Abstract
Background: Despite growing access to effective therapies, asthma control still needs improvement. Many non-drug factors, such as allergens, air pollutants and stress also affect asthma control and patient quality of life, but an overview of the effectiveness of non-drug interventions on asthma control was lacking.
Objectives: To identify non-drug interventions likely to improve asthma control.
Methods: A systematic review of the available literature in Medline and the Cochrane Library was conducted in March 2017, without any time limit. Initial searching identified 884 potentially relevant clinical trial reports, literature reviews and meta-analyses, which were screened for inclusion using criteria of quality, relevance, and reporting outcomes based on asthma control.
Results: Eighty-two publications met the inclusion criteria. In general, the quality of the studies was low. Patient education programmes (22 studies) significantly improved asthma control. Multifaceted interventions (10 studies), which combined patient education programmes with decreasing exposure to indoor allergens and pollutants, significantly improved asthma control based on clinically relevant outcomes. Renovating homes to reduce exposure to allergens and indoor pollutants improved control (two studies). Air filtration systems (five studies) were effective, especially in children exposed to second-hand smoke. Most measures attempting to reduce exposure to dust mites were ineffective (five studies). Dietary interventions (eight studies) were ineffective. Promoting physical activity (five studies) tended to yield positive results, but the results did not attain significance.
Conclusion: Twenty-six interventions were effective in asthma control. Simultaneously combining several action plans, each focusing on different aspects of asthma management, seems most likely to be effective.
KEY MESSAGES
Therapeutic patient education programmes significantly improve disease control.
Multifaceted interventions, combining patient education programmes with measures to decrease exposure to indoor allergens and pollutants, significantly improved disease control.
These results call for a stronger emphasis on patient-focused care in asthma, in particular on their information needs and self-management skills.
Introduction
About 300 million people have asthma worldwide, including 30 million in Europe [Citation1,Citation2]. Asthma mortality has decreased in recent years, most likely because of new treatments and the spread of clinical guidelines but there is still room for improvement [Citation3,Citation4]. Patients with asthma often suffer from comorbidities and these comorbid diseases may hinder asthma control [Citation5–8].
The publication of the Global Initiative for Asthma (GINA) recommendations for asthma in 2004 marked a shift from the concept of severity to that of control [Citation9]. Control of asthma is evaluated based on disease activity in the last four weeks, assessed by the frequency of respiratory symptoms and their impact on daily living.
The effectiveness of drug treatments for asthma is well-recognized, with inhaled corticosteroids the cornerstone of treatment [Citation9]. But many other factors are also associated with asthma control, including allergens, air pollutants, viral infections, foods, drugs—non-steroidal anti-inflammatory drugs (NSAIDS), beta-blockers—obesity and emotional stress [Citation9,Citation10]. Addressing them could help to improve asthma control and patient quality of life.
Studies that attempt to measure the effectiveness of interventions aimed at correcting these factors are more difficult to perform than drug trials, may suffer from contamination bias, and be of doubtful generalizability. However, as a chronic disease, asthma calls for comprehensive care. The goal of this review is to identify and summarize the published evidence concerning non-drug interventions that aim to improve asthma control in adults and children.
Method
A systematic review of the available literature was conducted in March 2017. There were no time limits.
Information sources
The Medline database (PubMed) and the Cochrane Library were used to identify relevant published articles.
Search strategy
We searched PubMed to find all articles indexed using the MeSH terms: ‘asthma,’ ‘risk factors’ and ‘prevention and control,’ then limited the search to include only those articles classified as a ‘clinical trial,’ ‘review,’ ‘systematic review’ or ‘meta-analysis.’ In the Cochrane Library, the term ‘asthma’ was sought in titles, abstracts and keywords. When an original intervention study had already been aggregated into a review, we excluded the original study.
Inclusion criteria
Articles were judged potentially relevant to our review if they:
studied a population of adults and children with asthma. Participants could be on medication, as long as the medication were not part of the intervention;
were clinical trials, reviews or meta-analyses of non-drug interventions for asthma;
studied interventions of non-drug therapies, though we included vaccination studies as our goal was to build a comprehensive overview of all the available preventive strategies;
reported outcomes based on asthma control, including at least one defined by GINA—day or night symptoms, physical activity, exacerbations, absence from work or school, use of short-acting (rescue) β2-agonists, forced expiratory volume in one second (FEV1) or peak expiratory flow (PEF), and circadian variation of the PEF. Each study’s primary outcome measure(s) was used to judge the effectiveness of the intervention. The lack of standard outcome measures across the different studies meant that we were unable to define clinically relevant improvements in each outcome in advance;
were written in English or French.
