circulatory support at baseline was determined by any claim indicating extracorporeal membrane oxygenation (ECMO), percutaneous ventricular assistance devices (pVAD), temporary ventricular assistance devices (tVAD), or intra-aortic balloon pump (IABP). Factors associated with a decreased rate of death were evidence of a durable VAD at baseline, (HR 0.4 [95% CI, 0.2–0.7]; p < 0.0021) and non-ischemic cardiomyopathy (HR 0.6 [95% CI, 0.5–0.8]; p < 0.0003) (Supplement 4).
Secondary outcomes
In the study cohort, 10.45% (N = 236) experienced a pump thrombus, 10.27% (N = 232) experienced an ischemic stroke, 3.63% (N = 82) experienced a hemorrhagic stroke, 9.03% (N = 204) underwent VAD explant after the index VAD insertion hospitalization, and 6.29% (N = 142) underwent heart transplantation. A sub-analysis was conducted for only the infection cohort to categorize the outcomes that occurred after the patient’s first VAD infection date. Among the 369 patients, 6.50% (N = 24) experienced an ischemic stroke with a mean time from first VAD infection date of 70 days; 4.34% (N = 16) experienced a hemorrhagic stroke with a mean time from first infection date of 54 days; 5.15% (N = 19) experienced pump thrombus following with a mean time from first infection date of 57 days.
Economic outcomes
Patients in the infection cohort experienced a mean of five re-hospitalizations with a mean of 78 days in the hospital. For the non-infection cohort, the mean number of re-hospitalizations was three with a mean of 57 days in the hospital. The cost per patient of the index VAD insertion procedure plus all HCU during follow-up for the non-infection cohort had a mean of $354,339 and a median of $302,627 (10th percentile $179,046, 90th percentile $586,547). The cost per patient of the index VAD insertion procedure plus all HCU during follow-up for the infection cohort had a mean of $397,546 and a median of $347,693 (10th percentile $214,555, 90th percentile $655,013), equating to roughly $43,207 greater cost per person in the infection cohort as compared to the non-infection cohort (p < 0.0001) (). Five patients in the non-infection cohort indicated a date of death on the same day as their index VAD insertion and were excluded from the HCU analysis. An additional 2 patients in the non-infection cohort were excluded from the cost analysis due to missing cost data in the administrative claims database.
Discussion
This analysis leverages a large U.S. claims dataset to characterize the impact of VAD-specific infections, finding that VAD infection increased costs by $43K per patient. In keeping with prior literature, a 16.1% cumulative incidence of VAD infection was observed, with a correlation between younger age and higher rate of VAD infection. Furthermore, patients with VAD infections were 2.1 times more likely to die than those without infection during the follow-up period. The data were analyzed through the end of 2019, and thus was not affected by COVID-19 related effects.
While traditional clinical trials achieve a high degree of internal validity, they can sometimes be less reflective of therapy application and outcomes in real-world settings. Analyses such as this have a complementary role, as they have less control and specificity over data collection, but typically have larger datasets that are more representative of actual practice. This analysis is additive to and corroborative of the current understanding of the impacts of VAD infections.
There is considerable variability in the literature on the incidence of VAD infections. One multicenter report following 150 HeartMate II recipients for up to 1-year post implant found that 22% (n = 33) experienced a VAD-specific infection, with DLI making up 28 of 33 infected patientsCitation15. Other reports analyzed DLI specifically, with one noting that 16.9% of HeartWare HVAD recipients in the ADVANCE Bridge to Transplant (BTT) trial completing at least 6 months of follow-up experienced DLICitation5, and another finding that 34.7% of 75 patients at a single center experienced DLI16. A systematic literature review of DLI reported a range of estimates from as low as 6% to as high as 92.5%, with a preponderance of values in the range of 12%–44%Citation7. Here, we showed a 16.1% cumulative incidence of VAD-specific infection, which aligns with the existing literature. Based on the methodology used in the current study, it was not possible to differentiate the types of VAD-specific infections (DLI, pump, pump pocket, cannula infections) but as reported from other studies, DLI is expected to comprise the majority.
