ABSTRACT
Introduction
Previous research showed that methadone maintenance treatment (MMT) is linked to impulsivity, with higher impulsivity levels being associated with for example, increased drug use. One aspect of impulsivity, most commonly studied in rodent research, is premature responding, the failure to wait for a starting signal. Premature responding is of high translational significance since it predicts the development of addiction-like behaviors in rodents.
Methods
We assessed 45 MMT patients and 46 demographically matched (age, sex, education, and handedness) healthy volunteers (HVs) on premature responding alongside action and inhibition of instructed and intentional trials using the Intentional Hand Task (IHT).
Results
The results showed markedly enhanced premature responses in the MMT vs. the HV group, which correlated positively with methadone dosage in the MMT patients. Throughout the task, MMT patients were faster across all trial parts and less accurate in response to instructed trials compared to HVs.
Conclusions
The increase in premature motor reactions during variable waiting periods alongside increased motion speed and lower accuracy might reflect a specific motor inhibition deficit in MMT, a subcomponent of impulsivity not previously assessed in MMT. Incorporating an experimentally defined measure of impulsivity, such as premature responding, into existing test batteries used by clinicians might enable more tailored treatments addressing the increased impulsivity levels and associated dysfunctional behaviors in MMT.
1. Methadone maintenance treatment and impulsivity: premature responding
Methadone maintenance treatment (MMT) is effective in reducing opioid intake and relapse rates in opioid use disorders (Joseph et al., Citation2000). Compared to heroin treatment, MMT is associated with better psychomotor performance (Soyka et al., Citation2011), but when contrasted with healthy participants, a range of cognitive deficits persist in patients on MMT. These deficits include reduced psychomotor speed, working memory, and decision-making, as well as abnormalities in impulsivity-related mechanisms (Butler & Le Foll, Citation2019; Mintzer & Stitzer, Citation2002).
Impulsivity is a multifaceted construct and is of high importance for the development and maintenance of addictions (Dalley & Robbins, Citation2017; Kreek et al., Citation2005; Lee et al., Citation2019). Increased self-reported impulsivity is also one of the factors predicting relapse in MMT, in addition to lower social support and other personality aspects (Mahu et al., Citation2019; Zhu et al., Citation2018). However, the concept of impulsivity involves several related, but separable, processes, including delay discounting, response inhibition, response interference, and premature responding (e.g., Buss & Plomin, Citation1975; Dalley & Robbins, Citation2017; Sharma et al., Citation2013). While to our knowledge premature responding has not yet been investigated in MMT, MMT patients usually present with steeper delay discounting rates compared to controls (Li et al., Citation2021; Robles et al., Citation2011; Scherbaum et al., Citation2018), but with less severe discounting deficits when compared to heroin users (Karakula et al., Citation2016). When assessing response inhibition as measured by the Stop Signal Task (SST; Logan et al., Citation1984), inhibition-related findings are less consistent with MMT. Patients on MMT are either found to be better, worse, or not different relative to control participants on measures of inhibition, with little consistency across studies (e.g., Li et al., Citation2021; Liao et al., Citation2014; Zeng et al., Citation2016).
Premature responding, or the tendency to respond before the “start” signal, is a concept of high translational significance and is commonly utilized in rodent research assessing impulsivity (e.g., Amitai & Markou, Citation2010). In rodents, premature responding is assessed with the five-choice serial reaction time task (5-CSRT; Robbins, Citation2002), where rodents are trained to perform a nose poke onto targets after a starting signal such as the illumination of the target (e.g., Paterson et al., Citation2012; Wang et al., Citation2017). Premature responses are operationally defined as nose pokes before the illumination of the target resulting in a time-out (an additional delay before the delivery of a food pellet) and are seen as an indicator of impulsive performance (e.g., Cope et al., Citation2016; Dalley & Robbins, Citation2017; Jiménez-Urbieta et al., Citation2019). In line with the notion of impulsivity consisting of related, but separable processes, previous research found that premature responding relates to a different aspect of impulsivity than response inhibition as measured with the Stop Signal task or delay discounting in both humans and rodents (Bailey et al., Citation2021; Dalley & Robbins, Citation2017; Eagle et al., Citation2009; Morris et al., Citation2016; Paterson et al., Citation2012; Pattij et al., Citation2009; Robinson et al., Citation2009; Van Dessel et al., Citation2019). In rodents, baseline premature responding predicts addictive behaviors such as compulsive drug-seeking, e.g., the persistence of cocaine seeking despite punishment in the form of electric shock (Belin et al., Citation2008; Voon, Citation2014). Baseline premature responding also uniquely predicted levels of substance intake and drug-primed reinstatement of cocaine-seeking following abstinence in a longitudinal rodent study comparing the predictive utility of premature responding to that of risk-related impulsive choice (Arrondeau et al., Citation2023). Similarly, in humans, the level of premature responding correlated positively with the amount of alcohol consumed (Marinkovic et al., Citation2012), further highlighting the importance of premature responding for the maintenance of addiction.
While the effects of the mu-opioid agonist methadone on premature responses are yet to be determined, previous rodent research points toward increased premature responding following acute administration of other mu-opioid agonists as well as stimulants (Maguire & France, Citation2019; Maguire et al., Citation2016; Pattij et al., Citation2009; Wiskerke et al., Citation2011, Citation2012; Zhong et al., Citation2018). Heroin, for example, increases premature responses in the rodent 5-CSRT (Zhong et al., Citation2018), while acute administration of the mu-opioid agonist morphine had either no effect (Maguire & France, Citation2019; Maguire et al., Citation2016) or increased premature responses (Pattij et al., Citation2009). However, following stabilization on subchronic morphine for a 3-week period, premature responses in the 5-CSRT in rodents increased approximately fourfold (Maguire & France, Citation2019). Similarly, psychostimulants such as nicotine, methylphenidate, and amphetamine were found to increase premature responding in rodents despite their effects relating to different receptor types (Maguire & France, Citation2019; Maguire et al., Citation2016; Wiskerke et al., Citation2011, Citation2012). Administration of mu-opioid antagonists, such as Naloxone, has no effect on baseline premature responding, but is able to abolish the increase in premature responding seen following morphine and amphetamine administration (Pattij et al., Citation2009; Wiskerke et al., Citation2011), but not the increase in premature responding following nicotine intake, which was found to be dependent on cannabinoid, but not opioid receptors (Wiskerke et al., Citation2012).
