Abstract
Aim
To evaluate cost-effectiveness of upadacitinib (targeted synthetic-disease modifying anti-rheumatic drug [ts-DMARD]) as first-line (1 L) treatment versus current treatment among patients with rheumatoid arthritis (RA) in the Kingdom of Saudi Arabia (KSA), who had an inadequate response to prior conventional-synthetic (csDMARDs) and/or biologic-DMARDs (bDMARDs).
Methods
This Excel-based model included patients with moderate (Disease Activity Score [DAS28]: >3.2 to ≤5.1) or severe RA (DAS28 > 5.1). Cost-effectiveness of current treatment (1 L: adalimumab-originator/biosimilar; second-line (2 L): other bDMARDs/tofacitinib) was compared against a new treatment involving two scenarios (1 L: upadacitinib, 2 L: adalimumab-biosimilar [scenario-1]/adalimumab-originator [scenario-2]) for a 10-year time-horizon from societal perspective. Model outcomes included direct and indirect costs, quality-adjusted life-years (QALYs), hospitalization days, number of orthopedic surgeries, and incremental cost-utility ratio (ICUR) per QALY.
Results
With the current pathway, estimated total societal costs for 100 RA patients over 10-year period were Saudi Riyal (SAR) 50,450,354 (United States dollars [USD] 13,453,428) (moderate RA) and SAR50,013,945 (USD13,337,052) (severe RA). New pathway (scenario-1) showed that in patients with moderate-to-severe RA, upadacitinib led to higher QALY gain (+8.99 and +15.63) at lower societal cost (cost difference: -SAR2,023,522 [-USD539,606] and -SAR3,373,029 [-USD899,474], respectively). Thus, as 1 L, upadacitinib projects “dominant” ICUR per QALY over current pathway. Moreover, in alternate pathway (scenario-2), upadacitinib also projects “dominant” ICUR per QALY for patient with severe RA (QALY gain: +15.63; cost difference: -SAR 164,536 [-USD43,876]). However, moderate RA was associated with additional cost of SAR1,255,696 (USD334,852) for improved QALY (+8.99) over current pathway (ICUR per QALY: SAR139,742 [USD37,264]). Both scenarios resulted in reduced hospitalization days (scenario-1: −14.83 days; scenario-2: −11.41 days) and number of orthopedic surgeries (scenario-1: −8.36; scenario-2: −6.54) for moderate-to-severe RA over the current treatment pathway.
Conclusion
Upadacitinib as 1 L treatment in moderate-to-severe RA can considerably reduce healthcare resource burden in KSA, majorly due to reduced drug administration/monitoring/hospitalization/surgical and indirect costs.
Introduction
Rheumatoid arthritis (RA) is a chronic aggressive inflammatory and disabling multisystem autoimmune disease that is characterized by symmetric inflammation of synovial joints, leading to irreversible joint deformities [Citation1–5]. This can have a major impact on the functional abilities of patients that can lead to debilitating consequences on patient’s overall wellbeing, as well as higher comorbidity and mortality rates [Citation1,Citation2,Citation4–6]. RA is the most common inflammatory joint disease in adults, with an estimated global mean point prevalence of 0.56% (range 0–2.7%) between 1986–2014 [Citation7]. An annual incidence rate of 20–50 cases per 100,000 has been documented in the American and North European population [Citation8], with total costs of RA treatment being around €41 billion and €45 billion in the United States (US) and Europe, respectively [Citation9]. In the Kingdom of Saudi Arabia (KSA) alone, the point prevalence of RA is estimated to be 0.22% [Citation10], lower than the global prevalence likely due to differences in age distribution of the general population and the association of genetic factors and RA. It has also been reported that the prevalence rates are more common in women as compared to men (3:1) and may occur at any age, with a peak in incidence occurring at 50-60 years of age [Citation10,Citation11].
The aim of RA treatment is to achieve remission or reduction in disease activity by preventing inflammation, progression of joint damage, and disability [Citation12–14]. Currently, the standard endpoint to measure the efficacy of RA treatment is the American College of Rheumatology (ACR) response rate [Citation14,Citation15]. The ACR20, ACR50, and ACR70 responses are defined as reduction of ≥20%, ≥50%, and ≥70%, respectively, in the number of tender and swollen joints and in at least three of the ACR core measures, which are: 1) patient’s assessment of pain, 2) physician’s global assessment of disease, 3) patient’s global assessment of disease, 4) physical function and 5) the level of acute-phase reactants: erythrocyte sedimentation rate or C-reactive protein [Citation14,Citation15]. The treatment landscape of RA has advanced greatly in the last decade and the availability of novel treatment options has brought a paradigm shift in the management of RA [Citation11,Citation16]. These treatment targets have evolved to not only achieve clinical remission but also structural remission and functional remission [Citation17]. Clinical remission is a widely accepted therapeutic target for patients with RA, with low disease activity (LDA) as a best possible alternative [Citation17–19]. Evidence suggests that achieving clinical remission or LDA is associated with reduction in risks of all-cause hospitalization, emergency department visits, mortality, and medical costs. Additionally, it is associated with better patients-reported outcomes [Citation6,Citation17,Citation20,Citation21].
Conventional synthetic disease-modifying anti-rheumatic drugs (csDMARD) slow the progression of RA [Citation16,Citation22] and are classified into antimalarials (hydroxychloroquine), gold salts, azathioprine, minocycline cyclophosphamide and chlorambucil, which are rarely used and have notable side effects [Citation13,Citation22]. On the other hand, methotrexate (MTX), sulfasalazine and leflunomide are commonly used at initial disease diagnosis due to their high efficacy and safety [Citation13,Citation22]. Of these, MTX is the most widely used csDMARD and is part of all current RA treatment strategies [Citation22–25]. The guidelines from the Saudi Society for Rheumatology also recommend MTX over sulfasalazine in DMARD-naive patients with LDA [Citation26].