We found a correlation between VAD infection and increased mortality, with infected patients being 2.1 times more likely to die during follow-up. A previous analysis involving HeartMate II patients also concluded that VAD infection increased 1-year mortality, with an adjusted hazard ratio of 5.615. The MOMENTUM 3 trial included HeartMate II and III patients and reported that more patients died in the infection group than in the no infection groupCitation17. The specific cause of death in those with a VAD infection is not well defined in these studies, or the current study, and may be due to direct effects of the infection itself or indirect effects such as stroke and/or pump thromboses.
The impact of VAD infections on the frequency and intensity of healthcare utilization have not been previously reported, however, some aspects of cost have. Akhter and colleagues found that the median cost for a single readmission specific to DLI was $11,506Citation6. A cost-effectiveness analysis of LVAD implanted via thoracotomy estimated the overall cost of treating DLI to be $13,681Citation8. Our estimate of $43,207 in total costs is higher compared to previous reports, but this could be explained by a few factors. First, this analysis aimed specifically at measuring overall costs for VAD infections; second, this analysis captured both direct and indirect costs associated with VAD infection; and third, this analysis looked at all VAD-specific infections rather than DLI specifically. If approximately 58% of Medicare patients are covered by Medicare Fee-for-ServiceCitation18 and 91.1% of VAD patients are covered by Medicare rather than private insuranceCitation19, total costs associated with VAD infections over a 3-year period in the U.S. are estimated to be over $185 M.
There is some prior evidence on risk factors for VAD infections. The study of HeartMate II patients found a history of depression and elevated baseline serum creatinine to be independent predictors of VAD infectionCitation15. The ADVANCE BTT trial found that patients with DLI had higher body mass index (BMI) and were more frequently diabetic than those withoutCitation5. Significant predictors of VAD infection in HeartMate II and HeartMate 3 patients included female gender, pre-implant use of intra-aortic balloon pump, pre-implant history of cardiac surgery, and a body mass index ≥ 3017. An intensive care unit (ICU) stay greater than 2 weeks was found to be protective against DLI in a single-center studyCitation16. This analysis showed that age (65+) was the only covariate that was significantly associated with VAD infection. While this finding may seem counterintuitive at first, it is consistent with findings from other implantable cardiac devices. Birnie and colleagues identified that younger age was a significant predictor of infection in prospective clinical trial data.Citation20 This result was confirmed in a separate large retrospective datasetCitation21 and noted in a national registry analysis.Citation22 This result may be further explained in this population by the fact that younger patients, 43.6% < 65 years in this study, are more active and may have a higher rate of driveline trauma and subsequent infection. However, our risk factor analysis has limitations based on the methodology of using claims data, and before conclusions can be made, these findings need to be replicated in studies that are specifically designed for risk factor analyses.
Limitations
The current study has several other limitations. This was a retrospective analysis so causal inferences cannot be established. This analysis was based on an claims database, which may have included coding errors and lacked the scale of clinical detail in baseline characteristics, treatments, medications (e.g. antimicrobials), lab pathology, and outcomes that would be possible with a clinical trial. This database largely represents the age population of patients 65 years or older and does not include claims from skilled nursing facilities or long-term care settings, therefore it is possible the rates of infection in this manuscript are lower than the true rate. This database is from the U.S. Medicare population and may not be generalizable to other patient populations or healthcare systems. The VAD insertion code represented a general “heart assist system”, which may include left, right, and bi-ventricular assist devices, however LVAD is expected to be the most common system represented. Furthermore, these systems have common components such as a transcutaneous driveline that would similarly affect infection risk. The specific manufacturer of each LVAD could not be ascertained in the claims dataset. This analysis only included infections within one year post implant, however later infections are likely to have a similar economic impact which is driven by VAD replacement costs. The infection code used for this study can be associated with different cardiac devices, but this was mitigated by requiring VAD insertion for inclusion in the analysis and accounting for the presence of secondary cardiac devices in the sensitivity analysis. The cost difference analyzed all-cause costs rather than infection-specific costs.