Human research on premature responses is limited, with no data existing on the association between methadone usage and premature responding. Existing evidence related to addictions implies a similar picture as in rodent studies, with higher levels of premature responding in patients with stimulant dependencies such as amphetamine and cocaine addiction (Zhukovsky et al., Citation2020). Additionally, Voon et al. (Citation2014) found increased premature responding in relation to recreational cannabis use, as well as in abstinent methamphetamine, and alcohol-dependent participants. Similarly, premature responses are more common in binge drinkers when compared to non-binge drinkers (Morris et al., Citation2016; Sanchez-Roige et al., Citation2014) and administering methylphenidate acutely increases the number of premature responses (Voon et al., Citation2016).
Measuring premature responses in humans and rodents includes a time-out as a negative consequence following the execution of premature responses which is variably found in human translational versions (e.g., Sanchez-Roige et al., Citation2014; Voon et al., Citation2014). Following this rationale, a recently developed paradigm, the Intentional Hand Task (IHT; Weidacker et al., Citation2021) assesses premature responding alongside action and inhibition in instructed and intentional trials. In the IHT, participants are required to start moving a computer mouse from one side to a predefined location on the other side of the screen upon presentation of a start sign. Failure to wait for the start sign, operationally defined as premature responding similar to the rodent 5CSRT, is followed by the need for the mouse to be moved back to the starting position and for participants to restart the trial. Premature responses in the IHT hence have a similarly negative effect as the time-out punishment that usually follows on premature responses in rodent research (e.g., Sanchez-Roige et al., Citation2014). In the IHT, after successful initiation of a trial and moving the mouse for a predetermined distance, the cursor changes color (Cue) to indicate either the requirement to stop the motion (Instructed Stop), to move the mouse past the end point (Instructed Go), or to freely choose the performed motion between a correct Stop and Go response.
In the current study, we assessed premature responding and IHT action and inhibition measures in MMT patients and demographically matched (age, sex, education, and handedness) healthy volunteers (HVs). Based on previous rodent research highlighting a general increase in premature responding due to disinhibition effects of mu-opioid agonists, we hypothesized a higher level of premature responding in MMT patients.
2. Materials and methods
2.1 Participants
MMT participants were outpatients recruited from MMT clinics in Xuhui, Hongkou, and Yangpu districts in Shanghai, China. HVs were recruited by means of advertisements posted in Ruijin Hospital and Hongkou MMT clinic in Shanghai, China. This study was approved by the ethics committee of Ruijin Hospital, School of Medicine Shanghai Jiaotong University. A total of 52 HVs and 53 MMT patients were originally recruited for this study and provided demographic information. Two participants per group were left handed. Inclusion criteria for MMT patients were between 18 and 65 years of age, and daily MMT for at least 1 month. The patients’ medical history was assessed, and participants were not enrolled in the study when exclusion criteria were met. The exclusion criteria included severe psychiatric disorders such as psychotic disorders or bipolar disorder, neurological illnesses (e.g., Parkinson’s, epilepsy, and dementia), or head injury, while current nicotine dependence was not an exclusion criterion. The last methadone intake was required to be at least 24 h before the start of the experiment and only participants not in withdrawal were enrolled in the study, as assessed by a clinician. At the clinics, random drug tests were carried out to ensure the absence of illegal substance use, and all enrolled patients self-reported no illegal substance use.
To ascertain that only participants who acted according to the task instructions were included, participants were excluded when their Percentage Choice Go trials were beyond 2 standard deviations (SDs) of their group mean, leading to exclusion of four HVs and six MMT patients. Thereafter, outlier removal was based on the percentage correct responses in the instructed Go condition (beyond 2 SDs from the group mean) leading to the final sample of 46 HVs and 45 patients included in this report.
2.2 Intentional hand task (IHT)
The IHT was based on a previously published version of this task (Weidacker et al., Citation2021) and programmed using the software Presentation (Version 20.0, Neurobehavioral Systems, Inc., Berkeley, CA, http://www.neurobs.com). The task is comprised of 120 trials in total, subdivided into 30 instructed Stop, 30 instructed Go, and 60 Choice trials. Trial presentation was pseudo-randomized with the restriction to not present more than five trials of the same trial type in a row.
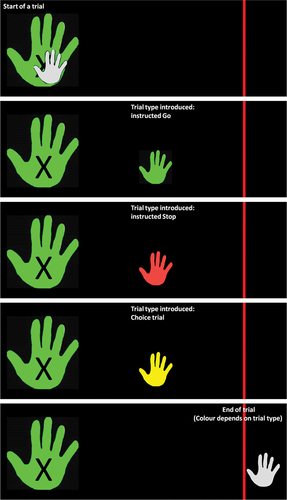
In this task, participants use a computer mouse on a wooden-sliding board (allowing only mouse movements in the horizontal plane) with their right hand. The goal of the task is to move the cursor of the mouse from the far left (position is indicated by the picture of a hand) to the far-right end point (indicated by a red finishing line) in one smooth motion. See for a graphical representation of the trial types. The cursor of the mouse has been replaced with a picture of a white hand (cursor-hand). At the start of each trial, the cursor-hand is at the far left of the screen on top of a bigger white hand (start-hand, marked with an X). The trial starts when the start-hand turns from white to green (the delay between the appearance of the green hand and the onset of the start-hand was randomized and jittered between 500 ms and 1 sec, in steps of 50 ms). Moving the mouse before this color change indicates a premature response, which is followed by presenting “You moved the mouse too early. Move the mouse back to the starting position and click the left button.” When the participant started the trial successfully, thus upon color change of the start-hand, the cursor-hand moves toward the right with the speed determined by the participant motion. To indicate the upcoming trial type (instructed Go, instructed Stop, and Choice trial), the cursor-hand changes color at a randomized location (but, across all trials at equal distance to the red line). The cursor-hand can become either green (indicating an instructed Go trial where the goal is to move the cursor hand past the red line as fast as possible) or red (indicating an instructed Stop trial with the goal to stop the cursor hand as fast as possible before the red line) or yellow (indicating Choice trials). In Choice trials, participants were instructed to decide between stopping to move the cursor as quickly as possible (choice Stop trials) or move it as fast as possible past the red line (choice Go trials). Choice trials were given the additional instruction of trying to balance the decision 50:50 between stopping and going without counting or alternating choices and without making their decision in advance of receiving the trial-type instruction. A trial finished either after the full cursor-hand passed the red line or when the cursor-hand stopped moving for one refresh rate (16 ms). The participants were then instructed to move the mouse back to the starting position on the sliding board (the cursor-hand was automatically set to its predefined starting point on the screen). Before the experimental trials, two practice sessions were performed. First, 15 practice trials were presented having an equal amount of trials per trial type to familiarize the participants with the mouse motion and the task layout. Second, the Choice trials were practiced for 15 trials to further familiarize the participants with the task design, motion required and multi-tasking requirement (deciding while keeping the cursor-hand moving). In both practice sessions, participants received feedback upon completion of a trial. For instructed trials the feedback was based on the correctness of the response and for Choice trials the feedback incorporated their choice and a counter reflecting the total number of choices made toward Go and Stop trials.