We excluded reports of interventions which only targeted exacerbations, or which were primarily concerned with the effectiveness of one or more drugs.
Selection process
The list of articles identified in the database was established, and duplicate entries were eliminated. Each article was analysed for inclusion by two independent investigators. Disagreements were resolved by consensus.
Data extraction and analysis
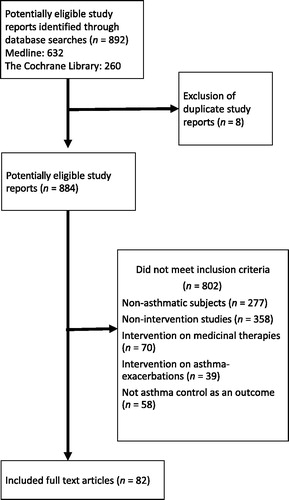
Eight hundred and ninety-two references were identified. Eight duplicate study reports were excluded. Eighty-two references were included. The selection process is summarized in . The name of the first author, year of publication, country where the study was conducted, study design, number and age of participants, type and description of intervention, primary outcomes and estimated effect size with corresponding 95% confidence intervals were extracted and recorded in an Excel spreadsheet.
Results
The original articles reported studies conducted mainly in the US, Canada, Australia and Northern Europe, which included both children and adults. Most were performed in primary care.
Indoor environment
A meta-analysis of 23 randomized controlled trials (RCTs) focused on physical methods (such as air aspiration systems) or chemical methods (miticides) to reduce exposure to dust mite allergens in the homes of adults or children with asthma who were sensitive to acarids (), [Citation11–27]. These interventions were found not to improve asthma control when used in an isolated manner11. Renovating homes affected by dampness or mould improved adults’ breathing symptoms and reduced emergency treatment delivered to children [Citation13]. Workplace interventions attempting to reduce or eliminate exposure to airborne pathogens significantly improved symptoms [Citation15].
Table 1. Description of selected studies evaluating indoor living environment interventions to improve asthma control (n = 17).
The use of air purifiers in living rooms and children’s rooms was assessed in several studies [Citation16–22]. A meta-analysis of air filtration reported an association with fewer symptoms, but none of the trials had employed validated scales to measure outcomes [Citation18]. Two RCTs showed a reduction of symptoms in inner-city children [Citation16] or on the use of unscheduled asthma visits in children exposed to second-hand smoke at home [Citation17]. Systems using ionizers or dehumidifiers were not effective [Citation21,Citation22]. Adapting bedding as the sole measure to reduce exposure to dust mite allergens showed no positive effect on asthma control [Citation23–25].
Diet and exercise
Fourteen literature reviews and two meta-analyses focused on diet or physical activity (), [Citation28–43]. Diets enriched with vitamin C, marine fatty acids, or selenium did not have any effect on asthma control [Citation28–30]; neither did restricting sodium or eliminating monosodium glutamate [Citation31,Citation32]. Caffeine improved the peak expiratory flow (PEF), but only for four hours after consumption [Citation33].
Table 2. Description of selected studies evaluating dietary and physical activity interventions to improve asthma control (n = 15).
Physical activity did not cause any side effects and did not exacerbate symptoms. Although the evidence lacked strength, the authors suggested that promoting physical activity improved quality of life [Citation34,Citation35]. One study of 38 patients included in a 2002 Cochrane review reported that low-calorie diets had beneficial effects on asthma control; however, the review authors considered that the evidence was inconclusive [Citation36]. A Cochrane review of weight loss interventions in patients with asthma retrieved a controlled study which showed a short-term reduction in doses of rescue medication in the treatment group. Weight loss was associated with a statistically significant improvement in FEV1 and FVC in one study, but this was considered clinically unimportant; there was no improvement in PEF. No data were reported on healthcare utilization and adverse effects. The reviewers concluded that poor study methodology meant that any positive effect of obesity treatment on asthma control was uncertain [Citation43].