The advent of biologic DMARDs (bDMARDs) revolutionized the field of treatment of RA by neutralizing key immune system signals that lead to inflammation and joint damage [Citation11,Citation22,Citation27]. They include anti-tumor necrosis factor alpha (TNF-α) antagonists such as infliximab, etanercept, adalimumab, golimumab and certolizumab, which were approved by US Food and Drug Administration (USFDA) [Citation11,Citation22,Citation27]. Subsequently, other bDMARDs such as rituximab and tocilizumab were developed that do not block TNF-α but other inflammatory signals [Citation22].
Following bDMARDs, a new class of targeted synthetic DMARDs (tsDMARDs) emerged as an alternative advanced treatment option in RA, which targeted a particular molecular structure and were labeled as small molecule inhibitors [Citation22,Citation27]. There are several advantages of tsDMARDs over bDMARDs. Firstly, the route of administration is oral as opposed to intravenous (IV) or via subcutaneous (SC) injection [Citation27]. Secondly, being small molecule inhibitors (≤500 Da size), tsDMARDs offer wide protection against pro-inflammatory cytokines as compared to bDMARDs (>1000 Da size) which block specific extracellular molecules [Citation27]. The first tsDMARD drug approved by the USFDA in 2012 and subsequently by the European Medicines Agency (EMA) in 2017 was tofacitinib, which inhibits Janus kinases (JAK) family of enzymes (JAK3, JAK1 and to a lesser extent JAK2) [Citation28]. Upadacitinib, recently approved by USFDA in 2019, has shown relatively greater inhibitory potency for JAK1 as compared to JAK2, JAK3, and TYK2 [Citation28,Citation29].
Both bDMARDs and tsDMARDs have been approved for use either as monotherapy or in combination with MTX for patients who do not respond to MTX or other csDMARDs [Citation11,Citation28,Citation30]. Upadacitinib treatment, has demonstrated significantly higher rates of remission and LDA in all its pivotal trials [Citation14,Citation27,Citation28,Citation31–38]. However, these clinical advantages which might seem self-evident to the rheumatologists at large [Citation14,Citation39,Citation40], are yet to be perceived given the high healthcare cost of upadacitinib [Citation14,Citation41–43]. Thus, an assessment of specific cost-effectiveness analysis (CEA) will help inform payers on the benefits that upadacitinib has to offer in view of its high cost. A few CEAs have been performed in US, UK and Canada [Citation14,Citation32,Citation37,Citation41,Citation43]; however, no such analyses have been performed in the Middle-East region. After the establishment of the health technology assessment (HTA) entity in the KSA, a need for rigorous economic evaluations has emerged that will drive optimal allocation of financial resources towards value-based health decisions [Citation44].
Therefore, the objective of this study was to evaluate the cost-effectiveness of upadacitinib (tsDMARD) as the first-line (1 L) treatment versus current treatment pathway among patients with moderate-to-severe RA who had inadequate response (IR) to prior csDMARDs and/or bDMARDs, from a societal perspective in the KSA.
Methods
Modelling approach
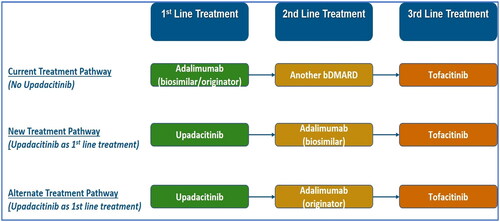
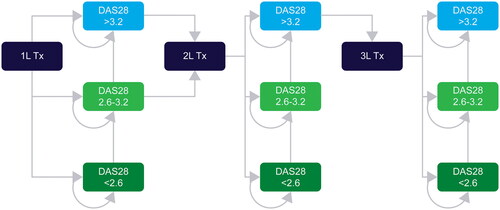
An Excel-based, Markov model was developed to assess the cost-effectiveness of upadacitinib as a treatment option in comparison to the current treatment pathway (). The modelling methodology was aligned with the handbook on decision modelling for health economic evaluation [Citation45] and the manuscript followed the Consolidated Health Economic Evaluation Reporting Standards developed by The Professional Society for Health Economics and Outcomes Research [Citation46].
Figure 1. Model structure.
Abbreviations: 1L: First-line; 2L: Second-line; 3L: Third-line; DAS28: Disease Activity Score 28; Tx: Treatment
The present analysis took a societal perspective with a model cycle length of 6 months. Projected outcomes of interest included total costs which was divided into direct costs and indirect costs, quality-adjusted life-years (QALYs), hospitalization days, and the number of orthopedic surgeries. Cost-effectiveness was described in terms of the incremental cost-utility ratio (ICUR), which is the cost per additional unit of QALY gained for the intervention versus the alternative over a 10-year time horizon. In the current analyses, a willingness-to-pay (WTP) threshold of Saudi Riyal (SAR) 260,000 (United States dollars [USD] 69,333) per QALY gained was assumed based on conventional practices (equivalent to the Gross Domestic Product per inhabitant in KSA). Both costs and health outcomes were discounted by 3.0% annually according to Saudi guidelines, and all prices were stated in SAR and USD (using exchange rate of USD1 = SAR3.75 [Citation47]). All analyses were simulated in a cohort of 100 patients.
Model inputs
The cost-effectiveness model was adapted in accordance with local treatment practice based on data retrieved from literature and inputs from key clinical experts in the KSA (Figure S1).
Patient population
The baseline characteristics incorporated in the cost-effectiveness model was based on a cohort size of 100 patients with moderate-to-severe RA who experienced a prior IR to csDMARDs and/or bDMARDs. The target patient population was further divided into two groups based on Disease Activity Score-28 (DAS28): RA with moderate activity (3.2 > DAS28 ≤ 5.1) and RA with severe activity (DAS28 > 5.1) (). These baseline characteristics were based upon the pooled SELECT trial data (SELECT-NEXT [Citation32,Citation35,Citation38], SELECT-MONO [Citation32,Citation35,Citation38], SELECT-COMPARE [Citation31,Citation32,Citation34,Citation35], SELECT-SUNRISE [Citation48,Citation49]) and included the following: mean age (years), mean health assessment questionnaire (HAQ) score, mean weight, mean DAS, disease duration (years) and female proportion (%). A summary of the baseline characteristics of individuals accounted in the model is provided in Table S1.