Figure 1. Example trial parts for the IHT. While the start-hand (marked with an x) turns green upon trial start, the cursor-hand (small) is initially white till it is moved to a predefined location on the right, the cursor-hand then changes color depending on the trial type: green for instructed Go trials (instructed to move the cursor-hand past the red line), red for instructed Stop (instructed to stop moving the cursor-hand as fast as possible), yellow for Choice trials (the participant chooses to either move the cursor-hand past the red line or stop moving as fast as possible). The trial ends when the cursor-hand is fully moved past the red line.
2.3 Data analysis
Statistical analyses were carried out using SPSS v26 (IBM SPSS Statistics). First, we assessed group differences in IHT performance indicators that are independent of the trial type, such as premature responses (failures to wait for the color change of the start-hand), movement delay (from color change of the start-hand to actually moving the mouse), and average speed (distance/time) during the pre-Cue phase (from initiating the mouse motion to the color change of the cursor-hand indicating the trial type). Normality in these variables was assessed using skewness and kurtosis cutoffs of > ±3 and > ±10, respectively (Kline, Citation2015). Normality was only violated for the premature responses and movement delay in MMT patients; hence, Mann–Whitney U tests were conducted to assess group differences in these variables. Group differences in average speed were analyzed using independent-sample t-tests. The equality of variances was assessed via Levene’s tests and corrected statistics are reported if applicable.
Second, post-Cue response times were analyzed separately for Go (from color change of the cursor-hand to moving the mouse past the finishing line) and Stop trials (from color change of the cursor-hand to stopping the mouse) using two repeated-measures (rm) ANOVAs. Each rmANOVA included group as between-subject factor and instructed vs. choice as a within-subject factor. Accuracy for instructed trials was analyzed in a rmANOVA, using group as between-subject factor and the within-subject factor instructed trial type (Go, Stop). Between-group differences on the percentage of Choice Stop trials were assessed with an independent-sample t-test. All t-tests were corrected for inhomogeneity of variances when applicable.
3. Results
3.1 Demographic data
The demographic information of the MMT patients and HVs is presented in . Education level, gender composition, and age did not differ significantly between groups. The MMT group received between 5 and 150 ml/day (M = 42.98, SD = 30.49) and the MMT duration ranged between 2 and 204 months (M = 42.98, SD = 30.49). The Spearman rho correlation between dosage and MMT duration was not significant (r(43) = −.09, p = .567).
Table 1. Demographics and methadone descriptives.
3.2 Premature responses
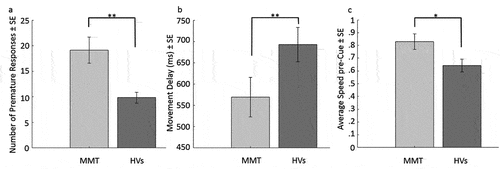
The number of premature responses differed significantly between groups (Mann–Whitney U = 1436.00, p = .001, r = .33) due to the patients making about twice as many premature responses (M = 19.11, SD = 17.02) than the HVs (M = 9.85, SD = 7.03), see . On an exploratory level, we assessed whether the number of premature responses was associated with methadone-related descriptives. While the Spearman rho correlation between premature responding and MMT duration was not significant (r(43) = .036, p = .812), we found a positive Spearman rho correlation between methadone dosage (ml/day) and premature responding (r(43) = .38, p = .011).
Figure 2. Bar graph of the significant between-group differences on pre-Cue performance. 2a) shows the number of premature responses in the task, 2b) shows the movement delay and 2c) shows the average speed per group before Cue presentation. ** indicates significance at p < .01, and * significance at p < .05. MMT = methadone maintenance patients, HVs = healthy volunteers.
3.3 Movement delay and average speed pre-cue
MMT patients (M = 569.46, SD = 312.87) were significantly faster than HVs (M = 692.92, SD = 272.01) in initiating the motion following the color change that indicated the start of the trial (Mann–Whitney U = 682.00, p = .005, r = .29), see . Similarly, the average speed pre-Cue (i.e., the time period between participants starting to move the mouse and the appearance of the Cue for the current trial type) was higher in MMT (M = .83, SD = .40) compared to HVs (M = .64, SD = .35) and the difference was significant (t(89) = 2.37, p = .020, Hedges' g = .492), see .
3.4 Response times
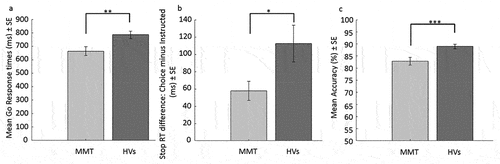
Analyzing the response times to Go trials revealed a significant main effect of Instructed vs. Choice (F(1,89) = 9.60, p = .003, ηp2 = .097) and a significant main effect of group (F(1,89) = 8.20, p = .005, ηp2 = .084), while the interaction between group and trial type was not significant (F(1,89) < .001, p = .99). The main effect of Instructed vs. Choice was related to significantly slower response times during Choice (M = 742.01, SD = 235.84) than Instructed (M = 707.71, SD = 194.67) Go trials. The main effect of group was due to MMT patients having faster Go response times (M = 663.60, SD = 215.14), regardless of whether they were Instructed or Choice trials, compared to HVs (M = 784.79, SD = 187.94), see . Analyzing the response times during Stop trials indicated a similar pattern, a significant main effect of Instructed vs. Choice (F(1,89) = 50.27, p < .001, ηp2 = .361) and a significant main effect of group (F(1,89) = 22.92, p < .001, ηp2 = .205), but additionally, the interaction between group and trial type was significant (F(1,89) = 5.17, p = .03, ηp2 = .055). The main effect of Instructed vs. Choice again showed significantly slower response times during Choice (M = 547.67, SD = 149.98) than Instructed (M = 462.17, SD = 88.20) Stop trials. The main effect of group indicated faster stopping response times across both trial types in MMT (M = 455.70, SD = 98.14) compared to HVs (M = 553.07, SD = 95.86). Further, the interaction between group and trial type was due to the MMT patients having a smaller response time increase when comparing Instructed to Choice Stop trials (M = 57.88, SD = 73.44) than HVs (M = 112.53, SD = 143.92), see . However, repeating the analyses with only right-handed participants revealed that the interaction was no longer significant (F(1,84) = 3.52, p = .064), while all other results reported for this study resembled those observed in only right-handed participants.