Vaccinations
A review of the use of the pneumococcal vaccine in patients with asthma found only one study, of 80 children aged 2 to 6 years. The authors considered that there was insufficient evidence to recommend pneumococcal vaccine for patients with asthma [Citation44].
Eighteen articles were included in a recent Cochrane review of flu vaccination for patients with asthma. Only two high-quality articles assessed the impact of the vaccine on the number of exacerbations, but their results did not demonstrate any beneficial effects of flu vaccination on patients with asthma [Citation45].
Alternative or additional therapies
Several Cochrane reviews have studied alternative therapies, such as acupuncture, homeopathy or herbal medicine [Citation46–48]. There was no evidence to support the use of these therapies in treating asthma. A review of speleotherapy (a method based on giving treatment in an underground environment) demonstrated non-significant improvement on the PEF [Citation49].
Physiotherapy
Two Cochrane reviews studied physiotherapy. The first review, of breathing exercises in patients of all ages, included seven articles. The heterogeneous nature of the interventions and effectiveness criteria precluded the authors from drawing any robust conclusions for practice [Citation50]. Two articles about manual therapies, such as osteopathy, were included in another review, and found no evidence of effectiveness [Citation51].
Psychological treatment
Four meta-analyses focused on psychological treatment. The use of relaxation techniques was found to decrease consumption of drugs ‘on demand’ significantly and increased the PEF by 31.73 L/min (P < 0.0001). Cognitive behavioural therapy significantly improved the quality of life, as measured by the asthma quality of life questionnaire. Methodological limitations prevented the authors from drawing definite conclusions about the effect these interventions could have on asthma control [Citation52].
A second review devoted to psychological treatment of children with asthma yielded similar conclusions [Citation53], though children undergoing family therapy and receiving pharmacological treatment were less likely to limit their activities and reported fewer days with dyspnoea and wheezing [Citation54].
The use of written emotional disclosure did not improve asthma control [Citation55].
Therapeutic education
Therapeutic patient education (TPE) aims to help patients acquire and maintain the necessary skills to self-manage their chronic disease, [Citation56–78]. Four Cochrane reviews reported a positive impact of TPE on asthma control. TPE decreased night-time symptoms, the number of days of restricted activity, and increased quality of life. It reduced the numbers of days lost at work or school, the use of ambulances, and emergency department (ED) visits, though it did not significantly reduce ED re-presentations. While the trend in effect favours educational interventions, the pooled results were not statistically significant. TPE seemed to reach higher effectiveness in uncontrolled asthma, especially in children [Citation57–60].
Table 3. Description of selected studies evaluating therapeutic patient education interventions to improve asthma control (n = 22).
In another Cochrane review, a restricted health education programme, which included only information related to asthma, its causes, and treatments, did not seem to improve control [Citation61]. The use of written action plans (WAPs) seemed to have a positive impact on night-time symptoms and the number of ED attendances, but the risk of methodological bias (in a before/after study) prompted caution [Citation62]. The result stood in contrast to a Cochrane review that concluded that providing WAPs to adult patients offered no advantages over routine care [Citation63]. Using WAPs with children reduced the number of exacerbations requiring intensive care. WAPs based on symptoms seem preferable to WAPs based on PEF measurement [Citation64].
Healthcare organization
Four meta-analyses focused on healthcare organization. Nurse-led asthma clinics in primary care settings seem to offer few advantages over standard care [Citation79]. A meta-analysis comparing care delivered by a specialized nurse to that delivered by a GP did not reveal any significant difference in control or quality of life [Citation80].
Pharmacy advice in low- and middle-income countries improved the quality of life (an increase of 0.31 points on a 1 to 5 scale; P < 0.001) and decreased GP consultations (P = 0.01) [Citation81].
Telemedicine interventions reduced the risk of hospitalization (RR: 0.25; 95%CI: 0.09–0.66), particularly for patients with severe asthma [Citation82].
An RCT comparing nurse-led care in a school setting with routine care found that the intervention decreased night-time symptoms (1.68 nights with symptoms vs. 2.20; P = 0.02) and school absence (0.37 days vs. 0.85; P = 0.03) [Citation83]. Another RCT of an educational intervention following ED attendance for asthma showed no difference in the number of subsequent ED visits, medication use, or quality of life [Citation84].