Treatment Algorithm
In the current treatment pathway, all patients are initiated on a simulation by receiving adalimumab-originator/biosimilar as 1 L treatment since they were intolerant to prior csDMARDs. Disease progression was determined by DAS28-CRP score, segregated into remission (DAS28 ≤ 2.6) or LDA (2.6 < DAS28 ≤ 3.2) or moderate-high disease activity (HDA) state (DAS28 > 3.2). It may be observed while on the same drug regimen, requiring intensification of therapy to regain remission and LDA. In the present analysis, patients switch to escalation therapy (second-line [2L]), which is any other bDMARD (70% patients receive bDMARDS equally split between abatacept, rituximab, tocilizumab and 30% patients receive tofacitinib [tsDMARD]). However, in the new and alternate treatment pathways, all patients start a simulation by receiving upadacitinib as 1 L treatment and 2 L treatment will comprise of adalimumab-biosimilar and adalimumab-originator ().
Efficacy inputs
Clinical efficacy and safety were obtained from secondary literature, clinical trials, indirect comparison studies. The study cohort was split into 3 health states: remission, LDA, M/HDA based on initial treatment response (induction period) and a fixed probability was applied in the following cycles to account for loss of treatment effectiveness in the long term. Owing to the unavailability of published studies comparing the effectiveness of upadacitinib to other bDMARDs, our model relied on the SELECT clinical trial results [Citation31,Citation32,Citation34,Citation35,Citation38] and the indirect comparison study [Citation50] to assess the responses rate of RA patients to adalimumab, tofacitinib and upadacitinib. Rates of achieving remission (DAS28 < 2.6) and LDA (DAS28 < 3.2) for biologic-naïve patients for the induction and maintenance phase in 1 L treatment were assumed to be same and sourced from Park et al. study [Citation51] (Table S2) which evaluated long-term efficacy and safety of using biologics for patients with RA. Treatment success criteria for 1 L was achieving remission and for 2 L was achieving LDA i.e. patients progressed from 1 L to 2 L if DAS-28 > 2.6 and 2 L to 3 L if DAS28 > 3.2. This criterion was selected based on a survey of clinicians regarding the thresholds applied to assess treatment success in their clinical practice (Table S3). A 0.84 hazard ratio (HR) was applied on the treatment efficacy based on a report published by the Institute for Clinical and economic review (ICER) to estimate the relative reduction in achieving remission using a subsequent treatment after failure of 1 L [Citation14,Citation52]. This approach was adopted due to lack of reliable comparable estimates for all comparators. Maintenance phase efficacy was derived from the study by Park et.al [Citation51]. Other efficacy inputs such as mean improvements from baseline HAQ were sourced from initial reduction in HAQ by European Alliance of Associations for Rheumatology (EULAR) response based on Phase III trials of upadacitinib [Citation14,Citation31–35,Citation38], with mean HAQ change indicating good remission (-0.76) and LDA (-0.48) (Table S4). These two efficacy parameters were used in the current analysis to estimate QALY gained for each scenario.
Utilities
According to Hernandez et al. a four-class mixture model was used to estimate the EQ-5D [Citation53]. HAQ, which correlates well with EQ-5D, is a commonly used measure in RA clinical studies. Thus, the model utility inputs were estimated over the entire time horizon by mapping the baseline and health state specific HAQ scores to EQ-5D; consistent with discrete-event simulation (DES) model framework.
The mapping algorithm applied in the model used a 6-step process:
Pain value mapped from current HAQ based on cohort characteristics (Age, male trigger, RA duration in years, baseline HAQ, Pain score from Visual Analogue Scale [VAS])
Estimate the probability for patients to be classified to the four latent classes using coefficients from Hernandez et al. [Citation53]
Estimate mean utility by class
Rescale the estimated mean EQ-5D by class
Calculate the probability weighted average EQ-5D
Output
Utility score for each health state and gender are summarized in Table S5.
Cost data
Drug acquisition cost of upadacitinib was obtained from AbbVie. On the other hand, drug acquisition cost of adalimumab-originator/biosimilar and tofacitinib, was obtained from Saudi Food and Drug Authority. Drug dosing schedules were aligned as per their respective EMA labels and will be further validated by Key opinion leaders (KOLs) and clinical experts. Drug administration costs were applied to IV and SC treatments, with the assumption that the administration cost for oral drugs was zero based on KOLs inputs. Drug monitoring costs included costs of quarterly tests including comprehensive metabolic test panel, complete blood cell count, lipid panel, acute hepatitis panel, and an additional annual tuberculosis test. The estimated frequency of monitoring and follow-up care was based on KOLs inputs. Cost of health-care resources/test was based on Ministry of health service costs or internal IQVIA database which was validated with KOLs/experts. Rate of joint surgery and hospitalization per cycle was obtained from the literature [Citation54]. Distribution of surgeries by surgery and joint type was also sourced from relevant literature [Citation55]. However, costs related to surgery and hospitalization were obtained from KOLs inputs. Total productivity loss was defined as an aggregate of the missed workdays (absenteeism) and low productivity days (presentism) due to higher levels of disease activity and functional disability. We utilized the disease activity level specific (i.e. DAS28) estimates from two published studies evaluating the relationship of work productivity with disease activity, functional capacity, life quality and radiological damage due to RA [Citation56,Citation57]. More information on direct costs (associated with drug-administration, monitoring, surgical and hospitalization cost) and indirect costs [Citation56–59] (such as loss of productivity) is available in and SCitation7 respectively.
Table 6. Cost breakdown and health outcomes with Upadacitinib - Scenario 2 (severe RA).
Analytical approach
This model calculated and compared the cost-effectiveness of upadacitinib as treatment option in the current KSA market for two scenarios:
Scenario 1: Current treatment pathway (1L: adalimumab-biosimilar, 2L: other bDMARDs/tofacitinib) versus New treatment pathway (1L: upadacitinib, 2L: adalimumab‑biosimilar)
Scenario 2: Current treatment pathway (1L: adalimumab-biosimilar, 2L: other bDMARDs/tofacitinib) versus Alternate treatment pathway (1L: upadacitinib, 2L: adalimumab‑originator)
Sensitivity analyses
To assess the parameter uncertainty in our model, we conducted one-way sensitivity analysis (OWSA) and probabilistic sensitivity analysis (PSA). For OWSA, the input parameters were varied within 20% of their mean values and the results were presented as tornado diagrams depicting the top 20 parameters that affected the net monetary benefit (NMB) outcomes. NMB was chosen for this analysis to avoid showing negative ICUR values that would have been non-interpretable. The PSA was run for 1,000 simulations and the results were presented as the cost-effectiveness plane and cost-effectiveness acceptability curves.
