 ?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.ABSTRACT
Introduction
Atypical visual and social attention has often been associated with clinically diagnosed autism spectrum disorder (ASD), and with the broader autism phenotype. Atypical social attention is of particular research interest given the importance of facial expressions for social communication, with faces tending to attract and hold attention in neurotypical individuals. In autism, this is not necessarily so, where there is debate about the temporal differences in the ability to disengage attention from a face.
Method
Thus, we have used eye-tracking to record saccadic latencies as a measure of time to disengage attention from a central task-irrelevant face before orienting to a newly presented peripheral nonsocial target during a gap-overlap task. Neurotypical participants with higher or lower autism-like traits (AT) completed the task that included central stimuli with varied expressions of facial emotion as well as an inverted face.
Results
High AT participants demonstrated faster saccadic responses to detect the nonsocial target than low AT participants when disengaging attention from a face. Furthermore, faster saccadic responses were recorded when comparing disengagement from upright to inverted faces in low AT but not in high AT participants.
Conclusions
Together, these results extend findings of atypical social attention disengagement in autism and highlight how differences in attention to faces in the broader autism phenotype can lead to apparently superior task performance under certain conditions. Specifically, autism traits were linked to faster attention orienting to a nonsocial target due to the reduced attentional hold of the task irrelevant face stimuli. The absence of an inversion effect in high AT participants also reinforces the suggestion that they process upright or inverted faces similarly, unlike low AT participants for whom inverted faces are thought to be less socially engaging, thus allowing faster disengagement.
Autism Spectrum Disorder (ASD) has often been reported to be characterized by atypical patterns of visual processing and attention (Dakin & Frith, Citation2005; Keehn et al., Citation2013). Similar perceptual and attention differences have also been linked to the expression of autism-like traits, which are normally distributed throughout the general population and commonly referred to as the Broader Autism Phenotype (BAP; Landry & Chouinard, Citation2016; Piven et al., Citation1997). Many of these visual anomalies appear to be associated with temporal aspects of social behaviors such as the rate of processing of faces, social orienting, and joint visual attention (Dawson et al., Citation2005; Keehn et al., Citation2013). This in turn suggests that this link between social perception and anomalies in visual attention may be of importance to understanding the widespread anomalies of social behavior seen in those with ASD. Thus, the current study addressed this issue by employing eye tracking technology to examine how attentional disengagement in a gap/overlap paradigm was affected by different face stimuli amongst adults from the general population displaying varying expressions of autism traits.
Socially salient face processing in the neurotypical population and in ASD
Faces are highly salient and evolutionarily important visual stimuli from which information required for optimal social interactions are drawn (Bailly et al., Citation2010). Hence, impairments in face and facial emotion processing capacity as reported in ASD (Dawson et al., Citation2005; Lozier et al., Citation2014; Weigelt et al., Citation2012) and in the BAP (Becker et al., Citation2021; Poljac et al., Citation2013; Tardif et al., Citation2007) are thought to contribute to the development of social difficulties (Schultz, Citation2005) and impairments in social interactions (Farah et al., Citation2000; Hoehl & Striano, Citation2013). Such arguments are supported by evidence demonstrating weak or reduced activation of neural regions associated with face processing in brain imaging of adults with autism compared to neurotypical individuals (Dalton et al., Citation2005; McPartland et al., Citation2004; Pierce et al., Citation2001; Sabatino et al., Citation2013)
One demonstration of the special nature of faces in the general population is referred to as the face inversion effect, in which preferable processing of upright compared with inverted faces is evidenced (Farah et al., Citation1995). The face inversion effect has been measured in terms of reduced face identity accuracy (Farah et al., Citation1995; Rossion, Citation2009b), persistence of face representations in face-selective visual areas during brain imaging (Strother et al., Citation2011), as well as increased electrophysiological and saccadic latencies (Latinus & Taylor, Citation2006; Rousselet et al., Citation2008; Sadeh & Yovel, Citation2010). Impaired performance for inverted faces has also been hypothesized to be explained by a shift from holistic and configural processing to a featural processing strategy, similar to processing of nonsocial objects (Rossion, Citation2009b).
By comparison, the face inversion effect is controversial in ASD, with some studies finding an attenuation of this effect (McPartland et al., Citation2004; Tantam et al., Citation1989) and others finding performance comparable to that of neurotypical individuals (Hedley et al., Citation2015; Teunisse & de Gelder, Citation2003). Evidence from eye-tracking (Van der Geest et al., Citation2002) has demonstrated that children with ASD show similar duration of fixations on upright and inverted faces, whereas neurotypical children spend less time fixating on inverted than upright faces. Laycock, Wood et al. (Citation2020) have also shown much reduced inversion effects in the saccadic reaction time when orienting toward upright or inverted faces in a BAP population, supporting the hypothesis that individuals with ASD and those from the general population with high autistic traits do not rely on the same holistic and configural face processing strategies as neurotypical individuals (Dawson et al., Citation2005). Other studies indicate that a bias toward local, more detailed face information may mediate differences in susceptibility to face inversion (Lahaie et al., Citation2006; Mottron et al., Citation2006). Taken together, these findings suggest a need to further examine the conditions in which a reduced face inversion effect may be observed in ASD. While most investigations of the face inversion effect focus on explicit face identity recognition (Rossion, Citation2009a), the recent finding of differences in reflexive saccadic orienting to upright/inverted faces in the broader autism phenotype suggests that more automatic aspects of face processing may be more susceptible to anomalies in autistic populations. While attention orienting is one important component of a functioning attention system, there is currently little research to determine if reduced face inversion effects are specific to attention orienting.
Hence, we chose to use the Gap/Overlap Paradigm as a useful approach for separating the orienting and disengagement of attention processes. In this paradigm, a central fixation stimulus, such as a cross, is either maintained during the appearance of a peripheral target (overlap condition) or is removed prior to the appearance of the target (gap condition; Kingstone & Klein, Citation1993). Individuals in the neurotypical population exhibit longer saccadic onset times (SOTs) during the overlap compared to the gap condition (Kingstone & Klein, Citation1993; Taylor et al., Citation1998). This difference between the gap and overlap conditions is referred to as the gap-effect (Saslow, Citation1967). It is thought to reflect the inhibition of saccadic responses to the target while attention is disengaged from fixation in the overlap condition, compared to the facilitation of saccadic responses created by removal of the fixation stimulus in the gap condition, allowing participants to disengage attention from fixation before the target stimulus is presented (Taylor et al., Citation1998). Previous gap/overlap studies incorporating central stimuli consisting of fixation crosses or other similar nonsocial stimuli have identified a pattern of slower saccadic responses associated with ASD in the overlap condition (Elsabbagh et al., Citation2013; Keehn et al., Citation2013; Landry & Bryson, Citation2004; Sacrey et al., Citation2014; Zwaigenbaum et al., Citation2005), leading to the suggestion that ASD in children and adults may be uniquely associated with a reduced speed of attentional disengagement that is more pronounced with degree of severity of the symptoms (Kawakubo et al., Citation2007).
Although the support for an association between atypical visual orienting in ASD and a deficit in attentional disengagement is compelling (Keehn et al., Citation2013), some gap/overlap studies have found seemingly contradictory results (Kawakubo et al., Citation2004; Sacrey et al., Citation2014; Van der Geest et al., Citation2001). More recently, Wilson and Saldaña (Citation2019) also found no evidence for disengagement deficits for nonsocial stimuli, with faster saccadic responses in the gap condition for ASD children of mixed ability aged 4–9 years compared with neurotypical participants, and no group differences in the overlap condition.