Conclusion
This large, claims-based, real-world analysis complements the understanding of the incidence, cost and impact of VAD infections as seen in clinical trials and single-center analyses. VAD infections are a frequent complication of VAD system implants and are associated with an increased rate of mortality, greater healthcare utilization, and increased costs in the U.S. healthcare system. Elimination of the driveline is the future of safer VAD systems, but until that occurs other strategies to minimize VAD infections could lead to significant improvements in clinical and economic outcomes.
Transparency
Declaration of financial/other relationships
AYP: investigator-initiated research grant from MSD outside the submitted work; EF: advisor/consultant/advisory boards/honoraria Medtronic outside the submitted work; MM: advisor for Medtronic outside the submitted work; SSE: consultant Medtronic, Abiomed, Inspired Therapeutics, BioVentrix outside the submitted work; DM: consultant for Abbott outside the submitted work; DZ: consultant/research/grants/speaker fees Abbott, Medtronic, Berlin Heart, Abiomed, Edwards outside the submitted work; RH, JM, SS: employment Medtronic; NM: consultant Abbott, Medtronic, SynCardia, Carmat, Xylocor outside the submitted work.
Author contributions
AP, NM, EF, MM, SES, DM, DZ, RH, JM, and SS were members of the writing committee that drafted the manuscript. JM conducted the analysis. All authors had access to the full data through Medtronic. All authors reviewed and approved the manuscript, and all authors agree to be accountable for all aspects of the work. No other individuals met the International Committee of Medical Journal Editors authorship guideline criteria for this work. AP and NM had final responsibility for submitting the manuscript.
Supplemental Material
Download MS Word (62.1 KB)Acknowledgements
The authors would like to acknowledge the contributions of Luke Jacobsen, Pat Zimmerman, Evan Stanelle, and Colleen Longacre for consulting advice on statistical modeling, Lucas Higuera for data validation, Denise Griesmann for coding definitions, and Leslie Sweet for clinical perspective during the design of the study (Medtronic contributors).
Additional information
Funding
References
- Ponikowski P, Voors AA, Anker SD, et al. 2016 ESC guidelines for the diagnosis and treatment of acute and chronic heart failure: the task force for the diagnosis and treatment of acute and chronic heart failure of the European society of cardiology (ESC). developed with the special contribution of the heart failure association (HFA) of the ESC. Eur J Heart Fail. 2016;18(8):891–975. doi: 10.1002/ejhf.592.
- Miller LW, Pagani FD, Russell SD, et al. Use of a continuous-flow device in patients awaiting heart transplantation. N Engl J Med. 2007;357(9):885–896. doi: 10.1056/NEJMoa067758.
- Phadke VK, Pouch SM. Contemporary management strategies in VAD infection. Curr Heart Fail Rep. 2020;17(4):85–96. doi: 10.1007/s11897-020-00459-x.
- O’Horo JC, Abu Saleh OM, Stulak JM, et al. Left ventricular assist device infections: a systematic review. Asaio J. 2018;64(3):287–294. doi: 10.1097/MAT.0000000000000684.
- John R, Aaronson KD, Pae WE, et al. Drive-line infections and sepsis in patients receiving the HVAD system as a left ventricular assist device. J Heart Lung Transplant. 2014;33(10):1066–1073. doi: 10.1016/j.healun.2014.05.010.
- Akhter SA, Badami A, Murray M, et al. Hospital readmissions after continuous-flow left ventricular assist device implantation: incidence, causes, and cost analysis. Ann Thorac Surg. 2015;100(3):884–889. doi: 10.1016/j.athoracsur.2015.03.010.