Figure 3. Bar graphs of significant group effects on response times and accuracy. 3a) shows the significant group differences on response times across Go trials (Choice and instructed). 3b) shows the significant group by trial type interaction on Stop response times, as indicated by Choice Stop response times minus instructed Stop response times. 3c) shows the accuracy rates across instructed Go and Stop trials. *** indicates significance at p < .001, ** indicates significance at p < .01, and * indicates significance at p < .05. MMT = methadone maintenance patients, HVs = healthy volunteers.
3.5 Accuracy rates
The rmANOVA on accuracy rates in the instructed conditions revealed a significant main effect of trial type (F(1,89) = 117.87, p < .001, ηp2 = .570) and a significant main effect of group (F(1,89) = 12.91, p < .001, ηp2 = .127), while the interaction between group and trial type was not significant (F(1,89) = 3.91, p = .05). The main effect of trial type was due to generally lower accuracy rates to instructed Go (M = 77.12, SD = 13.75) than Stop trials (M = 94.07, SD = 10.35). The main effect of group indicated lower accuracy across trial types in MMT (M = 82.19, SD = 10.73) compared to HVs (M = 88.93, SD = 6.76), see .
3.6 Intentional stop trials
Assessing between-group differences in the percentage of performed Choice Stop trials revealed no significant differences between HVs and MMT (t(67.48) = .16, p = .87).
4. Discussion
This first assessment of premature responding and IHT performance in MMT patients revealed significant impairments in the ability to inhibit premature responses. Within MMT patients, premature responses were about twice as common as in HVs. The number of premature responses further increased at higher methadone dosage. Additionally, MMT patients were faster across all trial parts than HVs, but this came at the cost of overall reduced accuracy rates. Finally, while the tendency to inhibit their responses on Choice trials did not differ across groups, MMT patients’ increase in response times between Instructed and Choice Stop trials was reduced compared to HVs.
The main finding from the report, premature responses being significantly increased in patients on MMT and positively related to dosage, mirrors previous reports on premature responding in addictions. In rodents, heroin as well as psychostimulant injections increase premature responding (Maguire & France, Citation2019; Maguire et al., Citation2016; Pattij et al., Citation2009; Wiskerke et al., Citation2011, Citation2012; Zhong et al., Citation2018). In humans, patients with cocaine or amphetamine addiction as well as binge drinkers and cannabis users were previously found to show increased premature responding, in line with the notion of increased impulsivity levels in these populations (Morris et al., Citation2016; Sanchez-Roige et al., Citation2014; Voon et al., Citation2014; Zhukovsky et al., Citation2020).
According to Näätänen (Citation1971), premature responses can be subdivided into premature expectant reactions and premature motor reactions. Premature expectant reactions arise from the high predictability of the waiting interval which induces strong motor excitation. In contrast, premature motor reactions do not rely on strong expectancy, but occur due to cognitive failure in adjusting the correct amount of control over motor readiness (Näätänen, Citation1971). Premature responses in the IHT occurred before the presentation of a starting signal that appeared after a variable and randomized delay (500 ms to 1 sec; in steps of 50 ms); hence, the predictability of the starting signal is lower than during fixed fore-periods and than needed for Näätänen’s (Citation1971) premature expectant reactions. Instead, the type of premature responses recorded during this IHT design likely reflects premature motor reactions. In line with this, accurate time estimation seems most important for premature expectant reactions and previous research hypothesized failed time perception to underlie premature responses, especially during fixed delays (Cope et al., Citation2016). Time perception during fixed waiting intervals is affected by drug use, with amphetamine speeding up time perception and cannabis use slowing it down. Importantly, amphetamine also increases premature responding and cannabis use decreases them, adding to the evidence that premature responses during fixed delays are associated with failures in time perception (Cope et al., Citation2016; de Wit et al., Citation2002; Hayton et al., Citation2012; Lake & Meck, Citation2013).
A variable waiting interval, on the other hand, induces a decrease in premature responding in response to amphetamine in rodents (Hayton et al., Citation2012). In human binge drinkers, premature responses were more common during fixed waiting periods than in controls, while premature responding was not significantly different during the variable waiting period (Sanchez-Roige et al., Citation2014). We employed only variable waiting periods, indicating a low predictability of the waiting interval and hence a minimal contribution from accurate time perception. Of note, previous research on time estimation in MMT suggests no differences compared to HVs (Mintzer & Stitzer, Citation2002; Wang et al., Citation2014). Instead, our reported increase in premature responding in MMT likely relates to Näätänen (Citation1971) premature motor reactions arising from a cognitive failure in controlling motor readiness, in other words, an inhibition deficit that is independent from timing ability, which correlates positively with methadone dosage. Importantly, differences between addictions in the type of premature responses exhibited could be clinically relevant. Previous research found no increase in premature responding when studying binge drinkers under variable delays (Sanchez-Roige et al., Citation2014), while the current investigation revealed a clear deficit in inhibiting premature responses under variable delay periods in MMT. This exemplifies that even within the impulsivity aspect of premature responding, addiction-related disorders differ in the type of pronounced inhibition deficit. For MMT, the increased rate of premature responding under variable delay periods likely indicates a cognitive control failure according to Näätänen’s (Citation1971) model. Neurally, the dorsal premotor cortex (PMd) is likely involved in the failed inhibition of premature motor reactions. According to Duque et al. (Citation2012) inhibition of the dorsal premotor cortex (PMd) by means of repetitive transcranial magnetic stimulation (rTMS) attenuates the inhibition of motor output during the waiting period. Similarly, direct injection of a GABAA antagonist into the primate PMd reduced the cortical inhibition of motoric outputs during waiting periods (Sawaguchi et al., Citation1996). Taking this previous research into account likely indicates that MMT patients’ increase in premature responding might be accompanied by alterations in the functioning of the PMd, including insufficient inhibition of motor outputs.