Unfortunately, studies that provide conflicting evidence regarding a deficit in attentional disengagement in ASD have included small sample sizes (Keehn et al., Citation2013). This is of particular concern given the large heterogeneity regarding IQ and comorbid psychiatric diagnoses common to ASD (Brookman-Frazee et al., Citation2018; Bruining et al., Citation2010; Neuhaus et al., Citation2018). In studies requiring attentional disengagement from faces in toddlers (Chawarska et al., Citation2010) and adolescents (Kikuchi et al., Citation2011), those with ASD have demonstrated a paradoxically faster disengagement of attention compared to neurotypical individuals, whereas Fischer et al. (Citation2014) found no differences in the response times of ASD or neurotypical children in an overlapping condition employing centralized face stimuli. Methodological differences may explain these discrepancies and the evidence appears to suggest that task-irrelevant faces have a uniquely strong tendency to capture and hold the attention of neurotypical individuals (Cao et al., Citation2013) in comparison to those with ASD.
The current study took a dimensional approach to understanding the broader autism spectrum. Interestingly, many visual processing anomalies that are reported in ASD populations, such as impaired motion, global, and face processing (Atherton & Cross, Citation2022; Crewther et al., Citation2015; Laycock et al., Citation2014; Van Boxtel et al., Citation2017; Wyer et al., Citation2012), and superior performance on visual search and tasks that require local rather than global visual processing (Almeida et al., Citation2013; Cribb et al., Citation2016; Gonzalez et al., Citation2013; Jarrold et al., Citation2005), have also been established as atypical in the broader autism phentotype as measured throughout the general population (Crewther et al., Citation2015; Cribb et al., Citation2016; Laycock et al., Citation2014; Muth et al., Citation2014). Thus, we refer to anomalous perception in the sense of being different from the norm (i.e., atypical), given that autistic perception is characterized by both inferior and superior (but different) aspects.
A number of advantages to examining quantitative autistic traits in the general, non-clinical, population have been proposed (Landry & Chouinard, Citation2016). One such advantage is the possibility of modeling autism, while controlling for the substantial comorbidity with other psychiatric disorders (Simonoff et al., Citation2008), as well as for the wide range of intellectual functioning found in ASD (Brown et al., Citation2017; Elsabbagh et al., Citation2012). By studying the broader spectrum, the confounding effect of these factors on task performance may be better controlled. Finally, while some of the etiological underpinnings and phenotypic features of sub-clinical autistic traits appear to be continuous with the clinical ASD population (Folstein & Rosen-Sheidley, Citation2001), a skill pattern observed in only one of these populations (e.g., impairment on a cognitive task in those with ASD but not those with high autistic features) may indicate distinct causative features (Landry & Chouinard, Citation2016). This enables the study of the validity of clinical cutoffs for different cognitive and behavioral features of ASD.
Thus, the current study sought to assess attentional disengagement from photographs of faces, as indexed by saccadic onset times in a gap/overlap task, in individuals from the general population ranked as high or low in expressions of autism traits. We hypothesized that high-autism trait individuals would disengage attention from faces more quickly in the overlap condition than would low-autism trait individuals. In addition, because an earlier study (Laycock, Wood et al., Citation2020) had shown that participants with higher autism traits demonstrated a reduced face inversion effect when measuring the saccadic response time for orienting toward face targets, we also explored the effect of face inversion on attentional disengagement in the gap/overlap task. We hypothesized that participants with low autism traits would demonstrate a face inversion effect, with slower saccadic responses when disengaging from upright compared with inverted faces in the overlap condition, while participants with high autism traits would show a reduced face inversion effect. Finally, we examined the effects of emotionally expressive faces, which might be more likely to engage attention than faces with a neutral expression. We hypothesized that participants with low autism traits would be slower to disengage attention from expressive compared to neutral faces, while high autism traits would show no effect of expression.
Methods
Participants
A total of 60 adults participated in the study, a sample size determined based on the results of Kikuchi et al. (Citation2011). In this earlier study, a stimulus by group attentional disengagement effect was shown, indicating 22 participants per autism trait group would be required to detect an equivalent effect assuming 80% power and alpha of .05. The convenience sample was recruited through social media, posters displayed around the university campus, and a university department participant registry. Participants were aged between 18 and 50, with normal or corrected vision, and no history of epilepsy or neurological disorders. Signed, informed consent was obtained from all individual participants included in the study. Upon completion of the study participants received a $20 voucher as compensation for their time. All procedures were approved by the La Trobe University Human Ethics Committee (approval no. FHEC14/R89) and were in accordance with the Declaration of Helsinki.
Materials
Scores on the Subthreshold Autism Trait Questionnaire (SATQ) was used to divide participants into high autism trait (AT) and low AT groups. The SATQ is a 24-item, self-report scale designed to measure the full range of the BAP in the general population (Kanne et al., Citation2012). The autism trait domains measured by the SATQ include social interactions, oddness, the reading of facial expressions, expressive language, and rigidity. Each item is rated on a four-point Likert scale ranging from “not at all” to “very true.” The full scale numerical scores range from 0 to 72. The scale has been shown to demonstrate good reliability with a Cronbach’s alpha of .73 and test-retest reliability of .79, as well as good convergent validity with other measures of ASD and autism traits (Kanne et al., Citation2012). Although the Autism Quotient (AQ) is more commonly used in research (Landry & Chouinard, Citation2016), the SATQ is shorter in length and demonstrates slightly better reliability than the AQ (Nishiyama et al., Citation2014).
Non-verbal intelligence
An abbreviated form of the Raven’s Standard Progressive Matrices (RSPM-9), a nine-item measure of non-verbal fluid intelligence, was used to confirm that intelligence did not differentiate autism trait groups (Bilker et al., Citation2012). The RSPM-9 is a non-time-limited, multiple-choice test designed to assess nonverbal mental ability associated with abstract reasoning or fluid intelligence, with total scores ranging from 0 to 9. Bilker et al. (Citation2012) found a .98 correlation between performance on Form A of the RSPM-9 (used in this study) and the 60-item RSPM, thus predicting total scores for the 60-item RSPM with good accuracy, while substantially reducing testing time.
Gap/overlap task
An EyeLink 1000 Eye Tracker (SR Research, Ottawa, Canada) was used to record participants’ saccades toward target locations on the gap/overlap task. This task was performed on a Dell computer with a 22-inch screen and a 1024 × 768-pixel resolution. The peripheral target consisted of a simple black square (0.5° × 0.5°) that appeared approximately 14° to the left or right of central fixation. The central stimuli consisted of 80 color images derived from the Karolinska Directed Emotional Faces (KDEF) inventory (Lundqvist et al., Citation1998). These included 80 forward facing male and female faces, with 20 neutral, 20 (different) inverted-neutral, 20 fearful, and 20 happy faces. For each condition, the 20 KDEF faces chosen were selected based on an analysis by Goeleven et al. (Citation2008) to determine the most accurately identified emotional expressions from the larger KDEF inventory.
Participants were instructed to fixate on the face in the center of the screen before directing their gaze toward a subsequent peripheral target as quickly and as accurately as possible. illustrates the event time sequence for alternate trial structure for gap and overlap conditions.
Figure 1. Trial structure for the gap/overlap task. In the gap condition the trial commenced with a 400 ms blank screen, after which participants fixated on a central face. Faces were shown for a randomized duration of 600–1200 ms, with a 200 ms gap presented before a target appeared randomly to the left or right. In the overlap condition the trial sequence was identical except that the central face was presented for 800–1400 ms and remained on the screen after the target appeared. In both conditions the target was presented for 400 ms. Participants were required to fixate the face and look toward the target as soon as it appeared.