Multifaceted interventions
Several RCTs focused on multifaceted interventions (i.e. those combining several interventions) conducted in primary care in a community context (school, home, local services), or in the ED , [Citation85–94]. The interventions were heterogeneous, but most included a TPE action plan. Interventions were directed towards children with asthma sensitive to dust mites or exposed to passive smoking, to reduce indoor pollution within patients’ homes [Citation86]. Other measures, such as administering treatment at school, offering telephone follow-up, or delivering patient-centred care regardless of coordination by the GP, were also assessed.
Table 4. Description of selected studies evaluating multifaceted interventions to improve asthma control (n = 10).
These multifaceted interventions were not conducted by doctors but were led by social workers or community health workers. Among the 10 studies included, only two did not exhibit any improvement of clinical signs related to control [Citation92, Citation94]. One study showed a decrease in the number of hospitalizations [Citation93]. Six interventions reduced symptoms and one improved daily activities [Citation85–87, Citation91].
Discussion
Main findings
A total of 82 publications met the inclusion criteria. In general, study methodological quality was low. Out of 68 interventions studied, 26 were effective in asthma control according to the authors’ prospective criteria. Patient education programmes (22 studies) significantly improved asthma control but identifying the most effective type of programme proved difficult. Multifaceted interventions (10 studies), which typically combined therapeutic patient education programmes with decreasing exposure to indoor allergens and pollutants, significantly improved asthma control based on clinically relevant outcomes. Totally or partially renovating homes to reduce exposure to allergens and indoor pollutants improved control (two studies). Air purification systems by filtration (five studies) were effective on asthma control especially in children exposed to second-hand smoke. Most measures attempting to reduce exposure to dust mites were ineffective (five studies). Dietary interventions (eight studies) were ineffective. Physical activity (five studies) had encouraging but insignificant results. Psychological interventions (four studies) and physiotherapy (two studies) were not effective.
Comparison with existing literature
International guidelines now emphasize the importance of patients being educated to develop the skills to manage their asthma. The GINA components for effective guided asthma self-management include self-monitoring of symptoms and/or peak flow, written asthma action plans and regular review of asthma control, treatment and skills [Citation9]. Our review confirms that therapeutic patient education programmes significantly improve asthma control. However, identifying the most effective therapeutic patient education programmes remains difficult.
Although coordination of care is considered to be part of a GP’s general skill [Citation95], our study shows that effective multifaceted interventions in asthma were generally not conducted by GPs, and that GP-only interventions rarely had a significant impact. Interventions by multi-professional teams seem necessary. In France, some of these multifaceted interventions have materialized in the recent engagement of health advisors in indoor environment.
Most of the measures to reduce exposure to dust mites proved ineffective, but renovating homes, partly or fully, to reduce exposure to allergens and indoor pollution was an effective method to improve control. GINA guidelines also report these data but avoidance strategies are often complicated and expensive [Citation9]. Our study also suggests that air purification using a filter system has a positive impact on asthma control in children exposed to air pollution or second-hand smoke. Further studies employing personal monitoring devices for allergen, pollutant, and microbial exposure may clarify the importance of environmental interventions [Citation96]. There was no evidence to support the use of ionizers. These devices release nitric oxide, which is an asthma trigger [Citation97].
According to GINA, there is a heterogeneous level of evidence regarding isolated measures to reduce outdoor allergens or air pollution [Citation9]. As expected, no strong evidence was found in our study regarding these risk factors but avoiding physical activity in unfavourable environmental conditions seems advisable.
There was no conclusive evidence for specific dietary interventions, and physical activity showed encouraging but non-significant results. However, GINA recommends a healthy diet and regular physical activity for their general health benefits, even if the evidence for one form of physical activity over another remains limited [Citation9].
Respiratory viruses trigger asthma exacerbations [Citation98]. For this reason, GINA logically recommends flu vaccination while acknowledging that it has not proved effective in asthma control [Citation9]. GINA does not recommend the pneumococcal vaccine, and the present review has not identified sufficient evidence to recommend it [Citation9].
Specific interventions included in this review did not address tobacco cessation. Some trials assessing the impact of tobacco cessation on asthma control have been published. The few studies that we identified did not meet our inclusion criteria. In one study participants used medication (oral nicotine) for tobacco cessation and in another, the primary outcome was the change in reported smoking habits after the intervention. It can be assumed that this intervention has mostly been studied by observational and cohort studies that were not included in our review [Citation99]. Nevertheless, tobacco cessation was a frequent element in the multifaceted interventions we did include and helping smokers to quit must remain a key issue for primary care professionals, especially among patients suffering from chronic respiratory conditions.