When trials were started in the absence of the cortical failure to inhibit the motor output during the delay phase, MMT patients showed a clear speed advantage over HVs. MMT patients were faster in initiating the task and had a higher average speed during the pre-Cue phase, as well as faster Go and Stop response times across instructed vs. choice trials, but this was accompanied by lower accuracy rates across instructed trial types. Previous research on performance speed in MMT patients often found no differences to HVs in terms of simple motor speed (Mazhari et al., Citation2015; Wang et al., Citation2014), but slower performance on tasks that require additional processes such as cognitive flexibility and selective attention (Bracken et al., Citation2012; Mazhari et al., Citation2015; Mintzer & Stitzer, Citation2002; Verdejo et al., Citation2005). The pre-Cue measures of the IHT, such as task initiation speed and average speed pre-Cue, are free from such confounds and hence reveal faster response times in MMT patients at basic motor speed. This higher speed, however, came at the cost of reduced accuracy rates in instructed trials, perhaps indicating a faster but more carefree response style in MMT.
Despite the promising findings of increased premature responding, a positive association between dosage and premature responding, and an enhanced focus on speed over accuracy in MMT patients, this study has several limitations. First, we only tested patients who used methadone as replacement of opioids, as such our findings cannot discern the source of these behavioral abnormalities as opioid dependence or MMT/usage of methadone. However, given that cognitive impairments are commonly reduced under MMT compared to opioid use (Karakula et al., Citation2016; Soyka et al., Citation2011), future research would benefit from including non-MMT opioid groups, e.g., opioid dependence/recreational users, to further investigate the generalizability of the current findings. In line with this, it is further unclear whether these inhibitory deficits were present before MMT or opioid addiction. Previous research suggests that SST-based response inhibition deficits at baseline predicts the amount of alcohol consumption over a period of 8 years in alcohol-naïve teenagers (Jones et al., Citation2021) indicating predictive utility for some measures of impulsivity. However, similar human longitudinal research on impulsivity measures, including premature responding, is lacking for MMT or opioid use disorder and should be investigated further (Christensen et al., Citation2023).
Secondly, for the current sample treatment adherence measures were not available, a limitation that should be addressed in future research. Previous research assessed treatment adherence by, for example, the duration of abstinence (Zhu et al., Citation2018). In humans with opioid use disorder, prolonged abstinence from opioids is predicted, among others, by lower self-reported impulsivity levels (Zhu et al., Citation2018). In MMT patients, research showed that self-reported sensation seeking, an aspect of impulsivity, is inversely related to recent substance use, indicating treatment relapse (Mahu et al., Citation2019). Assessing impulsivity in terms of behaviorally defined variables, e.g., premature responding, might provide a more objective measure of impulsive tendencies and its utility in predicting treatment, as well as addiction-related outcomes, should be subject of future research. Additionally, future research would benefit from assessing illegal substance use biochemically and withdrawal states objectively in all participants before the initiation of the task.
Further, acute effects of methadone were not investigated; patients performed the experiment before the daily methadone intake, which was approximately 24 h after the last dose. Additionally, the dosage varied between MMT patients and higher dosage is often associated with higher severity, something that was not explicitly assessed in the current study. As such, the observed effects might relate to the time-course of methadone levels or addiction severity, which warrants further investigation. Of note, premature responses in the IHT were followed by the punishment to have to restart the trial and the associated waiting period. This suggests that, even when the premature responses do not lead to a performance advantage, premature responding in MMT is increased when compared to HV.
We here report a strong increase in premature responding, a marker of impulsivity commonly utilized in rodent research. Previous research showed that those with elevated premature responses have stronger drug-primed reinstatement and higher levels of drug and alcohol intake (Arrondeau et al., Citation2023; Marinkovic et al., Citation2012). Behaviorally, a rodent meta-analysis showed that rodents with high levels of premature responding make more economically disadvantageous choices, e.g., are drawn to large rewards that are associated with disproportionally large negative consequences (Barrus et al., Citation2015). In line with this, premature responses are likely driven by reward sensitivity including incentive salience (Toschi et al., Citation2022; Zhukovsky et al., Citation2020). Importantly, previous research on MMT patients showed no clear inhibition deficit when, for example, using the SST, which exemplifies that premature responding is a separate aspect of impulsivity from response inhibition (e.g., Van Dessel et al., Citation2019). While the current results await replication, an experimental measure that captures the increased impulsivity in patients on MMT could be utilized to provide a more complete clinical picture of the patients’ needs and tailor treatment accordingly. For example, a patient with high levels of premature responses might co-present with higher reward sensitivity and hence has a more economically disadvantageous decision-making style (Barrus et al., Citation2015). In rodents, increased drug abuse and drug-primed reinstatement were associated with high levels of premature responding (Arrondeau et al., Citation2023), and if translatable to humans, this likely indicates increased relapse risk. Assessing premature responding in MMT patients would hence provide an additional tool for identifying those in need of tailored intervention, e.g., cognitive control training and additional relapse prevention measures, to mitigate the effects of these dysfunctional correlates.
In sum, this first report on premature responding in MMT patients revealed markedly increased impulsivity levels alongside faster and less accurate responding. Premature responding was strongly increased during a variable waiting period, indicating failed motor inhibition, which was positively related to methadone dosage. Throughout the task, MMT patients expressed a faster, but more inaccurate response style than HVs. The findings hint toward a specific motoric inhibition deficit in MMT patients following a buildup of cortical preparedness, which differs from those previously seen in, for example, binge drinkers.