- Pavlovic NV, Randell T, Madeira T, et al. Risk of left ventricular assist device driveline infection: a systematic literature review. Heart Lung. 2019;48(2):90–104. doi: 10.1016/j.hrtlng.2018.11.002.
- Mahr C, McGee E, Jr., Cheung A, et al. Cost-effectiveness of thoracotomy approach for the implantation of a centrifugal left ventricular assist device. Asaio J. 2020;66(8):855–861. doi: 10.1097/MAT.0000000000001209.
- Shah P, Birk SE, Cooper LB, et al. Stroke and death risk in ventricular assist device patients varies by ISHLT infection category: an INTERMACS analysis. J Heart Lung Transplant. 2019;38(7):721–730. doi: 10.1016/j.healun.2019.02.006.
- Vandenbroucke JP, von Elm E, Altman DG, et al. Strengthening the reporting of observational studies in epidemiology (STROBE): explanation and elaboration. Int J Surg. 2014;12(12):1500–1524. doi: 10.1016/j.ijsu.2014.07.014.
- Motheral B, Brooks J, Clark MA, et al. A checklist for retrospective database studies–report of the ISPOR task force on retrospective databases. Value Health. 2003;6(2):90–97. doi: 10.1046/j.1524-4733.2003.00242.x.
- Eby EL, Bengtson LGS, Johnson MP, et al. Economic impact of cardiac implantable electronic device infections: cost analysis at one year in a large U.S. health insurer. J Med Econ. 2020;23(7):698–705. doi: 10.1080/13696998.2020.1751649.
- Hannan MM, Husain S, Mattner F, et al. Working formulation for the standardization of definitions of infections in patients using ventricular assist devices. J Heart Lung Transplant. 2011;30(4):375–384. doi: 10.1016/j.healun.2011.01.717.
- U.S. Bureau of Labor Statistics. All Urban US city average cpi for medical care services 2012-2022);https://www.bls.gov/data/#prices.
- Gordon RJ, Weinberg AD, Pagani FD, et al. Prospective, multicenter study of ventricular assist device infections. Circulation. 2013;127(6):691–702. doi: 10.1161/CIRCULATIONAHA.112.128132.
- Juraszek A, Smólski M, Kołsut P, et al. Prevalence and management of driveline infections in mechanical circulatory support - a single center analysis. J Cardiothorac Surg. 2021;16(1):216. doi: 10.1186/s13019-021-01589-6.
- Patel CB, Blue L, Cagliostro B, et al. Left ventricular assist systems and infection-related outcomes: a comprehensive analysis of the MOMENTUM 3 trial. J Heart Lung Transplant. 2020;39(8):774–781. doi: 10.1016/j.healun.2020.03.002.
- Freed M, Fuglesten Biniek J, Damico A, et al. Medicare advantage in 2021: enrollment update and key trends. https://wwwkfforg/medicare/issue-brief/medicare-advantage-in-2021-enrollment-update-and-key-trends/.
- Modi K, Pannu AK, Modi RJ, et al. Utilization of left ventricular assist device for congestive heart failure: inputs on demographic and hospital characterization from nationwide inpatient sample. Cureus. 2021;13(7):e16094. doi: 10.7759/cureus.16094.
- Birnie DH, Wang J, Alings M, et al. Risk factors for infections involving cardiac implanted electronic devices. J Am Coll Cardiol. 2019;74(23):2845–2854. doi: 10.1016/j.jacc.2019.09.060.
- Ahmed FZ, Blomström-Lundqvist C, Bloom H, et al. Use of healthcare claims to validate the prevention of arrhythmia device infection trial cardiac implantable electronic device infection risk score. Europace. 2021;23(9):1446–1455. doi: 10.1093/europace/euab028.
- Olsen T, Jørgensen OD, Nielsen JC, et al. Incidence of device-related infection in 97 750 patients: clinical data fromthe complete danish device-cohort (1982–2018). Eur Heart J. 2019;40(23):1862–1869. doi: 10.1093/eurheartj/ehz316.