Strengths and limitations
To the best of our knowledge, this is the first attempt to synthesize knowledge about the impact of non-drug therapies on asthma control. All the interventions included used patient-centred clinical criteria for asthma control as defined by GINA.
The main study limitation lies within the Medline and Cochrane focus. We chose to include original studies as well as systematic reviews and meta-analysis, though for each intervention, we did not include any original study that had already been systematically reviewed. The alternative would have been to perform a meta-review, i.e., a review of reviews, to ensure the homogeneity of included material. However, this would have limited our attempt to build a comprehensive overview of the topic, in particular regarding multifaceted interventions.
Implications for clinical practice, research and policy
Simultaneously combining several action plans, each focusing on different factors of asthma control, seems to be the most effective measure. Involvement of both patients and of healthcare professionals is essential, building effective, thorough and multidimensional care for patients with asthma. An example is the French EPODE programme. This programme is a coordinated, capacity-building approach aimed at reducing childhood obesity through a societal process in which local environments, childhood settings, and family norms are directed and encouraged to facilitate the adoption of healthy lifestyles in children [Citation100]. The EPODE programme has demonstrated a global diminution on overweight and obesity prevalence [Citation101], an efficiency across all socioeconomic levels, and the capacity to decrease health inequities [Citation102]. This programme is derived from Wagner’s model, which is otherwise known as the Chronic Care Model [Citation103]. In the case of asthma, an action plan was designed directly from Wagner’s model intended for a sample of children living in a precarious environment [Citation104]; the results of which corroborate the effectiveness of this model on clinical outcomes [Citation90].
Future recommendations regarding asthma should certainly take the efficacy of multifaceted and multidisciplinary interventions into account as well as comorbidity, which mostly affects patients with chronic diseases.
Conclusion
Most of the effective asthma control interventions focused either on patient education or a combination of a patient education programme with measures to reduce the exposure to allergens and indoor pollution. Recent studies have shown that these interventions can be successfully adapted into primary care settings, reducing the morbidity of asthma in these populations [Citation105]. Future non-drug intervention studies should acknowledge the necessity of a multifaceted approach, and the engagement of a multidisciplinary team. This review may also serve as a summary of the effectiveness of non-drug therapies for asthma.
Supplemental Material - PRISMA 2009 Checklist
Download MS Word (26.6 KB)Disclosure statement
The authors report no conflicts of interests. The authors alone are responsible for the content and writing of the paper.
References
- Masoli M, Fabian D, Holt S, et al. The global burden of asthma: executive summary of the GINA Dissemination Committee report. Allergy. 2004;59:469–478.
- To T, Stanojevic S, Moores G. et al. Global asthma prevalence in adults: findings from the cross-sectional world health survey. BMC Public Health. 2012;12:204.
- Tual S, Godard P, Bousquet J, et al. Diminution de la mortalité par asthme en France. Rev Mal Respir. 2008;25:814–820.
- Demoly P, Paggiaro P, Plaza V, et al. Prevalence of asthma control among adults in France, Germany, Italy, Spain and the UK. Eur Respir Review. 2009;18:105–112.
- Soriano JB, Visick GT, Muellerova H, et al. Patterns of comorbidities in newly diagnosed COPD and asthma in primary care. Chest. 2005;128:2099–2107.
- Wijnhoven HAH, Kriegsman DMW, Hesselink AE, et al. The influence of co-morbidity on health-related quality of life in asthma and COPD patients. Respir Med. 2003;97:468–475.
- Lehrer PM, Karavidas MK, LuS-E, et al. Psychological treatment of comorbid asthma and panic disorder: a pilot study. J Anxiety Disord. 2008;22:671–683.
- Deshmukh VM, Toelle BG, Usherwood T, et al. The association of comorbid anxiety and depression with asthma-related quality of life and symptom perception in adults. Respirology. 2008;13:695–702.
- Global Initiative for Asthma [Internet]. Global Strategy for Asthma Management and Prevention, 2016. [cited 2019 Jan 22]. Available from: www.ginasthma.org.