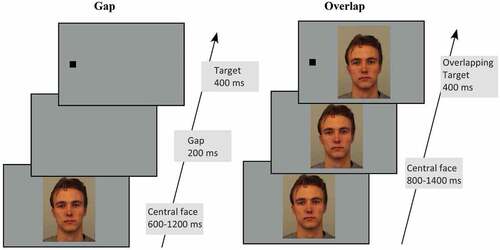
The task consisted of a total of 400 gap and 400 overlap trials, with 100 each for upright-neutral, inverted-neutral, upright-fearful, and upright-happy faces in both gap and overlap conditions. The different face conditions were randomly interspersed within alternating gap or overlap blocks of 50 trials. The order of blocks was counterbalanced such that half of the participants completed a gap block first, and the other half completed an overlap block first.
Procedures
After making contact via e-mail or social media, participants were provided with an online participant information statement, and electronically gave consent before completing demographics questions and the SATQ. Upon arrival at the laboratory, participants provided signed informed consent. Testing was divided into four sections: first, participants completed the first half (8 blocks) of the gap/overlap task, then the RSPM and a brief psychophysical task not part of the current project, and finally the second half (8 blocks) of the gap/overlap task. To complete the gap/overlap task, participants were positioned in a chin and forehead rest 60 cm from the screen and the eye tracker was calibrated for the participants’ right eye. A 5-point calibration procedure was performed at the start of the task, and if the participant readjusted their head position following any breaks away from the head rest. The total testing time took approximately one hour and 20 minutes, with the total time spent on the gap/overlap task being approximately 50 minutes, including short breaks when needed.
Data analysis
To dichotomize the data and enable a quantile analysis similar to that recommended by Cribb et al. (Citation2016), the SATQ data from participants who fell in the middle 20% of ranked SATQ scores (n = 12) were removed from analysis. This left 24 participants (40%) in each of the high- and low AT groups. Descriptive statistics, including gender, age, SATQ and RSPM-9 scores for participants in each group are presented in . These groups did not differ in terms of age (p = .275) or intelligence (p = .170). Participants were not asked whether they had an ASD diagnosis. For context, our original sample (n = 60) had a mean SATQ score of 22.4 (SD = 7.8) which is similar to the sample characteristics reported by Kanne et al. (Citation2012), with mean SATQ score of 23.1 (SD = 7.1). Our high AT group had a SATQ score range of 24 to 41 (M = 29.8, SD = 5.1), which as expected is higher than the population mean, though it is lower than an ASD sample (M = 40.8, SD = 13.6) as reported in Kanne et al. (Citation2012). In fact, a t-test comparing these different samples was significant (t(39) = 3.63, p < .001, d = 1.15.) This suggests that although it cannot be ruled out that our high AT sample included anyone with a diagnosed ASD, it does not appear that the sample reflects the SATQ scores of an ASD sample.
Table 1. Demographic characteristics for low and high autism trait (AT) groups.
For the gap/overlap task, saccade onset time (SOT) was defined as the first saccade following appearance of the target. Saccades were removed from analysis if they occurred prior to the targets’ appearance, if the gaze prior to the saccade did not fall within one degree of central fixation, or if the saccade was less than four degrees toward the target. Saccadic onset times were defined as the time interval between the onset of the target and the onset of a saccadic eye movement toward the target.
To test the hypotheses regarding the effect of autism trait group, face inversion, and face emotion on attentional disengagement as assessed by the SOT, a 2 (autism trait group: high, low) ×2 (fixation condition: gap, overlap) ×4 (face condition: happy, fearful, neutral, inverted neutral) mixed model ANOVA was conducted. Simple effect analyses were conducted where a significant interaction was observed. Finally, in order to explore face inversion effects for the rate of saccadic disengagement for autism trait groups, planned comparisons were performed to determine differences in inversion effect SOTs for the two AT groups separately for gap and overlap conditions using one sample t tests.
Results
One participant in the low autism trait group was identified as an outlier based on SOT scores (greater than 3 × the interquartile range) for both fear and happy faces in the gap condition. It was decided to not exclude this participant in order to retain the sample size, although the pattern of significance in the results described below was unchanged with this participant excluded.
Gap/overlap effects with different fixation conditions
A 2 × 2 × 4 mixed model ANOVA was conducted to investigate the effect of autism trait group and gap/overlap condition on SOTs for different face conditions. shows mean SOTs for high and low autism trait groups across each fixation and face condition.
Figure 2. Mean Saccade Onset Time (SOT) in the a) gap, and b) overlap conditions across low and high AT groups. Error bars indicate SEM.
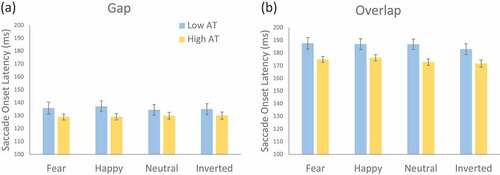
A significant main effect was found for autistic trait (AT) group, F (1,46) = 4.74, p = .035, = .09, with high AT having faster SOTs than low AT participants. A significant main effect was found for gap/overlap condition, with longer SOTs found for overlap compared with gap conditions, F (1, 46) = 355.06, p < .001,
= .89, indicating that the expected gap-effect was observed. A significant main effect was also found for face condition, F (3, 138) = 4.88, p = .003,
= .10, with post hoc tests indicating this was mainly driven by the faster SOTs for inverted compared with happy (p = .004), fearful (p = .010), neutral faces (p = .090), while neutral faces showed faster SOTs than for happy faces (p = .041). All other pairwise comparisons were not significant (p’s > .175). The effect of face condition, however, was qualified by a significant interaction with gap/overlap condition, F (3, 138) = 3.05, p = .031,
= .06. No interaction was found between gap/overlap condition and group, F (1, 46) = 1.47, p = .232,
= .03, between face condition and group, F(3, 138), = 0.56, p = .644,
= .01, nor for the three-way interaction between gap/overlap condition, group, and face condition, F (3, 138) = 1.75, p = .159,
= .04.
Simple effects analysis was used to further examine the interaction between gap/overlap condition and face condition. shows the estimated marginal mean SOTs for face condition within gap or overlap conditions, irrespective of autism trait group.
Figure 3. Estimated marginal mean Saccade Onset Times (SOTs) for fearful, happy, neutral, and inverted neutral faces within gap or overlap conditions. Error bars indicate SEM. * p < .05.
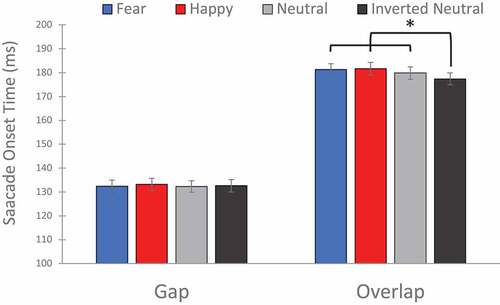
Within the gap condition, no significant difference in SOTs between different face conditions were observed (all p’s > .33). However, within the overlap condition significantly shorter SOTs were observed for inverted faces compared to fearful (p = .003), happy (p = .001) and neutral (p = .001) faces, indicating attentional disengagement was faster from inverted faces than upright faces. No other significant differences were observed between face conditions.