Compliance with ethics guidelines
This CEA was based on existing literature findings and completed clinical trials and did not involve any studies on human participants and animals directly performed by any of the authors.
Results
Cost-effectiveness analysis (base-case)
In Scenario 1, the new treatment pathway led to higher QALYs gain (moderate RA: +8.99; severe RA: +15.63) at lower societal cost (moderate RA: -SAR2,023,522 [-USD539,606]; severe RA: -SAR3,373,029 [-USD899,474]) over a 10-year time horizon (). Thus, as 1 L, upadacitinib projects “dominant” ICUR per QALY over current treatment pathway (). Also, the new treatment pathway resulted in reduced hospitalization days (-14.83 days and −11.41 days) and the number of orthopedic surgeries (-8.36 and −6.54) among the moderate and severe RA patients, respectively (). The cost savings associated with the new treatment pathway over current treatment pathway were majorly driven by drug acquisition, drug administration, hospitalization, monitoring, orthopedic surgeries, and indirect costs for moderate RA () and severe RA ().
Figure 3. Total societal cost and QALY with upadacitinib – Scenario 1.
Abbreviations: RA: Rheumatoid Arthritis; SAR: Saudi Riyal; QALY: Quality-adjusted Life-year
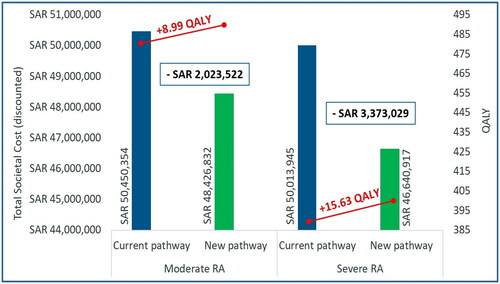
Table 1. Health outcomes with Upadacitinib - Scenario 1.
Table 2. Cost breakdown and health outcomes with Upadacitinib - Scenario 1 (moderate RA).
Table 3. Cost breakdown and health outcomes with Upadacitinib - Scenario 1 (severe RA).
In Scenario 2, for patients with severe RA the alternate treatment pathway led to a higher QALYs gain (+15.63) at lower societal cost (cost difference: -SAR164,536 [-USD43,876]) over a 10-year time horizon (). Thus, the alternate treatment pathway also projects “dominant” ICUR per QALY for patient with severe RA (). However, treatment of moderate RA was associated with additional societal cost of SAR1,255,696 (USD334,852) for improved QALY (+8.99) over current treatment pathway resulting in an ICUR per QALY: SAR139,742 (USD37,264) ( and ). This was due to the higher drug acquisition cost associated with alternate treatment pathway over current treatment pathway (cost difference: +SAR1,713,332 [USD456,888]) which was partially offset by decrease in costs related to orthopedic surgeries, administration, monitoring, hospitalization, and indirect costs (). On the other hand, the cost savings associated with the alternate treatment pathway over current treatment pathway for severe RA patients were majorly driven by drug administration, hospitalization, monitoring, orthopedic surgeries, and indirect costs (). Overall, the alternate treatment pathway resulted in reduced hospitalization days (-14.83 days and −11.41 days) and number of orthopedic surgeries (-8.36 and −6.54) among moderate and severe RA patients, respectively ().
Figure 4. Total societal cost and QALY with upadacitinib – Scenario 2.
Abbreviations: RA: Rheumatoid Arthritis; SAR: Saudi Riyal; QALY: Quality-adjusted Life-year
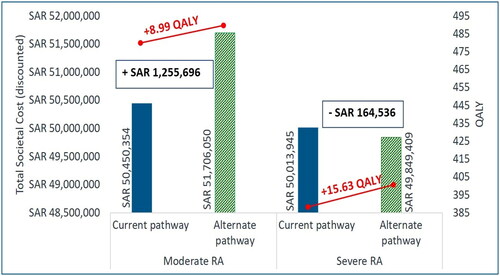
Table 4. Health outcomes with Upadacitinib - Scenario 2.
Table 5. Cost breakdown and health outcomes with Upadacitinib - Scenario 2 (moderate RA).
Sensitivity analysis results
The tornado diagrams showed that the parameters that maximally affected the NMB for each scenario were the drug acquisition cost of upadacitinib, utility values for DAS <2.6, and the efficacy parameters during remission for both moderate and severe RA (Figure S2).
The PSA analysis results suggested that most of the simulation were in southeast quadrants for the new scenario versus current treatment pathway (Figure S3). While for the comparison of alternate treatment pathway versus current treatment pathway, majority of the simulations were in southeast and northeast quadrants (Figure S4). The cost-effectiveness acceptability curves showed that there was 50%–60% probability of new or alternate treatment pathway being cost-effective compared to the current treatment pathway (Figures S3 and S4).
Discussion
To the best of our knowledge, this the first study conducting CEA of upadacitinib as 1 L versus current treatment pathway among patients with RA in the KSA, having an IR to prior csDMARDs and/or bDMARDs. The results of this study showed that the new and alternate therapy comprising of upadacitinib as 1 L treatment was dominant or cost-effective and resulted in reduced hospitalization days and orthopedic surgeries in patients with moderate-to-severe RA as compared to current treatment pathway from a societal perspective over a time horizon of 10 years. The major driver of study outcomes were the costs related to hospitalization, monitoring, orthopedic surgeries, and indirect costs. The sensitivity analyses confirmed robustness of our study results.