- Yawn B. The role of the primary care physician in helping adolescent and adult patients improve asthma control. Mayo Clin Proc. 2011;9:894–902.
- Howden-Chapman P, Pierse N, Nicholls S, et al. Effects of improved home heating on asthma in community dwelling children: randomised controlled trial. BMJ. 2008;337:1411.
- Dietemann A, Bessot JC, Hoyet C, et al. A double-blind, placebo controlled trial of solidified benzyl benzoate applied in dwellings of asthmatic patients sensitive to mites: clinical efficacy and effect on mite allergens. J Allergy Clin Immunol. 1993;91:738–746.
- Sauni R, Uitti J, Jauhiainen M, et al. Remediating buildings damaged by dampness and mould for preventing or reducing respiratory tract symptoms, infections and asthma (Review). Evid Based Child Health. 2013:8;944–1000.
- Takaro TK, Krieger J, Song L, et al. The breathe-easy home: the impact of asthma-friendly home construction on clinical outcomes and trigger exposure. Am J Public Health. 2011;101:55–62.
- De Groene GJ, Pal TM, Beach J, et al. Workplace interventions for treatment of occupational asthma: a Cochrane systematic review. Occup Environ Med. 2012;69:373–374.
- Butz AM, Matsui EC, Breysse P, et al. A randomized trial of air cleaners and a health coach to improve indoor air quality for inner-city children with asthma and secondhand smoke exposure. Arch Pediatr Adolesc Med. 2011;165:741–748.
- Lanphear BP, Hornung RW, Khoury J, et al. A. Effects of HEPA air cleaners on unscheduled asthma visits and asthma symptoms for children exposed to secondhand tobacco smoke. Pediatrics. 2011;127:93–101.
- McDonald E, Cook D, Newman T, et al. Effect of air filtration systems on asthma: a systematic review of randomized trials. Chest. 2002;122:1535–1542.
- Warner JA, Frederick JM, Bryant TN, et al. Mechanical ventilation and high-efficiency vacuum cleaning: A combined strategy of mite and mite allergen reduction in the control of mite-sensitive asthma. J Allergy Clin Immunol. 2000;105:75–82.
- Kilburn SA, Lasserson TJ, McKean MC. Pet allergen control measures for allergic asthma in children and adults. Cochrane Database Syst Rev. 2003;1CD002989.
- Singh M, Jaiswal N. Dehumidifiers for chronic asthma. Cochrane Database Syst Rev. 2013;6:CD003563.
- Blackhall K, Appleton S, Cates CJ. Ionisers for chronic asthma. Cochrane Database Syst Rev. 2012;9:CD002986.
- Woodcock A, Forster L, Matthews E, et al. Control of exposure to mite allergen and allergen-impermeable bed covers for adults with asthma. N Engl J Med. 2003;349:225–236.
- Glasgow NJ, Ponsonby A-L, Kemp A, et al. Feather bedding and childhood asthma associated with house dust mite sensitisation: a randomised controlled trial. Arch Dis Child. 2011;96:541–547.
- Carter MC, Perzanowski MS, Raymond A, et al. Home intervention in the treatment of asthma among inner-city children. J Allergy Clin Immunol. 2001;108:732–737.
- Gøtzsche PC, Hammarquist C, Burr M. House dust mite control measures in the management of asthma: meta-analysis. BMJ. 1998;317:1105–1110.
- Schatz M, Zeiger RS. Ineffectiveness of telephone-based environmental control intervention to improve asthma outcomes. J Allergy Clin Immunol. 2010;126:873–875.
- Kaur B, Rowe BH, Stovold E. Vitamin C supplementation for asthma. Cochrane Database Syst Rev. 2013;3:CD000993.
- Woods RK, Thien FC, Abramson MJ. Dietary marine fatty acids (fish oil) for asthma in adults and children. Cochrane Database Syst Rev. 2002;3:CD001283.
- Allam MF, Lucane RA. Selenium supplementation for asthma. Cochrane Database Syst Rev. 2004;2:CD003538.
- Pogson Z, McKeever T. Dietary sodium manipulation and asthma. Cochrane Database Syst Rev. 2011;3:CD000436.
- Zhou Y, Yang M, Dong BR. Monosodium glutamate avoidance for chronic asthma in adults and children. Cochrane Database Syst Rev. 2012;6:CD004357.