Group differences in SOTs for upright and inverted faces
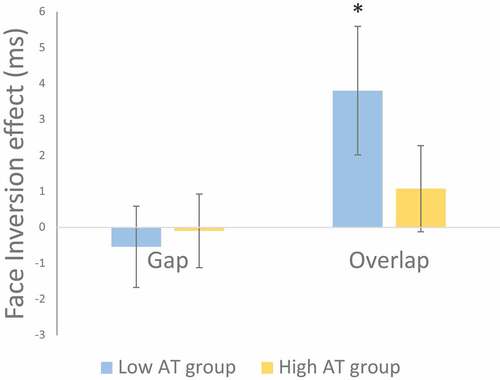
Given the a priori hypothesis concerning differences in the saccadic face inversion effect between autism trait groups, and with respect to the earlier simple effects analysis revealing a difference between upright and inverted faces in the overlap condition (regardless of group), an inversion effect was calculated separately for gap and overlap conditions for each participant. This was defined as the inverted neutral face SOT subtracted from the upright neutral face SOT for each gap/overlap condition. A one-sample t-test, tested against 0, determined if this inversion effect was significant (see, ). For the gap condition, neither the low AT group (t(23) = 0.48, p = .637, d = 0.10), nor the high AT group (t(23 = 0.09, p = .927, d = 0.02) demonstrated a saccadic inversion effect. However, in the overlap condition, the low AT group demonstrated a significant inversion effect (t(23) = 2.13, p = .044, d = 0.43), while the high AT group did not (t(23) = 0.90, p = .380, d = 0.18).
Figure 4. Saccadic face inversion effects were calculated by subtracting the Saccade Onset Time (SOT) for inverted neutral face from upright (neutral) face conditions. A positive inversion effect indicates faster disengagement from inverted compared with upright fixation faces. Only the low AT group demonstrated a significant face inversion effect in the overlap condition. Error bars indicate SEM. * indicates p < .05.
Finally, given the above observation, across all participants, of differences between SOTs for inverted and upright stimuli in the overlap condition, along with the different saccadic inversion effects established in high and low AT groups, a further exploratory analysis compared saccade latency between AT groups separately for upright (i.e., combining across emotions) and inverted faces. Independent samples t tests were conducted and found that in the gap condition, AT groups showed no significant difference in saccade latency for upright faces, t(46) = 1.35, p = .183, d = 0.39, or for inverted faces, t(46) = 0.99, p = .328, d = 0.29. However, in the overlap condition, the high AT group showed significantly faster SOTs for both upright faces, t(46) = 2.51, p = .016, d = 0.72, and for inverted faces, t(46) = 2.27, p = .028, d = 0.65 when compared with the low AT group.
Discussion
The present study aimed to examine the relationship between autism traits in the general population and the ability to disengage attention from face stimuli. Results suggest that while all participants demonstrated the expected gap effect, with shorter SOTs for gap compared with overlap conditions, this depended on autism trait group membership and also on face type (i.e., facial expression or inverted conditions). As hypothesized, no differences in SOTs were observed between AT groups within the gap conditions. This indicates that when the face fixation is removed prior to the target appearance, attention is released and both groups of participants oriented to the peripheral target with a similar latency. As expected, face emotion did not affect SOT in the gap condition. Contrary to expectations that AT group differences would be observed in overlap conditions, this hypothesis was not supported by the findings in the main analysis (i.e., the 3-way interaction effect was not significant). However, planned a priori analysis of the saccadic inversion effect did suggest attention disengagement was different between AT groups in the overlap condition depending on the orientation of the face. Whereas the low AT group demonstrated an inversion effect with faster SOTs when required to disengage attention from inverted than upright faces, the high AT group did not demonstrate any inversion effect. A further exploratory analysis revealed that only in the overlap conditions the high AT group had significantly faster SOTs compared to the low AT group when disengaging attention from upright faces (i.e., excluding the inverted face condition), and faster SOTs for inverted faces.
Together, these findings suggest that high autistic trait scores are linked to different patterns of attention disengagement from faces. Specifically, those with high autism traits were faster to disengage from a face, regardless of emotional expression or orientation. However, there was also evidence that attention to inverted faces varied depending on AT group membership, with the high AT group apparently showing no evidence of faster attention disengagement from inverted compared with upright faces.
General population capacity to disengage attention from expressive and inverted faces
When considering the combined sample of high and low AT participants, irrespective of group membership, the observed shorter SOTs from inverted compared to all upright stimuli in overlap conditions, is consistent with research indicating that faces are given less attentional priority when inverted (Gong et al., Citation2014). As such, participants in the current study appeared to disengage more quickly from the inverted compared to upright faces (even when comparing the inverted-neutral faces to the upright-neutral face, which only differed in their orientation). Evidence from neuroimaging studies and studies indicating that inverted faces are processed with a more featural strategy compared to the holistic and configural processing that characterizes upright faces processing, suggests that inverted faces may be processed similarly to nonsocial objects within the adult neurotypical population (Farah et al., Citation1995; Schwarzer, Citation2000; Strother et al., Citation2011). Accordingly, the greater salience of the socially relevant information inherent in the upright compared to inverted faces could have driven the observed effect by capturing the social salience-driven attention of the participants.
The absence of a difference in SOTs between the neutral and the fearful or happy faces is a surprising result, given the large body of evidence indicating that individuals pay greater attention to faces displaying negative emotions (Mason & Capitanio, Citation2012; Vuilleumier, Citation2005), reflecting the greater social importance of prioritizing emotionally salient information throughout evolution (Bailly et al., Citation2010; Mason & Capitanio, Citation2012). However, although we expected that attentional disengagement would be slower for emotional faces compared to neutral faces, especially for faces displaying fear, the neural correlates of this type of processing has conflicting evidence in the literature. This conflicting evidence appears to be related to task relevance. Vuilleumier et al. (Citation2001) found that whilst fusiform activation was modulated by whether faces appeared in task relevant or task irrelevant locations (i.e., an attention manipulation), the left amygdala showed preferential activation for fearful faces, regardless of the attention manipulation. This latter finding suggests that some aspects of processing of facial emotions is automatic. On the other hand, Pessoa et al. (Citation2002) reported that the task relevance of fearful faces moderates neural responses in both fusiform and amygdala. Differences between these studies may be due to differences in the degree of success in the attention manipulation (Pessoa et al., Citation2002). In this respect, the findings of Pessoa et al. appear to fit with our findings, given that the faces were task irrelevant and attentional disengagement did not differ between emotional and neutral faces. In this case, task irrelevance may have attenuated the relationship between face emotions and SOTs.
Gap versus overlap conditions
The shorter SOTs observed in the gap compared to overlap conditions, irrespective of group or face condition, are consistent with past research employing gap/overlap paradigms with various populations and stimuli (Sacrey et al., Citation2014; Saslow, Citation1967). This gap-effect is thought to occur as a result of an anticipatory effect in the gap condition facilitating shorter SOTs, and the need to disengage attention in the overlap condition inhibiting saccadic orientating toward the target (Kingstone & Klein, Citation1993). The results indicated that the degree to which the gap facilitated shorter SOTs was not dependent on face condition nor on autism trait group. However, differences within overlap conditions indicated that the inhibitory effect of the overlap condition was dependent on both the face condition and the AT group. Hence, SOTs from the center of the screen were only dependent upon the group or stimuli when the disengaging operation of Posner and Dehaene’s (Citation1994) model of visual orienting was required, and no lingering effect of faces on SOTs was apparent in the gap conditions. This suggests that face condition and group differences related only to differences in attentional disengagement speed, rather than the speed of attentional shifts per se.