The clinical effectiveness and safety profile of upadacitinib were obtained from five global phase III randomized controlled trials that included patients with moderate-to-severe RA [Citation31–35,Citation38]. Of these, four trials compared the clinical effectiveness of upadacitinib compared with adalimumab, csDMARDs (including MTX) or placebo for moderate-to-severe RA that had responded inadequately to csDMARDs. In SELECT-MONOTHERAPY trials [Citation32,Citation35,Citation38], upadacitinib when compared with MTX, showed a significant improvement in ACR20 at 12 weeks (upadacitinib 68%, MTX 41%, p ≤ 0.001). Similar responses were also seen in SELECT-COMPARE [Citation31,Citation32,Citation34,Citation35] and SELECT-NEXT [Citation32,Citation35,Citation38] trials, where upadacitinib plus MTX and upadacitinib plus csDMARDs showed a significant improvement in ACR20 at 12 weeks when compared with adalimumab plus MTX or placebo plus MTX (63%; p ≤ 0.050 or 36%; p ≤ 0.001) and placebo plus csDMARDs (upadacitinib 64%, placebo 36%, p ≤ 0.001), respectively. In SELECT-EARLY, upadacitinib alone showed a statistically significant improvement in ACR50 at 12 weeks compared with MTX alone (upadacitinib 52.1%, MTX 28.3%, p ≤ 0.001) [Citation35]. On the other hand, SELECT-BEYOND showed that upadacitinib with csDMARDs was clinically more effective by significantly improving ACR20 at 12 weeks than placebo with csDMARDs (upadacitinib 65%, placebo 28%, p ≤ 0.001) for moderate to severe RA that had responded inadequately to bDMARDs [Citation32,Citation35,Citation38].
Recent network meta-analysis (NMA) compared (ACR)20/50/70 responses and DAS28-joint count C-reactive protein (DAS28- CRP) remission rates (DAS28-CRP\2.6) at 12 and 24 weeks for the three JAK inhibitors (Tofacitinib, Baricitinib & Upadacitinib) in patients with RA who had an IR to csDMARDs [Citation28]. Although no statistical differences were observed between different JAK inhibitors, the results of this NMA revealed numerically better efficacy of upadacitinib among combination therapies and monotherapies [Citation28]. These findings might provide greater insight into the comparative efficacy of JAK inhibitors at their approved doses and guide physicians in determining which inhibitor is better for efficient management of RA along with reimbursement decision making [Citation28]. In a separate meta-analysis [Citation36], it was also observed that baricitinib 4 mg + MTX and upadacitinib 15 mg + MTX showed the highest ACR response rates in RA patients with an IR to MTX as compared to adalimumab.
In lieu of these clinical benefits of upadacitinib, recent CEA results by National Institute for Health and Care Excellence (NICE) guidance showed that upadacitinib with MTX was not cost-effective in patients with moderate RA and cost-effective in patients with severe RA who had responded inadequately to csDMARDs in the UK [Citation32]. Moreover, the cost-effectiveness of upadacitinib monotherapy was similar to upadacitinib with MTX in patients with severe RA [Citation32]. In patients with severe RA, upadacitinib with MTX was not cost-effective who had responded inadequately to bDMARDs, but to rituximab [Citation32]. For the same patient group, upadacitinib with MTX turned out to be cost-effective for those who had responded inadequately to both bDMARDs and rituximab [Citation32].
Moreover, findings from a US based study determined the cost-effectiveness of upadacitinib versus MTX as a 1 L monotherapy in the treatment of RA patients [Citation37]. It was observed that upadacitinib was cost-effective as compared to MTX in achieving DAS28 score of ≤ 3.2 [Citation37].
The economic benefits of treating RA patients with upadacitinib were highlighted in a different study [Citation41] in the US where it was reported that in moderate to severe RA patients, upadacitinib 15 mg once daily in combination therapy substantially reduced the direct medical costs versus the combination therapy with tofacitinib 5 mg twice daily. Similar to combination therapy results, upadacitinib 15 mg once daily monotherapy majorly attributed to a decrease in direct medical costs as compared to MTX monotherapy in this particular patient population [Citation41].
Similar findings were obtained from a report published by ICER which suggested that upadacitinib with a standard DMARD conferred higher rates of disease remission and improved patient outcomes at similar costs compared with adalimumab plus a csDMARD in US [Citation14,Citation42].
However, as per recent pharmacoeconomic review report published by The Canadian Agency for Drugs and Technologies in Health (CADTH), upadacitinib as monotherapy or in combination with csDMARD was not considered a cost-effective treatment at conventionally accepted WTP thresholds in Canada [Citation43]. The results of this CEA should be interpreted with caution as several limitations were identified that could not be addressed or corrected in the submitted model, most notably being the lack of stratification of CEA results into moderate and severe RA disease conditions, uncertainty in the clinical estimates derived from NMA and the need of long-term extension of time horizon [Citation43].
The method of mapping utilities in this study was based on the study by Hernandez et al. and used a mixture model [Citation53]. In this method, the fit is far better than the linear regression model and the response mapping approach across the entire range of pain, EQ-5D, and HAQ (except when HAQ > 2.5) [Citation53]. These direct methods of estimating utilities do face certain challenges than the indirect response mapping approach in which weights from any country can be applied in the second stage rather than requiring the estimation of a new function. However, with the response mapping approach good fit may not be achieved with all the different sets of weights.
As with any modeling analysis, this study too has a few limitations that need to be considered while interpreting the results. First, is the availability of DAS28 response rates for all TNFs used as a Standard of Care (SoC). The SELECT trial compares only adalimumab to upadacitinib using various clinical outcomes such as ACR20/50/70 and DAS28 [Citation31,Citation32,Citation34,Citation35,Citation38]. This limitation was mitigated in the current modelling analysis by the use of adalimumab DAS responses as a representative for all bDMARDs due to the fact that adalimumab represents over 30% of overall bDMARDs in the RA management [Citation11,Citation27]. The model incorporated standard dose of adalimumab rather than the escalated dose. Second, is the availability of DAS28 response rates for the population of interest. The available literature limits the indirect comparison of tofacitinib and upadacitinib to RA patients with IR to csDMARDs [Citation14,Citation32,Citation37,Citation41–43] while our population of interest covers RA patients with IR to bDMARDs. Third is the availability of DAS28 response rates for longer time horizons. The SELECT trial only provides response rates up to a limited time period (48 weeks) [Citation31,Citation34], however patients may lose response in the longer time horizons. This limitation was mitigated in the current analysis by the use of model cycle length of 6 months. Moreover, as reported by ICER report [Citation14,Citation42], our model was also unable to draw a direct comparison of the health economic value between upadacitinib and the other JAK inhibitors, i.e. tofacitinib and baricitinib, in the patient populations due to a lack of published data. This limits the clinicians or policymakers in estimating the cost-effectiveness among the three JAK inhibitors.