Acknowledgments
This study was supported by the Medical Research Council Senior Clinical Fellowship (Grant No. MR/W020408/1), the National Natural Science Foundation of China (Grant No. T2250710686). The authors would like to thank the MMT clinics who helped immensely with recruitment. The authors would also like to thank all the participants who took part in this study.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Additional information
Funding
References
- Amitai, N., & Markou, A. (2010). Disruption of performance in the five-choice serial reaction time task induced by administration of N-methyl-D-aspartate receptor antagonists: Relevance to cognitive dysfunction in schizophrenia. Biological Psychiatry, 68(1), 5–16. https://doi.org/10.1016/j.biopsych.2010.03.004
- Arrondeau, C., Urueña-Méndez, G., Bellés, L., Marchessaux, F., Goutaudier, R., & Ginovart, N. (2023). Motor impulsivity but not risk-related impulsive choice is associated to drug intake and drug-primed relapse. Frontiers in Behavioral Neuroscience, 17, 1200392. https://doi.org/10.3389/fnbeh.2023.1200392
- Bailey, A. J., Romeu, R. J., & Finn, P. R. (2021). The problems with delay discounting: A critical review of current practices and clinical applications. Psychological Medicine, 51(11), 1799–1806. https://doi.org/10.1017/S0033291721002282
- Barrus, M. M., Hosking, J. G., Zeeb, F. D., Tremblay, M., & Winstanley, C. A. (2015). Disadvantageous decision-making on a rodent gambling task is associated with increased motor impulsivity in a population of male rats. Journal of Psychiatry and Neuroscience, 40(2), 108–117. https://doi.org/10.1503/jpn.140045
- Belin, D., Mar, A. C., Dalley, J. W., Robbins, T. W., & Everitt, B. J. (2008). High impulsivity predicts the switch to compulsive cocaine-taking. Science, 320(5881), 1352–1355. https://doi.org/10.1126/science.1158136
- Bracken, B., Trksak, G., Penetar, D., Tartarini, W., Maywalt, M., Dorsey, C., & Lukas, S. (2012). Response inhibition and psychomotor speed during methadone maintenance: Impact of treatment duration, dose, and sleep deprivation. Drug and Alcohol Dependence, 125(1–2), 132–139. https://doi.org/10.1016/j.drugalcdep.2012.04.004
- Buss, A. H., & Plomin, R. (1975). A temperament theory of personality development. Wiley-Interscience.
- Butler, K., & Le Foll, B. (2019). Impact of substance use disorder pharmacotherapy on executive function: A narrative review. Frontiers in Psychiatry / Frontiers Research Foundation, 10, 98. https://doi.org/10.3389/fpsyt.2019.00098
- Christensen, E., Brydevall, M., Albertella, L., Samarawickrama, S. K., Yücel, M., & Lee, R. S. (2023). Neurocognitive predictors of addiction-related outcomes: A systematic review of longitudinal studies. Neuroscience & Biobehavioral Reviews, 105295. https://doi.org/10.1016/j.neubiorev.2023.105295
- Cope, Z. A., Halberstadt, A. L., van Enkhuizen, J., Flynn, A. D., Breier, M., Swerdlow, N. R., Geyer, M. A., & Young, J. W. (2016). Premature responses in the five-choice serial reaction time task reflect rodents’ temporal strategies: Evidence from no-light and pharmacological challenges. Psychopharmacology (Berl), 233(19), 3513–3525. https://doi.org/10.1007/s00213-016-4389-4
- Dalley, J. W., & Robbins, T. W. (2017). Fractionating impulsivity: Neuropsychiatric implications. Nature Reviews Neuroscience, 18(3), 158–171. https://doi.org/10.1038/nrn.2017.8
- de Wit, H., Enggasser, J. L., & Richards, J. B. (2002). Acute administration of d-amphetamine decreases impulsivity in healthy volunteers. Neuropsychopharmacology, 27(5), 813–825. https://doi.org/10.1016/S0893-133X(02)00343-3
- Duque, J., Labruna, L., Verset, S., Olivier, E., & Ivry, R. B. (2012). Dissociating the role of prefrontal and premotor cortices in controlling inhibitory mechanisms during motor preparation. Journal of Neuroscience, 32(3), 806–816. https://doi.org/10.1523/JNEUROSCI.4299-12.2012
- Eagle, D. M., Lehmann, O., Theobald, D. E., Pena, Y., Zakaria, R., Ghosh, R., Dalley, J. W., & Robbins, T. W. (2009). Serotonin depletion impairs waiting but not stop-signal reaction time in rats: Implications for theories of the role of 5-HT in behavioral inhibition. Neuropsychopharmacology, 34(5), 1311–1321. https://doi.org/10.1038/npp.2008.202
- Hayton, S. J., Maracle, A. C., & Olmstead, M. C. (2012). Opposite effects of amphetamine on impulsive action with fixed and variable delays to respond. Neuropsychopharmacology, 37(3), 651–659. https://doi.org/10.1038/npp.2011.236
- Jiménez-Urbieta, H., Gago, B., Quiroga-Varela, A., Rodríguez-Chinchilla, T., Merino-Galán, L., Oregi, A., Belloso-Iguerategui, A., Delgado-Alvarado, M., Navalpotro-Gómez, I., Marin, C., & Fernagut, P. O. (2019). Pramipexole-induced impulsivity in mildparkinsonian rats: A model of impulse control disorders in Parkinson’s disease. Neurobiology of Aging, 75, 126–135. https://doi.org/10.1016/j.neurobiolaging.2018.11.021
- Jones, C. B., Meier, M. H., Corbin, W. E., & Chassin, L. (2021). Adolescent executive cognitive functioning and trait impulsivity as predictors of young-adult risky drinking and alcohol-related problems. Psychology of Addictive Behaviors, 35(2), 187. https://doi.org/10.1037/adb0000636
- Joseph, H., Stancliff, S., & Langrod, J. (2000). Methadone maintenance treatment (MMT): A review of historical and clinical issues. The Mount Sinai Journal of Medicine, New York, 67(5–6), 347–364.
- Karakula, S. L., Weiss, R. D., Griffin, M. L., Borges, A. M., Bailey, A. J., & McHugh, R. K. (2016). Delay discounting in opioid use disorder: Differences between heroin and prescription opioid users. Drug and Alcohol Dependence, 169, 68–72. https://doi.org/10.1016/j.drugalcdep.2016.10.009
- Kline, R. B. (2015). Principles and practice of structural equation modeling. Guilford publications.