Disengaging attention from faces across the autism spectrum
While previous studies have examined the performance of individuals with clinical diagnoses of ASD on gap/overlap tasks incorporating faces as the central stimuli (for a review see, Sacrey et al., Citation2014), no studies have examined social attention disengagement using a gap/overlap paradigm in relation to variation in autism traits within the neurotypical population. The current results are broadly consistent with previous ASD studies finding faster saccade latencies in children with ASD when required to disengage attention from social stimuli (Chawarska et al., Citation2010; Kikuchi et al., Citation2011). Conversely, Fischer et al. (Citation2014) used both social and nonsocial fixation, and also target stimuli manipulated orthogonally in a gap/overlap task, finding no evidence of impaired attention disengagement in ASD children.
Previous research has reported evidence for a general reduction in the face inversion effect in ASD (Falck-Ytter, Citation2008; McPartland et al., Citation2004; Rose et al., Citation2007) and also in adults with higher autism traits (Laycock, Wood et al., Citation2020; Wyer et al., Citation2012). However, these differences in face inversion effects have not always been replicated. Inconsistent findings may be a function of the paradigm and thus the specific aspect of face perception measured. For example, Hedley et al. (Citation2015) required participants to learn upright and inverted faces presented for 9 seconds in total across 3 viewings per face. Subsequently paricipants performed a recognition test with the presentation of target and foil faces presented in the same (i.e, upright or inverted) orientations. While overall face recognition was lower for ASD adults than a control group, there was a similar face inversion effect with lower recognition of inverted faces for both groups (Hedley et al., Citation2015). Thus, while early studies measuring the face inversion effect used paradigms involving recognition memory, or matching of face identity (Weigelt et al., Citation2012), some more recent studies have taken advantage of the phenomenon to explore the temporal aspects of face perception. Electrophysiological studies have established that fast processing mechanisms linked to the face inversion effect are different in ASD (McPartland et al., Citation2004; Vettori et al., Citation2019). Similarly, a reduction in the face inversion effect was recently shown in terms of timing of the saccadic reaction time in neurotypical adults with higher autism traits (Laycock et al., Citation2020). The current findings also observing reduced saccadic inversion effects with respect to attentional disengagement suggest that the temporal aspects of face perception, and the face inversion effect may be a useful approach to examining face perception in ASD.
Observable differences in social attention disengagement as reported here could have broader implications for understanding autism. It has previously been proposed that early developmental differences in the ability to orient to, and disengage attention from, socially relevant stimuli such as faces could lead to the social-communication challenges characteristic of ASD (Elsabbagh et al., Citation2011; Laycock, Crewther et al., Citation2020; Schultz, Citation2005). The finding of a similar pattern of difference in social attention disengagement that we report in the broader autism phenotype, could also have implications for determining a biomarker for the autism phenotype, and ultimately could assist in early detection of autism. For example, Elsabbagh et al. (Citation2013) showed that babies who at 36 months were diagnosed with ASD, had previously been found to be slower to disengage attention when tested at 14 months.
Limitations and future directions
It is important to acknowledge that the current findings relate directly to understanding the consequences of variation of autism traits in the general population and cannot be used to make direct inferences about populations with a diagnosed ASD. Future work should examine whether these effects, in particular the novel absence of an attention disengagement face inversion effect in the high autism trait group, can be replicated in an ASD population. While replication would elicit further insights into the broader cognitive differences in ASD, failure to replicate would also help establish a better understanding of the characteristics of ASD that are continuous with or distinct from the general population, potentially leading to a better categorization of the disorder and understanding of its etiological underpinnings.
The SATQ enables the analysis of autism traits by subdomains, as well as the full scale used to divide participants into high and low AT groups in this study (Kanne et al., Citation2012). While consideration of the type-one error rate for the participant numbers and expected power of the study indicated that it was not appropriate to conduct the number of additional statistical tests required to include an analysis of these subdomains in the present work, future research could benefit from the analysis of whether particular domains of autism traits are associated with the face processing anomalies established here.
The moderate sample size in the current study should also be acknowledged. While an a priori sample size estimation suggested that our achieved sample per group (n = 24) should be adequate to detect expected effects, larger samples are to be encouraged to increase confidence in replicable effects. For similar eye-tracking studies using a gap-overlap paradigm, this type of sample size is common in the literature (e.g., Bocca & Denise, Citation2006; Crawford, Citation2015; Drew et al., Citation2007; Kikuchi et al., Citation2011; Van der Geest et al., Citation2001). Importantly, the reported effect sizes in the current study (e.g., = .09 for the main effect of group in the main analysis indicating faster overall SOT by the low compared with high AT group; d = 0.72 for the group comparison of overlap upright faces indicating faster SOTs by the low AT group; d = 0.43 for the overlap inversion effect in the low AT group) indicates moderate-large effects (Cohen, Citation2013). Furthermore, the large number of trials (400 gap, 400 overlap) per participant increases confidence of a reliable estimate of performance per participant.
Finally, as suggested by an anonymous reviewer, the ecological validity of the utilized paradigm should be considered. Clearly, the rapid temporal dynamics of the paradigm in which faces were briefly presented and then suddenly removed (in the gap condition) before simple targets appeared in the periphery do not represent real-world social encounters. Of course, the gap-overlap paradigm was not designed as an ecologically valid assessment tool, but instead is intended for carefully designed behavioral experiments that tease apart different components of attention (Kingstone & Klein, Citation1993; Taylor et al., Citation1998). Thus, the gap-overlap paradigm allows investigation of the competition between different processes: the current fixation and the initiation of a saccade. The addition of a social (face) fixation point, as used here, adapts the paradigm to understand the attentional hold of faces, and the ability to disengage attention and initiate a saccade away from the face. A plausible direct implication of faster social attention disengagement is that while engaging in a conversation with a social partner, peripheral visual information could more easily disengage attentional focus away from the face. Future studies, perhaps using virtual reality, could measure eye-movements during social interactions and assess real-time attention disengagement when peripheral distracters are presented to test this idea.
Conclusion
The current study employed a saccadic gap/overlap paradigm to examine attentional disengagement from faces and the relationship between task performance and autism traits in the general population as to the best of our knowledge, no prior study looking at ASD or the broader autism phenotype has investigated this question using this paradigm. We found that the cost of disengaging from faces in the overlap condition was reduced overall by face inversion, though this effect was not significant amongst individuals with higher expressions of autism traits. These results demonstrate that some autistic characteristics, including a reduced attentional prioritization of faces, may be continuous across the broader autism phenotype in the general population. Thus, the present findings provide novel insights into the relationship between the broader autism spectrum, attentional disengagement, and social processing.
Acknowledgments
None.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Data availability statement
Data is available by request to the corresponding author.