The results published in this study may not be generalizable to other countries due to inherent differences in population characteristics. However, the current results may guide future rational drug use for the treatment of RA. Rational drug use is defined as the justified use of drugs according to patient’s clinical need, along with availability of these drugs at the lowest possible cost to patients and the community for betterment of healthcare. Therefore, health economic evaluations for rational drug use should be encouraged as they provide meaningful information on the clinical and cost-effectiveness of alternative treatment pathways over the current SoC.
Conclusion
Upadacitinib as a first-line treatment for the management of patients with moderate-to-severe RA projected improved health outcomes at lower budget over 10-year time horizon as compared to the current treatment pathway in the Kingdom of Saudi Arabia. This can be attributed to significant reduction that upadacitinib will bring in healthcare-resources utilization in the Kingdom of Saudi Arabia, majorly due to reduced cost of drug‑administration, monitoring, hospitalization, surgical-cost, and indirect-costs. Although the current CEA does not estimate cost savings with adalimumab-originator as second-line treatment for patients with moderate RA, it is perceived that this will not offset the use of adalimumab-originator over adalimumab-biosimilar. Given the enormous medical and societal costs associated with RA management, this CEA will help in guiding the optimal allocation of financial resources in the KSA.
Transparency
Author contributions
All authors have contributed to the development of this paper equally.
Previous presentation
Some parts of the study were presented at the Virtual ISPOR conference (ISPOR US May 15-18, 2022).
Reviewer disclosures
Peer reviewers on this manuscript have no relevant financial or other relationships to disclose.
Supplemental Material
Download MS Word (1.5 MB)Acknowledgements
The authors would like to thank all contributors for their commitment and dedication to the goals of RA. The authors would like to acknowledge the writing assistance provided by Prashee Peer, and Saurabh Trikha (IQVIA, India) and statistical analysis support provided by Shruti Patil (IQVIA, India) which was funded by AbbVie. The authors are fully responsible for all content and editorial decisions, were involved at all stages of manuscript development, and have approved the final version.
Declaration of financial/other relationships
ZA speaker and advisory honoraria from Pfizer, AbbVie, Lilly and Novartis, IA: speaker and advisory honorarium from Pfizer, Lilly, GSK, AbbVie, and Novartis, HAA, SA, WH, BAO have nothing to disclose, YS is full-time employee of IQVIA AG, OM was an employee at IQVIA AG, she has left her position in 2021, MA is a full-time employee at IQVIA Solutions Saudi Arabia, AA, TH and AJ are full-time employees at AbbVie Biopharmaceuticals GmbH and may hold company’s shares.
Data availability statement
The data that support the findings of this study are available from the corresponding author, MA, upon reasonable request.
Additional information
Funding
References
- Almoallim H, Hassan R, Cheikh M, et al. Rheumatoid arthritis Saudi database (RASD): disease characteristics and remission rates in a tertiary care center. Open Access Rheumatol. 2020;12:139–145. doi:10.2147/OARRR.S260426.
- Abu Al-Fadl EM, Ismail MA, Thabit M, et al. Assessment of health-related quality of life, anxiety and depression in patients with early rheumatoid arthritis. Egypt Rheumatol. 2014;36(2):51–56. doi:10.1016/j.ejr.2013.12.004.
- Almoallim HM, Alharbi LA. Rheumatoid arthritis in Saudi Arabia. Saudi Med J. 2014;35(12):1442–1454.
- Calabresi E, Petrelli F, Bonifacio AF, et al. One year in review 2018: pathogenesis of rheumatoid arthritis. Clin Exp Rheumatol. 2018;36(2):175–184.
- Myasoedova E, Davis JM, 3rd, Crowson CS, et al. Epidemiology of rheumatoid arthritis: rheumatoid arthritis and mortality. Curr Rheumatol Rep. 2010;12(5):379–385. doi:10.1007/s11926-010-0117-y.
- Katchamart W, Narongroeknawin P, Chanapai W, et al. Health-related quality of life in patients with rheumatoid arthritis. BMC Rheumatol. 2019;3(1):34. doi:10.1186/s41927-019-0080-9.
- Almutairi KB, Nossent JC, Preen DB, et al. The prevalence of rheumatoid arthritis: a systematic review of population-based studies. J Rheumatol. 2021;48(5):669–676. doi:10.3899/jrheum.200367.
- Alamanos Y, Drosos AA. Epidemiology of adult rheumatoid arthritis. Autoimmun Rev. 2005;4(3):130–136. doi:10.1016/j.autrev.2004.09.002.
- Manova M, Savova A, Vasileva M, et al. Comparative price analysis of biological products for treatment of rheumatoid arthritis. Front Pharmacol. 2018;9:1070. doi:10.3389/fphar.2018.01070.
- Al-Dalaan A, Al Ballaa S, Bahabri S, et al. The prevalence of rheumatoid arthritis in the Qassim region of Saudi Arabia. Ann Saudi Med. 1998;18(5):396–397. doi:10.5144/0256-4947.1998.396.
- Sanmartí R, Ruiz-Esquide V, Hernández MV. Rheumatoid arthritis: a clinical overview of new diagnostic and treatment approaches. Curr Top Med Chem. 2013;13(6):698–704. doi:10.2174/15680266113139990092.
- Gaujoux-Viala C, Gossec L, Cantagrel A, et al. Recommendations of the French society for rheumatology for managing rheumatoid arthritis. Joint Bone Spine. 2014;81(4):287–297. doi:10.1016/j.jbspin.2014.05.002.