- Welsh EJ, Bara A, Barley E, et al. Caffeine for asthma. Cochrane Database Syst Rev. 2010;1:CD001112.
- Chandratilleke MG, Carson KV, Picot J, et al. Physical training for asthma. Cochrane Database Syst Rev. 2012;5:CD001116.
- Beggs S, Foong YC, Le HCT. Swimming training for asthma in children and adolescents aged 18 years and under. Cochrane Database Syst Rev. 2013;4:CD009607.
- Cheng J, Pan T, Ye GH, et al. Calorie controlled diet for chronic asthma. Cochrane Database Syst Rev. 2005;3:CD004674.
- Milan SJ, Hart A, Wilkinson M. Vitamin C for asthma and exercise-induced bronchoconstriction. Cochrane Database Syst Rev. 2014;6.CD010391.
- Wilkinson M, Hart A, Milan SJ. Vitamins C and E for asthma and exercise-induced bronchoconstriction; Cochrane Database Syst Rev. 2014;6 CD010749.
- Carson KV, Chandratilleke MG, Picot J, et al. Physical training for asthma. Cochrane Database Syst Rev. 2013;9.CD001116.
- Freitas DA, Holloway EA, Bruno SS, et al. Breathing exercises for adults with asthma. Cochrane Database Syst Rev. 2013;10. CD001277.
- Silva IS, Fregonezi GA, Dias FA, et al. Inspiratory muscle training for asthma. Cochrane Database Syst Rev. 2013;9.CD003792.
- Grande AJ, Silva V, Andriolo BN, et al. Water-based exercise for adults with asthma. Cochrane Database Syst Rev. 2014;7.CD010456.
- Adeniyi FB, Young T. Weight loss interventions for chronic asthma. Cochrane Database Syst Rev. 2012;7:CD009339.
- Sheikh A, Alves B, Dhami S. Pneumococcal vaccine for asthma. Cochrane Database Syst Rev. 2002;1:CD002165.
- Cates CJ, Rowe BH. Vaccines for preventing influenza in people with asthma. Cochrane Database Syst Rev. 2013;2:CD000364.
- McCarney RW, Brinkhaus B, Lasserson TJ, et al. Acupuncture for chronic asthma. Cochrane Database Syst Rev. 2003;3:CD000008.
- McCarney RW, Linde K, Lasserson TJ. Homeopathy for chronic asthma. Cochrane Database Syst Rev. 2004;1:CD000353.
- Arnold E, Clark CE, Lasserson TJ, et al. Herbal interventions for chronic asthma in adults and children. Cochrane Database Syst Rev. 2008;1:CD005989.
- Beamon SP, Falkenbach A, Fainburg G, et al. Speleotherapy for asthma. Cochrane Database Syst Rev. 2001;2:CD001741.
- Holloway EA, Ram FS. Breathing exercises for asthma. Cochrane Database Syst Rev. 2009;1:CD001277.
- Hondras MA, Linde K, Jones AP. Manual therapy for asthma. Cochrane Database Syst Rev. 2005;2:CD001002.
- Yorke J, Fleming SL, Shuldham C. Psychological interventions for adults with asthma. Cochrane Database Syst Rev. 2006;1:CD002982.
- Yorke J, Fleming SL, Shuldham C. Psychological interventions for children with asthma. Cochrane Database Syst Rev. 2005;4:CD003272.
- Yorke J, Shuldham C. Family therapy for asthma in children. Cochrane Database Syst Rev. 2005;2:CD000089.
- Paudyal P, Hine P, Theadom A, et al. Written emotional disclosure for asthma. Cochrane Database Syst Rev. 2014;5:CD007676.
- World Health Organization. Therapeutic patient education – continuing education programmes for health care providers in the field of prevention of chronic diseases. Geneva: WHO, 1998. [cited 2019 Jan 22]. Available on: http://www.euro.who.int/__data/assets/pdf_le/0007/145294/E63674.pdf.
- Boyd M, Lasserson TJ, McKean MC, et al. Interventions for educating children who are at risk of asthma-related emergency department attendance. Cochrane Database Syst Rev. 2009;2:CD001290.
- Gibson PG, Powell H, Wilson A, et al. Self-management education and regular practitioner review for adults with asthma. Cochrane Database Syst Rev. 2002;3:CD001117.