- Kreek, M. J., Nielsen, D. A., Butelman, E. R., & LaForge, K. S. (2005). Genetic influences on impulsivity, risk taking, stress responsivity and vulnerability to drug abuse and addiction. Nature Neuroscience, 8(11), 1450–1457. https://doi.org/10.1038/nn1583
- Lake, J. I., & Meck, W. H. (2013). Differential effects of amphetamine and haloperidol on temporal reproduction: Dopaminergic regulation of attention and clock speed. Neuropsychologia, 51(2), 284–292. https://doi.org/10.1016/j.neuropsychologia.2012.09.014
- Lee, R. S., Hoppenbrouwers, S., & Franken, I. (2019). A systematic meta-review of impulsivity and compulsivity in addictive behaviors. Neuropsychology Review, 29(1), 14–26. https://doi.org/10.1007/s11065-019-09402-x
- Liao, D.-L., Huang, C.-Y., Hu, S., Fang, S.-C., Wu, C.-S., Chen, W.-T., Lee, T. S. H., Chen, P. C., & Li, C. S. R. (2014). Cognitive control in opioid dependence and methadone maintenance treatment. PLoS One, 9(4), e94589. https://doi.org/10.1371/journal.pone.0094589
- Li, J., Weidacker, K., Mandali, A., Zhang, Y., Whiteford, S., Ren, Q., Zhou, Z., Jiang, H., Du, J., Zhang, C., Sun, B., & Voon, V. (2021). Impulsivity and craving in subjects with opioid use disorder on methadone maintenance treatment. Drug and Alcohol Dependence, 219, 108483. https://doi.org/10.1016/j.drugalcdep.2020.108483
- Logan, G. D., Cowan, W. B., & Davis, K. A. (1984). On the ability to inhibit simple and choice reaction time responses: A model and a method. Journal of Experimental Psychology: Human Perception and Performance, 10(2), 276. https://doi.org/10.1037/0096-1523.10.2.276
- Maguire, D., & France, C. (2019). Effects of amphetamine, methylphenidate, atomoxetine, and morphine in rats responding under an adjusting stop signal reaction time task. Psychopharmacology (Berl), 236(6), 1959–1972. https://doi.org/10.1007/s00213-019-5183-x
- Maguire, D., Henson, C., & France, C. (2016). Daily morphine administration increases impulsivity in rats responding under a 5‐choice serial reaction time task. British Journal of Pharmacology, 173(8), 1350–1362. https://doi.org/10.1111/bph.13434
- Mahu, I., Conrod, P., Barrett, S., Sako, A., Swansburg, J., Lawrence, M., Laroque, F., Morin, J. F., Chinneck, A., Nogueira-Arjona, R., & Stewart, S. H. (2019). Specificity of personality relationships to particular forms of concurrent substance use among methadone maintenance therapy clients. Addictive Behaviors, 98, 106056. https://doi.org/10.1016/j.addbeh.2019.106056
- Marinkovic, K., Rickenbacher, E., Azma, S., & Artsy, E. (2012). Acute alcohol intoxication impairs top–down regulation of Stroop incongruity as revealed by blood oxygen level‐dependent functional magnetic resonance imaging. Human Brain Mapping, 33(2), 319–333. https://doi.org/10.1002/hbm.21213
- Mazhari, S., Keshvari, Z., Sabahi, A., & Mottaghian, S. (2015). Assessment of cognitive functions in methadone maintenance patients. Addiction and Health, 7(3–4), 109.
- Mintzer, M. Z., & Stitzer, M. L. (2002). Cognitive impairment in methadone maintenance patients. Drug and Alcohol Dependence, 67(1), 41–51. https://doi.org/10.1016/S0376-8716(02)00013-3
- Morris, L. S., Kundu, P., Baek, K., Irvine, M. A., Mechelmans, D. J., Wood, J.,Harrison, N. A., Robbins, T. W., Bullmore, E. T., & Voon, V. (2016). Jumping the gun: Mapping neural correlates of waiting impulsivity and relevance across alcohol misuse. Biological Psychiatry, 79(6), 499–507. https://doi.org/10.1016/j.biopsych.2015.06.009
- Näätänen, R. (1971). Non-aging fore-periods and simple reaction time. Acta Psychologica, 35(4), 316–327. https://doi.org/10.1016/0001-6918(71)90040-0
- Paterson, N. E., Wetzler, C., Hackett, A., & Hanania, T. (2012). Impulsive action and impulsive choice are mediated by distinct neuropharmacological substrates in rat. The International Journal of Neuropsychopharmacology, 15(10), 1473–1487. https://doi.org/10.1017/S1461145711001635
- Pattij, T., Schetters, D., Janssen, M. C., Wiskerke, J., & Schoffelmeer, A. N. (2009). Acute effects of morphine on distinct forms of impulsive behavior in rats. Psychopharmacology (Berl), 205(3), 489–502. https://doi.org/10.1007/s00213-009-1558-8
- Robbins, T. (2002). The 5-choice serial reaction time task: Behavioural pharmacology and functional neurochemistry. Psychopharmacology (Berl), 163(3), 362–380. https://doi.org/10.1007/s00213-002-1154-7
- Robinson, E., Eagle, D., Economidou, D., Theobald, D., Mar, A., Murphy, E., Robbins, T. W., & Dalley, J. W. (2009). Behavioural characterisation of high impulsivity on the 5-choice serial reaction time task: Specific deficits in ‘waiting’versus ‘stopping’. Behavioural Brain Research, 196(2), 310–316. https://doi.org/10.1016/j.bbr.2008.09.021
- Robles, E., Huang, B. E., Simpson, P. M., & McMillan, D. E. (2011). Delay discounting, impulsiveness, and addiction severity in opioid-dependent patients. Journal of Substance Abuse Treatment, 41(4), 354–362. https://doi.org/10.1016/j.jsat.2011.05.003
- Sanchez-Roige, S., Baro, V., Trick, L., Pena-Oliver, Y., Stephens, D. N., & Duka, T. (2014). Exaggerated waiting impulsivity associated with human binge drinking, and high alcohol consumption in mice. Neuropsychopharmacology, 39(13), 2919–2927. https://doi.org/10.1038/npp.2014.151
- Sawaguchi, T., Yamane, I., & Kubota, K. (1996). Application of the GABA antagonist bicuculline to the premotor cortex reduces the ability to withhold reaching movements by well-trained monkeys in visually guided reaching task. Journal of Neurophysiology, 75(5), 2150–2156. https://doi.org/10.1152/jn.1996.75.5.2150
- Scherbaum, S., Haber, P., Morley, K., Underhill, D., & Moustafa, A. A. (2018). Biased and less sensitive: A gamified approach to delay discounting in heroin addiction. Journal of Clinical and Experimental Neuropsychology, 40(2), 139–150. https://doi.org/10.1080/13803395.2017.1324022
- Sharma, L., Kohl, K., Morgan, T. A., & Clark, L. A. (2013). “Impulsivity”: Relations between self-report and behavior. Journal of Personality and Social Psychology, 104(3), 559. https://doi.org/10.1037/a0031181
- Soyka, M., Limmer, C., Lehnert, R., Koller, G., Martin, G., Küfner, H., Kagerer, S., & Haberthür, A. (2011). A comparison of cognitive function in patients under maintenance treatment with heroin, methadone, or buprenorphine and healthy controls: An open pilot study. The American Journal of Drug and Alcohol Abuse, 37(6), 497–508. https://doi.org/10.3109/00952990.2011.600381
- Toschi, C., El-Sayed Hervig, M., Burghi, T., Sell, T., Lycas, M. D., Moazen, P., Huang, L., Gether, U., Robbins, T. W., & Dalley, J. W. (2022). Dissociating reward sensitivity and negative urgency effects on impulsivity in the five-choice serial reaction time task. Brain and Neuroscience Advances, 6, 23982128221102256.