- Smolen JS, Landewé R, Bijlsma J, et al. EULAR recommendations for the management of rheumatoid arthritis with synthetic and biological disease-modifying antirheumatic drugs: 2016 update. Ann Rheum Dis. 2017;76(6):960–977. doi:10.1136/annrheumdis-2016-210715.
- Janus Kinase Inhibitors for Rheumatoid Arthritis. Effectiveness and Value October 11, 2019. Available from: https://icer.org/wp-content/uploads/2020/10/ICER_RA_Draft_Evidence_Report_101119.pdf.
- Singh JA, Saag KG, Bridges SL, Jr., et al. American college of rheumatology guideline for the treatment of rheumatoid arthritis. Arthritis Rheumatol. 2015;68(1):1–26.
- Fries JF. Current treatment paradigms in rheumatoid arthritis. Rheumatology (Oxford). 2000;39 Suppl 1(suppl_1):30–35. doi:10.1093/oxfordjournals.rheumatology.a031492.
- Bykerk VP, Massarotti EM. The new ACR/EULAR remission criteria: rationale for developing new criteria for remission. Rheumatology (Oxford). 2012;51(Suppl 6):vi16–20. doi:10.1093/rheumatology/kes281.
- Pinals RS, Masi AT, Larsen RA. Preliminary criteria for clinical remission in rheumatoid arthritis. Arthritis Rheum. 1981;24(10):1308–1315. doi:10.1002/art.1780241012.
- Aletaha D, Ward MM, Machold KP, et al. Remission and active disease in rheumatoid arthritis: defining criteria for disease activity states. Arthritis Rheum. 2005;52(9):2625–2636. doi:10.1002/art.21235.
- Lillegraven S, Prince FH, Shadick NA, et al. Remission and radiographic outcome in rheumatoid arthritis: application of the 2011 ACR/EULAR remission criteria in an observational cohort. Ann Rheum Dis. 2012;71(5):681–686. doi:10.1136/ard.2011.154625.
- Aletaha D, Landewe R, Karonitsch T, et al. Reporting disease activity in clinical trials of patients with rheumatoid arthritis: EULAR/ACR collaborative recommendations. Arthritis Rheum. 2008;59(10):1371–1377. doi:10.1002/art.24123.
- Treating Rheumatoid Arthritis with Disease-Modifying Drugs (DMARDs). Available from: https://www.webmd.com/rheumatoid-arthritis/dmard-rheumatoid-arthritis-treatment.
- Visser K, Katchamart W, Loza E, et al. Multinational evidence-based recommendations for the use of methotrexate in rheumatic disorders with a focus on rheumatoid arthritis: integrating systematic literature research and expert opinion of a broad international panel of rheumatologists in the 3E initiative. Ann Rheum Dis. 2009;68(7):1086–1093. doi:10.1136/ard.2008.094474.
- Smolen JS, Landewé RBM, Bergstra SA, et al. EULAR recommendations for the management of rheumatoid arthritis with synthetic and biological disease-modifying antirheumatic drugs: 2022 update. Ann Rheum Dis. 2023;82(1):3–18. doi:10.1136/ard-2022-223356.
- Fraenkel L, Bathon JM, England BR, et al. 2021 American college of rheumatology guideline for the treatment of rheumatoid arthritis. Arthritis Care Res (Hoboken). 2021;73(7):924–939. doi:10.1002/acr.24596.
- Omair MA, Al Rayes H, Khabsa J, et al. Recommendations for the treatment of rheumatoid arthritis in Saudi Arabia: adolopment of the 2021 American college of rheumatology guidelines. BMC Rheumatol. 2022;6(1):70. doi:10.1186/s41927-022-00301-y.
- Massalska M, Maslinski W, Ciechomska M. Small molecule inhibitors in the treatment of rheumatoid arthritis and beyond: latest updates and potential strategy for fighting COVID-19. Cells. 2020;9(8):1876. doi:10.3390/cells9081876.
- Pope J, Sawant R, Tundia N, et al. Comparative efficacy of JAK inhibitors for moderate-to-severe rheumatoid arthritis: a network meta-analysis. Adv Ther. 2020;37(5):2356–2372. doi:10.1007/s12325-020-01303-3.
- Rinvoq FDA Approval History. Available from: https://www.drugs.com/history/rinvoq.html.
- Smolen JS, Landewé R, Breedveld FC, et al. EULAR recommendations for the management of rheumatoid arthritis with synthetic and biological disease-modifying antirheumatic drugs. Ann Rheum Dis. 2010;69(6):964–975. doi:10.1136/ard.2009.126532.
- Fleischmann R, Pangan AL, Song IH, et al. Upadacitinib versus placebo or adalimumab in patients with rheumatoid arthritis and an inadequate response to methotrexate: results of a phase III, Double-Blind, randomized controlled trial. Arthritis Rheumatol. 2019;71(11):1788–1800. doi:10.1002/art.41032.
- Upadacitinib for treating moderate to severe rheumatoid arthritis. 2020. Available from: https://www.nice.org.uk/guidance/ta665/documents/129.
- Cohen SB, van Vollenhoven RF, Winthrop KL, et al. Safety profile of upadacitinib in rheumatoid arthritis: integrated analysis from the SELECT phase III clinical programme. Ann Rheum Dis. 2021;80(3):304–311. doi:10.1136/annrheumdis-2020-218510.
- Fleischmann R, Curtis JR, Charles-Schoeman C, et al. Safety and effectiveness of upadacitinib or adalimumab plus methotrexate in patients with rheumatoid arthritis over 48 weeks with switch to alternate therapy in patients with insufficient response. Ann Rheum Dis. 2019;82(9):1130–1141. doi:10.1136/annrheumdis-2019-215764.
- Tanaka Y. A review of upadacitinib in rheumatoid arthritis. Mod Rheumatol. 2020;30(5):779–787. doi:10.1080/14397595.2020.1782049.
- Lee YH, Song GG. Relative efficacy and safety of tofacitinib, baricitinib, upadacitinib, and filgotinib in comparison to adalimumab in patients with active rheumatoid arthritis. Z Rheumatol. 2020;79(8):785–796. doi:10.1007/s00393-020-00750-1.