Additional information
Funding
References
- Almeida, R. A., Dickinson, J. E., Maybery, M. T., Badcock, J. C., & Badcock, D. R. (2013). Visual search targeting either local or global perceptual processes differs as a function of autistic-like traits in the typically developing population. Journal of Autism and Developmental Disorders, 43(6), 1272–1286. https://doi.org/10.1007/s10803-012-1669-7
- Atherton, G., & Cross, L. (2022). Reading the mind in cartoon eyes: Comparing human versus cartoon emotion recognition in those with high and low levels of autistic traits. Psychological Reports, 125(3), 1380–1396. https://doi.org/10.1177/0033294120988135
- Bailly, G., Raidt, S., & Elisei, F. (2010). Gaze, conversational agents and face-to-face communication. Speech Communication, 52(6), 598–612. https://doi.org/10.1016/j.specom.2010.02.015
- Becker, C., Caterer, E., Chouinard, P. A., & Laycock, R. (2021). Alterations in rapid social evaluations in individuals with high autism traits. Journal of Autism and Developmental Disorders, 51(10), 3575–3585. https://doi.org/10.1007/s10803-020-04795-8
- Bilker, W. B., Hansen, J. A., Brensinger, C. M., Richard, J., Gur, R. E., & Gur, R. C. (2012). Development of abbreviated nine-item forms of the Raven’s standard progressive matrices test. Assessment, 19(3), 354–369. https://doi.org/10.1177/1073191112446655
- Bocca, M.-L., & Denise, P. (2006). Total sleep deprivation effect on disengagement of spatial attention as assessed by saccadic eye movements. Clinical Neurophysiology, 117(4), 894–899. https://doi.org/10.1016/j.clinph.2006.01.003
- Brookman-Frazee, L., Stadnick, N., Chlebowski, C., Baker-Ericzén, M., & Ganger, W. (2018). Characterizing psychiatric comorbidity in children with autism spectrum disorder receiving publicly funded mental health services. Autism, 22(8), 938–952. https://doi.org/10.1177/1362361317712650
- Brown, A. C., Chouinard, P. A., & Crewther, S. G. (2017). Vision research literature may not represent the full intellectual range of autism spectrum disorder. Frontiers in Human Neuroscience, 11, 57. https://doi.org/10.3389/fnhum.2017.00057
- Bruining, H., de Sonneville, L., Swaab, H., de Jonge, M., Kas, M., van Engeland, H., Vorstman, J., & Aziz, S. A. (2010). Dissecting the clinical heterogeneity of autism spectrum disorders through defined genotypes. PLoS One, 5(5), e10887. https://doi.org/10.1371/journal.pone.0010887
- Cao, R., Wang, S., Rao, C., & Fu, J. (2013). Task-irrelevant own-race faces capture attention: Eye-tracking evidence. Scandinavian Journal of Psychology, 54(2), 78–81. https://doi.org/10.1111/sjop.12027
- Chawarska, K., Volkmar, F., & Klin, A. (2010). Limited attentional bias for faces in toddlers with autism spectrum disorders. Archives of General Psychiatry, 67(2), 178–185. https://doi.org/10.1001/archgenpsychiatry.2009.194
- Cohen, J. (2013). Statistical power analysis for the behavioral sciences. Routledge.
- Crawford, T. (2015). The disengagement of visual attention in Alzheimer’s disease: A longitudinal eye-tracking study [original research]. Frontiers in Aging Neuroscience, 7, 7. https://doi.org/10.3389/fnagi.2015.00118
- Crewther, D. P., Crewther, D., Bevan, S., Goodale, M. A., & Crewther, S. G. (2015). Greater magnocellular saccadic suppression in high versus low autistic tendency suggests a causal path to local perceptual style. Royal Society Open Science, 2(12), 150226. https://doi.org/10.1098/rsos.150226
- Cribb, S. J., Olaithe, M., Di Lorenzo, R., Dunlop, P. D., & Maybery, M. T. (2016). Embedded figures test performance in the broader autism phenotype: A Meta-analysis. Journal of Autism and Developmental Disorders, 46(9), 2924–2939. https://doi.org/10.1007/s10803-016-2832-3
- Dakin, S., & Frith, U. (2005). Vagaries of visual perception in autism. Neuron, 48(3), 497–507. https://doi.org/10.1016/j.neuron.2005.10.018
- Dalton, K. M., Nacewicz, B. M., Johnstone, T., Schaefer, H. S., Gernsbacher, M. A., Goldsmith, H. H., Alexander, A. L., & Davidson, R. J. (2005). Gaze fixation and the neural circuitry of face processing in autism. Nature Neuroscience, 8(4), 519–526. https://doi.org/10.1038/nn1421
- Dawson, G., Webb, S. J., & McPartland, J. (2005). Understanding the nature of face processing impairment in autism: Insights from behavioral and electrophysiological studies. Developmental Neuropsychology, 27(3), 403–424. https://doi.org/10.1207/s15326942dn2703_6
- Drew, A. S., Langan, J., Halterman, C., Osternig, L. R., Chou, L.-S., & van Donkelaar, P. (2007). Attentional disengagement dysfunction following mTBI assessed with the gap saccade task. Neuroscience Letters, 417(1), 61–65. https://doi.org/10.1016/j.neulet.2007.02.038
- Elsabbagh, M., Divan, G., Koh, Y. J., Kim, Y. S., Kauchali, S., Marcín, C., Montiel-Nava, C., Patel, V., Paula, C. S., Wang, C., Yasamy, M. T., & Fombonne, E. (2012). Global prevalence of autism and other pervasive developmental disorders. Autism Research, 5(3), 160–179. https://doi.org/10.1002/aur.239
- Elsabbagh, M., Fernandes, J., Webb, S. J., Dawson, G., Charman, T., & Johnson, M. H. (2013). Disengagement of visual attention in infancy is associated with emerging autism in toddlerhood. Biological Psychiatry, 74(3), 189–194. https://doi.org/10.1016/j.biopsych.2012.11.030
- Elsabbagh, M., Holmboe, K., Gliga, T., Mercure, E., Hudry, K., Charman, T., Baron-Cohen, S., Bolton, P., & Johnson, M. H. (2011). Chapter 11 - Social and attention factors during infancy and the later emergence of autism characteristics. O. Braddick, J. Atkinson, & G. M. Innocenti(Eds.), Progress in brain research. (Vol. Vol. 189, pp. 195–207). Elsevier. https://doi.org/10.1016/B978-0-444-53884-0.00025-7
- Falck-Ytter, T. (2008). Face inversion effects in autism: A combined looking time and pupillometric study. Autism Research, 1(5), 297–306. https://doi.org/10.1002/aur.45
- Farah, M. J., Rabinowitz, C., Quinn, G. E., & Liu, G. T. (2000). Early commitment of neural substrates for face recognition. Cognitive Neuropsychology, 17(1), 117–123. https://doi.org/10.1080/026432900380526
- Farah, M. J., Tanaka, J. W., & Drain, H. M. (1995). What causes the face inversion effect? Journal of Experimental Psychology. Human Perception and Performance, 21(3), 628–634. https://doi.org/10.1037//0096-1523.21.3.628
- Fischer, J., Koldewyn, K., Jiang, Y. V., & Kanwisher, N. (2014). Unimpaired attentional disengagement and social orienting in children with autism. Clinical Psychological Science, 2(2), 214–223. https://doi.org/10.1177/2167702613496242
- Folstein, S. E., & Rosen-Sheidley, B. (2001). Genetics of autism: Complex aetiology for a heterogeneous disorder. Nature Reviews. Genetics, 2(12), 943–955. https://doi.org/10.1038/35103559
- Goeleven, E., De Raedt, R., Leyman, L., & Verschuere, B. (2008). The Karolinska directed emotional faces: A validation study. Cognition & Emotion, 22(6), 1094–1118. https://doi.org/10.1080/02699930701626582
- Gong, J., Zhang, Y., Huang, Y., Feng, J., & Zhang, W. W. (2014). Controversies in the facial inversion effect: Face specificity and expertise. Neurophysiology, 46(5), 438–443. https://doi.org/10.1007/s11062-015-9470-9