- Van Dessel, J., Morsink, S., Van der Oord, S., Lemiere, J., Moerkerke, M., Grandelis, M., Sonuga-Barke, E., & Danckaerts, M. (2019). Waiting impulsivity: A distinctive feature of ADHD neuropsychology? Child Neuropsychology, 25(1), 122–129. https://doi.org/10.1080/09297049.2018.1441819
- Verdejo, A., Toribio, I., Orozco, C., Puente, K. L., & Pérez-García, M. (2005). Neuropsychological functioning in methadone maintenance patients versus abstinent heroin abusers. Drug and Alcohol Dependence, 78(3), 283–288. https://doi.org/10.1016/j.drugalcdep.2004.11.006
- Voon, V. (2014). Models of impulsivity with a focus on waiting impulsivity: Translational potential for neuropsychiatric disorders. Current Addiction Reports, 1(4), 281–288. https://doi.org/10.1007/s40429-014-0036-5
- Voon, V., Chang-Webb, Y. C., Morris, L. S., Cooper, E., Sethi, A., Baek, K.,Grant, J., Robbins, T. W., & Harrison, N. A. (2016). Waiting impulsivity: the influence of acute methylphenidate and feedback. The International Journal of Neuropsychopharmacology, 19(1), yv074. https://doi.org/10.1093/ijnp/pyv074
- Voon, V., Irvine, M. A., Derbyshire, K., Worbe, Y., Lange, I., Abbott, S., Morein-Zamir, S., Dudley, R., Caprioli, D., Harrison, N. A., & Wood, J. (2014). Measuring “waiting” impulsivity in substance addictions and binge eating disorder in a novel analogue of rodent serial reaction time task. Biological Psychiatry, 75(2), 148–155. https://doi.org/10.1016/j.biopsych.2013.05.013
- Wang, G. Y., Wouldes, T. A., Kydd, R., Jensen, M., & Russell, B. R. (2014). Neuropsychological performance of methadone-maintained opiate users. Journal of Psychopharmacology, 28(8), 789–799. https://doi.org/10.1177/0269881114538541
- Wang, Y., Yin, F., Guo, H., Zhang, J., Yan, P., & Lai, J. (2017). The role of dopamine D1 and D3 receptors in N-methyl-D-aspartate (NMDA)/glycineB site-regulated complex cognitive behaviors following repeated morphine administration. The International Journal of Neuropsychopharmacology, 20(7), 562–574. https://doi.org/10.1093/ijnp/pyx010
- Weidacker, K., Kvamme, T. L., Whiteford, S., Guzman, N. V., & Voon, V. (2021). Incentives and voluntary stopping: The intentional hand task. Cognition, 206, 104504. https://doi.org/10.1016/j.cognition.2020.104504
- Wiskerke, J., Schetters, D., van Es, I. E., van Mourik, Y., den Hollander, B. R., Schoffelmeer, A. N., & Pattij, T. (2011). μ-opioid receptors in the nucleus accumbens shell region mediate the effects of amphetamine on inhibitory control but not impulsive choice. Journal of Neuroscience, 31(1), 262–272. https://doi.org/10.1523/JNEUROSCI.4794-10.2011
- Wiskerke, J., Van Mourik, Y., Schetters, D., Schoffelmeer, A. N., & Pattij, T. (2012). On the role of cannabinoid CB1-and μ-opioid receptors in motor impulsivity. Frontiers in Pharmacology, 3, 108. https://doi.org/10.3389/fphar.2012.00108
- Zeng, H., Su, D., Jiang, X., Zhu, L., & Ye, H. (2016). The similarities and differences in impulsivity and cognitive ability among ketamine, methadone, and non-drug users. Psychiatry Research, 243, 109–114. https://doi.org/10.1016/j.psychres.2016.04.095
- Zhong, H., Dang, J., Huo, Z., Ma, Z., Chen, J., Huang, Y., Zhu, Y., & Li, M. (2018). Effects of medial prefrontal cortex 5-HT7 receptor knockdown on cognitive control after acute heroin administration. Brain Research, 1678, 419–431. https://doi.org/10.1016/j.brainres.2017.11.002
- Zhu, Y., Evans, E. A., Mooney, L. J., Saxon, A. J., Kelleghan, A., Yoo, C., & Hser, Y.-I. (2018). Correlates of long-term opioid abstinence after randomization to methadone versus buprenorphine/naloxone in a multi-site trial. Journal of NeuroImmune Pharmacology, 13(4), 488–497. https://doi.org/10.1007/s11481-018-9801-x
- Zhukovsky, P., Morein‐Zamir, S., Meng, C., Dalley, J. W., & Ersche, K. D. (2020). Network failures: When incentives trigger impulsive responses. Human Brain Mapping, 41(8), 2216–2228. https://doi.org/10.1002/hbm.24941