- Jadhav S, Yang Y. Pms17 cost effectiveness analysis of upadacitinib and methotrexate for patients with methotrexate-resistant rheumatoid arthritis. Value Health. 2020;23:S217. doi:10.1016/j.jval.2020.04.704.
- Rubbert-Roth A, Combe B, Szekanecz Z, et al. POS0677 Consistency in time to response with upadacitinib as monotherapy or combination therapy and across patient populations with rheumatoid arthritis. ACR Convergence; 2021.
- Kerschbaumer A, Smolen JS, Dougados M, et al. EULAR recommendations for the management of psoriatic arthritis with pharmacological therapies: 2019 update. Ann Rheum Dis. 2020;79(6):778–786. doi:10.1136/annrheumdis-2020-217163.
- Singh JA, Guyatt G, Ogdie A, et al. Special article: 2018 American college of rheumatology/national psoriasis foundation guideline for the treatment of psoriatic arthritis. Arthritis Care Res (Hoboken). 2019;71(1):2–29. doi:10.1002/acr.23789.
- Bergman M, Tundia N, Yang M, et al. Economic benefit from improvements in quality of life with upadacitinib: comparisons with tofacitinib and methotrexate in patients with rheumatoid arthritis. Adv Ther. 2021;38(12):5649–5661. doi:10.1007/s12325-021-01930-4.
- ICER Releases Evidence Report on JAK Inhibitors to Treat Rheumatoid Arthritis. [Internet]. Available from: https://icer.org/news-insights/press-releases/jak_inhibitor_evidence_report/.
- CADTH Common Drug Reviews. Clinical review report: upadacitinib (rinvoq): (AbbVie): indication: for the treatment of adults with moderately to severely active rheumatoid arthritis who have had an inadequate response or intolerance to methotrexate. Ottawa (ON): Canadian Agency for Drugs and Technologies in Health Copyright © 2020 Canadian Agency for Drugs and Technologies in Health; 2020.
- Maraiki F, Bazarbashi S, Scuffham P, et al. Methodological approaches to cost-effectiveness analysis in Saudi Arabia: what can we learn? A systematic review. MDM Policy Pract. 2022;7(1):23814683221086869. doi:10.1177/23814683221086869.
- Briggs A, Sculpher M, Claxton K. Decision modelling for health economic evaluation. Oxford: Oxford University Press; 2006.
- Husereau D, Drummond M, Augustovski F, et al. Consolidated health economic evaluation reporting standards (CHEERS) 2022 explanation and elaboration: a report of the ISPOR CHEERS II good practices task force. Value Health. 2022;25(1):10–31. doi:10.1016/j.jval.2021.10.008.
- Saudi Central Bank. Finance and exchange rate. Available from: https://www.sama.gov.sa/en-US/FinExc/Pages/Currency.aspx.
- Kameda H, Takeuchi T, Yamaoka K, et al. Efficacy and safety of upadacitinib over 84 weeks in Japanese patients with rheumatoid arthritis (SELECT-SUNRISE). Arthritis Res Ther. 2021;23(1):9. doi:10.1186/s13075-020-02387-6.
- Kameda H, Takeuchi T, Yamaoka K, et al. Efficacy and safety of upadacitinib in japanese patients with rheumatoid arthritis (SELECT-SUNRISE): a placebo-controlled phase IIb/III study. Rheumatology (Oxford). 2020;59(11):3303–3313. doi:10.1093/rheumatology/keaa084.
- Edwards CJ, Sawant R, Garg V, et al. A Matching-Adjusted indirect comparison of upadacitinib versus tofacitinib in adults with moderate-to-severe rheumatoid arthritis. Rheumatol Ther. 2021;8(1):167–181. doi:10.1007/s40744-020-00257-w.
- Park MC, Matsuno H, Kim J, et al. Long-term efficacy, safety and immunogenicity in patients with rheumatoid arthritis continuing on an etanercept biosimilar (LBEC0101) or switching from reference etanercept to LBEC0101: an open-label extension of a phase III multicentre, randomised, double-blind, parallel-group study. Arthritis Res Ther. 2019;21(1):122. doi:10.1186/s13075-019-1910-2.
- Targeted immune modulators for rheumatoid arthritis: effectiveness & value. Available from: http://icerorg.wpengine.com/wp-content/uploads/2020/10/NE_CEPAC_RA_Evidence_Report_FINAL_040717.pdf.
- Hernández Alava M, Wailoo A, Wolfe F, et al. A comparison of direct and indirect methods for the estimation of health utilities from clinical outcomes. Med Decis Making. 2014;34(7):919–930. doi:10.1177/0272989X13500720.
- Boytsov N, Harrold LR, Mason MA, et al. Increased healthcare resource utilization in higher disease activity levels in initiators of TNF inhibitors among US rheumatoid arthritis patients. Curr Med Res Opin. 2016;32(12):1959–1967. doi:10.1080/03007995.2016.1222515.
- Boonen A, Matricali GA, Verduyckt J, et al. Orthopaedic surgery in patients with rheumatoid arthritis: a shift towards more frequent and earlier non-joint-sacrificing surgery. Ann Rheum Dis. 2006;65(5):694–695. doi:10.1136/ard.2005.047175.
- Radner H, Smolen JS, Aletaha D. Remission in rheumatoid arthritis: benefit over low disease activity in patient-reported outcomes and costs. Arthritis Res Ther. 2014;16(1):R56. doi:10.1186/ar4491.
- Chaparro Del Moral R, Rillo OL, Casalla L, et al. Work productivity in rheumatoid arthritis: relationship with clinical and radiological features. Arthritis. 2012;2012:137635–137637. doi:10.1155/2012/137635.
- General Authority for Statistics. Labor market statistics; 2020. Available from: https://www.stats.gov.sa/sites/default/files/LM_2Q2020%20%28Press%20release_EN%20%29_2.pdf.
- Based on World Bank estimate of GDP per capita in KSA for 2019. Available from: https://data.worldbank.org/indicator/NY.GDP.PCAP.CD?locations=SA.