- Gonzalez, C., Martin, J. M., Minshew, N. J., & Behrmann, M. (2013). Practice makes improvement: How adults with autism out-perform others in a naturalistic visual search task. Journal of Autism and Developmental Disorders, 43(10), 2259–2268. https://doi.org/10.1007/s10803-013-1772-4
- Hedley, D., Brewer, N., & Young, R. (2015). The effect of inversion on face recognition in adults with autism spectrum disorder. Journal of Autism and Developmental Disorders, 45(5), 1368–1379. https://doi.org/10.1007/s10803-014-2297-1
- Hoehl, S., & Striano, T. (2013). Further evidence for continuity in infants’ joint attention development. Human Development, 56(4), 249–253. https://doi.org/10.1159/00035105
- Jarrold, C., Gilchrist, I. D., & Bender, A. (2005). Embedded figures detection in autism and typical development: Preliminary evidence of a double dissociation in relationships with visual search. Developmental Science, 8(4), 344–351. https://doi.org/10.1111/j.1467-7687.2005.00422.x
- Kanne, S. M., Wang, J., & Christ, S. E. (2012). The Subthreshold Autism Trait Questionnaire (SATQ): Development of a brief self-report measure of subthreshold autism traits. Journal of Autism and Developmental Disorders, 42(5), 769–780. https://doi.org/10.1007/s10803-011-1308-8
- Kawakubo, Y., Kasai, K., Okazaki, S., Hosokawa-Kakurai, M., Watanabe, K., Kuwabara, H., Ishijima, M., Yamasue, H., Iwanami, A., Kato, N., & Maekawa, H. (2007). Electrophysiological abnormalities of spatial attention in adults with autism during the gap overlap task. Clinical Neurophysiology, 118(7), 1464–1471. https://doi.org/10.1016/j.clinph.2007.04.015
- Kawakubo, Y., Maekawa, H., Itoh, K., Hashimoto, O., & Iwanami, A. (2004). Spatial attention in individuals with pervasive developmental disorders using the gap overlap task. Psychiatry Research, 125(3), 269–275. https://doi.org/10.1016/j.psychres.2003.12.012
- Keehn, B., Muller, R. A., & Townsend, J. (2013). Atypical attentional networks and the emergence of autism. Neuroscience and Biobehavioral Reviews, 37(2), 164–183. https://doi.org/10.1016/j.neubiorev.2012.11.014
- Kikuchi, Y., Senju, A., Akechi, H., Tojo, Y., Osanai, H., & Hasegawa, T. (2011). Atypical disengagement from faces and its modulation by the control of eye fixation in children with autism spectrum disorder. Journal of Autism and Developmental Disorders, 41(5), 629–645. https://doi.org/10.1007/s10803-010-1082-z
- Kingstone, A., & Klein, R. M. (1993). Visual offsets facilitate saccadic latency: Does predisengagement of visuospatial attention mediate this gap effect? Journal of Experimental Psychology. Human Perception and Performance, 19(6), 1251–1265. https://doi.org/10.1037//0096-1523.19.6.1251
- Lahaie, A., Mottron, L., Arguin, M., Berthiaume, C., Jemel, B., & Saumier, D. (2006). Face perception in high-functioning autistic adults: Evidence for superior processing of face parts, not for a configural face-processing deficit. Neuropsychology, 20(1), 30–41. https://doi.org/10.1037/0894-4105.20.1.30
- Landry, R., & Bryson, S. E. (2004). Impaired disengagement of attention in young children with autism. Journal of Child Psychology and Psychiatry, 45(6), 1115–1122. https://doi.org/10.1111/j.1469-7610.2004.00304.x
- Landry, O., & Chouinard, P. A. (2016). Why we should study the broader autism phenotype in typically developing populations. Journal of Cognition and Development, 17(4), 584–595. https://doi.org/10.1080/15248372.2016.1200046
- Latinus, M., & Taylor, M. J. (2006). Face processing stages: Impact of difficulty and the separation of effects. Brain Research, 1123(1), 179–187. https://doi.org/10.1016/j.brainres.2006.09.031
- Laycock, R., Crewther, S. G., & Chouinard, P. A. (2020). Blink and you will miss it: A core role for fast and dynamic visual processing in social impairments in autism spectrum disorder. Current Developmental Disorders Reports, 7(4), 237–248. https://doi.org/10.1007/s40474-020-00220-y
- Laycock, R., Cross, A. J., Dalle Nogare, F., & Crewther, S. G. (2014). Self-rated social skills predict visual perception: Impairments in object discrimination requiring transient attention associated with high autistic tendency. Autism Research, 7(1), 104–111. https://doi.org/10.1002/aur.1336
- Laycock, R., Wood, K., Wright, A., Crewther, S. G., & Goodale, M. A. (2020). Saccade latency provides evidence for reduced face inversion effects with higher autism traits [original research]. Frontiers in Human Neuroscience, 13(470). https://doi.org/10.3389/fnhum.2019.00470
- Lozier, L. M., Vanmeter, J. W., & Marsh, A. A. (2014). Impairments in facial affect recognition associated with autism spectrum disorders: A meta-analysis. Development and Psychopathology, 26(4 Pt 1), 933–945. https://doi.org/10.1017/s0954579414000479.
- Lundqvist, D., Flykt, A., & Öhman, A. (1998). The Karolinska Directed Emotional Faces – KDEF [CD-ROM]. Department of Clinical Neuroscience, Psychology section, Karolinska Institute.
- Mason, W. A., & Capitanio, J. P. (2012). Basic emotions: A reconstruction. Emotion Review, 4(3), 238–244. https://doi.org/10.1177/1754073912439763
- McPartland, J., Dawson, G., Webb, S. J., Panagiotides, H., & Carver, L. J. (2004). Event-related brain potentials reveal anomalies in temporal processing of faces in autism spectrum disorder. Journal of Child Psychology and Psychiatry, 45(7), 1235–1245. https://doi.org/10.1111/j.1469-7610.2004.00318.x
- Mottron, L., Dawson, M., Soulieres, I., Hubert, B., & Burack, J. (2006). Enhanced perceptual functioning in autism: An update, and eight principles of autistic perception. Journal of Autism and Developmental Disorders, 36(1), 27–43. https://doi.org/10.1007/s10803-005-0040-7
- Muth, A., Honekopp, J., & Falter, C. M. (2014). Visuo-spatial performance in autism: A meta-analysis. Journal of Autism and Developmental Disorders, 44(12), 3245–3263. https://doi.org/10.1007/s10803-014-2188-5
- Neuhaus, E., Bernier, R. A., Tham, S. W., & Webb, S. J. (2018). Gastrointestinal and psychiatric symptoms among children and adolescents with autism spectrum disorder. Frontiers in Psychiatry, 9, 515. https://doi.org/10.3389/fpsyt.2018.00515
- Nishiyama, T., Suzuki, M., Adachi, K., Sumi, S., Okada, K., Kishino, H., Sakai, S., Kamio, Y., Kojima, M., Suzuki, S., & Kanne, S. M. (2014). Comprehensive comparison of self-administered questionnaires for measuring quantitative autistic traits in adults. Journal of Autism and Developmental Disorders, 44(5), 993–1007. https://doi.org/10.1007/s10803-013-2020-7
- Pessoa, L., McKenna, M., Gutierrez, E., & Ungerleider, L. G. (2002). Neural processing of emotional faces requires attention. Proceedings of the national academy of sciences of the United States of America, 99(17), 11458–11463. https://doi.org/10.1073/pnas.172403899
- Pierce, K., Müller, R. A., Ambrose, J., Allen, G., & Courchesne, E. (2001). Face processing occurs outside the fusiform ‘face area’ in autism: Evidence from functional MRI. Brain, 124(10), 2059–2073. https://doi.org/10.1093/brain/124.10.2059
- Piven, J., Palmer, P., Jacobi, D., Childress, D., & Arndt, S. (1997). Broader autism phenotype: Evidence from a family history study of multiple-incidence autism families. American Journal of Psychiatry, 154(2), 185–190. https://doi.org/10.1176/ajp.154.2.185
- Poljac, E., Poljac, E., & Wagemans, J. (2013). Reduced accuracy and sensitivity in the perception of emotional facial expressions in individuals with high autism spectrum traits. Autism, 17(6), 668–680. https://doi.org/10.1177/1362361312455703
- Posner, M. I., & Dehaene, S. (1994). Attentional networks. Trends in Neuroscience, 17(2), 75–79. https://doi.org/10.1016/0166-2236(94)
- Rose, F. E., Lincoln, A. J., Lai, Z., Ene, M., Searcy, Y. M., & Bellugi, U. (2007). Orientation and affective expression effects on face recognition in Williams syndrome and autism. Journal of Autism and Developmental Disorders, 37(3), 513–522. https://doi.org/10.1007/s10803-006-0200-4
- Rossion, B. (2009a). Distinguishing the cause and consequence of face inversion: The perceptual field hypothesis. Acta Psychologica, 132(3), 300–312. https://doi.org/10.1016/j.actpsy.2009.08.002
- Rossion, B. (2009b). Distinguishing the cause and consequence of face inversion: The perceptual field hypothesis. Acta Psychologica, 132(3), 300–312. https://doi.org/10.1016/j.actpsy.2009.08.002
- Rousselet, G. A., Husk, J. S., Bennett, P. J., & Sekuler, A. B. (2008). Time course and robustness of ERP object and face differences. Journal of Vision, 8(12), 3. https://doi.org/10.1167/8.12.3
- Sabatino, A., Rittenberg, A., Sasson, N. J., Turner-Brown, L., Bodfish, J. W., & Dichter, G. S. (2013). Functional neuroimaging of social and nonsocial cognitive control in autism. Journal of Autism and Developmental Disorders, 43(12), 2903–2913. https://doi.org/10.1007/s10803-013-1837-4
- Sacrey, L. A., Armstrong, V. L., Bryson, S. E., & Zwaigenbaum, L. (2014). Impairments to visual disengagement in autism spectrum disorder: A review of experimental studies from infancy to adulthood. Neuroscience and Biobehavioral Reviews, 47, 559–577. https://doi.org/10.1016/j.neubiorev.2014.10.011
- Sadeh, B., & Yovel, G. (2010). Why is the N170 enhanced for inverted faces? An ERP competition experiment. Neuroimage, 53(2), 782–789. https://doi.org/10.1016/j.neuroimage.2010.06.029
- Saslow, M. G. (1967). Effects of components of displacement-step stimuli upon latency for saccadic eye movement. Journal of the Optical Society of America, 57(8), 1024–1029. https://doi.org/10.1364/josa.57.001024
- Schultz, R. T. (2005). Developmental deficits in social perception in autism: The role of the amygdala and fusiform face area. International Journal of Developmental Neuroscience, 23(2–3), 125–141. https://doi.org/10.1016/j.ijdevneu.2004.12.012
- Schwarzer, G. (2000). Development of face processing: The effect of face inversion. Child Development, 71(2), 391–401. https://doi.org/10.1111/1467-8624.00152
- Simonoff, E., Pickles, A., Charman, T., Chandler, S., Loucas, T., & Baird, G. (2008). Psychiatric disorders in children with autism spectrum disorders: Prevalence, comorbidity, and associated factors in a population-derived sample. Journal of the American Academy of Child and Adolescent Psychiatry, 47(8), 921–929. https://doi.org/10.1097/CHI.0b013e318179964f
- Strother, L., Mathuranath, P. S., Aldcroft, A., Lavell, C., Goodale, M. A., & Vilis, T. (2011). Face inversion reduces the persistence of global form and its neural correlates. PLoS One, 6(4), e18705. https://doi.org/10.1371/journal.pone.0018705
- Tantam, D., Monaghan, L., Nicholson, H., & Stirling, J. (1989). Autistic children’s ability to interpret faces: A research note. Journal of Child Psychology and Psychiatry, 30(4), 623–630. https://doi.org/10.1111/j.1469-7610.1989.tb00274.x
- Tardif, C., Lainé, F., Rodriguez, M., & Gepner, B. (2007). Slowing down presentation of facial movements and vocal sounds enhances facial expression recognition and induces facial-vocal imitation in children with autism. Journal of Autism and Developmental Disorders, 37(8), 1469–1484. https://doi.org/10.1007/s10803-006-0223-x
- Taylor, T. L., Kingstone, A., & Klein, R. M. (1998). The disappearance of foveal and nonfoveal stimuli: Decomposing the gap effect. Canadian Journal of Experimental Psychology, 52(4), 192–200. https://doi.org/10.1037/h0087292
- Teunisse, J. P., & de Gelder, B. (2003). Face processing in adolescents with autistic disorder: The inversion and composite effects. Brain and Cognition, 52(3), 285–294. https://doi.org/10.1016/s0278-2626(03)
- van Boxtel, J. J. A., Peng, Y., Su, J., & Lu, H. (2017). Individual differences in high-level biological motion tasks correlate with autistic traits. Vision Research, 141, 136–144. https://doi.org/10.1016/j.visres.2016.11.005
- van der Geest, J. N., Kemner, C., Camfferman, G., Verbaten, M. N., & van Engeland, H. (2001). Eye movements, visual attention, and autism: A saccadic reaction time study using the gap and overlap paradigm. Biological Psychiatry, 50(8), 614–619. https://doi.org/10.1016/s0006-3223(01)
- van der Geest, J. N., Kemner, C., Verbaten, M. N., & van Engeland, H. (2002). Gaze behavior of children with pervasive developmental disorder toward human faces: A fixation time study. Journal of Child Psychology and Psychiatry, 43(5), 669–678. https://doi.org/10.1111/1469-7610.00055
- Vettori, S., Dzhelyova, M., Van der Donck, S., Jacques, C., Steyaert, J., Rossion, B., & Boets, B. (2019). Reduced neural sensitivity to rapid individual face discrimination in autism spectrum disorder. NeuroImage: Clinical, 21, 101613. https://doi.org/10.1016/j.nicl.2018.101613
- Vuilleumier, P. (2005). How brains beware: Neural mechanisms of emotional attention. Trends in Cognitive Science, 9(12), 585–594. https://doi.org/10.1016/j.tics.2005.10.011
- Vuilleumier, P., Armony, J. L., Driver, J., & Dolan, R. J. (2001). Effects of attention and emotion on face processing in the human brain: An event-related fMRI study. Neuron, 30(3), 829–841. https://doi.org/10.1016/s0896-6273(01)
- Weigelt, S., Koldewyn, K., & Kanwisher, N. (2012). Face identity recognition in autism spectrum disorders: A review of behavioral studies. Neuroscience and Biobehavioral Reviews, 36(3), 1060–1084. https://doi.org/10.1016/j.neubiorev.2011.12.008
- Wilson, C. E., & Saldaña, D. (2019). No evidence of atypical attentional disengagement in autism: A study across the spectrum. Autism, 23(3), 677–688. https://doi.org/10.1177/1362361318768025
- Wyer, N. A., Martin, D., Pickup, T., & Macrae, C. N. (2012). Individual differences in (non-visual) processing style predict the face inversion effect. Cognitive Science, 36(2), 373–384. https://doi.org/10.1111/j.1551-6709.2011.01224.x
- Zwaigenbaum, L., Bryson, S., Rogers, T., Roberts, W., Brian, J., & Szatmari, P. (2005). Behavioral manifestations of autism in the first year of life. International Journal of Developmental Neuroscience, 23(2–3), 143–152. https://doi.org/10.1016/j.ijdevneu.2004.05.001