- Wolf F, Guevara JP, Grum CM, et al. Educational interventions for asthma in children. Cochrane Database Syst Rev. 2003;4:CD000326.
- Tapp S, Lasserson TJ, Rowe BH. Education interventions for adults who attend the emergency room for acute asthma. Cochrane Database Syst Rev. 2007;3:CD003000.
- Gibson PG, Powell H, Coughlan J, et al. Limited (information only) patient education programs for adults with asthma. Cochrane Database Syst Rev. 2002;2:CD001005.
- D’Souza W, Burgess C, Ayson M, et al. Trial of a “credit card” asthma self-management plan in a high-risk group of patients with asthma. J Allergy Clin Immunol. 1996;97:1085–1092.
- Powell H, Gibson PG. Options for self-management education for adults with asthma. Cochrane Database Syst Rev. 2003;3:CD004107.
- Bhogal SK, Zemek RL, Ducharme F. Written action plans for asthma in children. Cochrane Database Syst Rev. 2006;3:CD005306.
- Peytremann-Bridevaux I, Arditi C, Gex G, et al. Chronic disease management programmes for adults with asthma. Cochrane Database Syst Rev. 2015;5.CD007988.
- Greineder DK, Loane KC, Parks P. Reduction in resource utilization by an asthma outreach program. Arch Pediatr Adolesc Med. 1995;149:415–420.
- Welsh EJ, Hasan M, Li P. Home-based educational interventions for children with asthma. Cochrane Database Syst Rev. 2011;10:CD008469.
- Bruzzese JM, Sheares BJ, Vincent EJ, et al. Effects of a school-based intervention for urban adolescents with asthma. A controlled trial. Am J Respir Crit Care Med. 2011;183:998–1006.
- Côté J, Bowie DM, Robichaud P. Evaluation of two different educational interventions for adult patients consulting with an acute asthma exacerbation. Am J Respir Crit Care Med. 2001;163:1415–1419.
- Cowie RL, Revitt SG, Underwood MF, et al. The effect of a peak flow-based action plan in the prevention of exacerbations of asthma. Chest. 1997;112:1534–1538.
- Coté J, Cartier A, Robichaud P, et al. Influence on asthma morbidity of asthma education programs based on self-management plans following treatment optimization. Am J Respir Crit Care Med. 1997;155:1509–1514.
- de Jongh T, Gurol-Urganci I, Vodopivec-Jamsek V, et l. Mobile phone messaging for facilitating self-management of long-term illnesses. Cochrane Database Syst Rev. 2012;12.CD007459.
- Runge C, Lecheler J, Horn M, et al. Outcomes of a web-based patient education program for asthmatic children and adolescents. Chest. 2006;129:581–593.
- Apter AJ, Wang X, Bogen DK, et al. Problem solving to improve adherence and asthma outcomes in urban adults with moderate or severe asthma: a randomized controlled trial. J Allergy Clin Immunol. 2011;128:516–523.
- Chang AB, Taylor B, Masters IB, et al. Indigenous healthcare worker involvement for Indigenous adults and children with asthma. Cochrane Database Syst Rev. 2010;5:CD006344.
- Bailey EJ, Cates CJ, Kruske SG, et al. Culture-specific programs for children and adults from minority groups who have asthma. Cochrane Database Syst Rev. 2009;2:CD006580.
- Canino G, Vila D, Normand S-LT, et al. Reducing asthma health disparities in poor Puerto Rican children: the effectiveness of a culturally tailored family intervention. J Allergy Clin Immunol. 2008;121:665–670.
- Marcano Belisario JS, Huckvale K, Greenfield G, et al. Smartphone and tablet self management apps for asthma. Cochrane Database Syst Rev. 2013;11.CD010013.
- Baishnab E, Karner C. Primary care based clinics for asthma. Cochrane Database Syst Rev. 2012;4:CD003533.
- Kuethe MC, Vaessen-Verberne AAPH, Elbers RG, et al. Nurse versus physician-led care for the management of asthma. Cochrane Database Syst Rev. 2013;2:CD009296.
- Pande S, Hiller JE, Nkansah N, et al. The effect of pharmacist-provided non-dispensing services on patient outcomes, health service utilisation and costs in low- and middle-income countries. Cochrane Database Syst Rev. 2013;2:CD010398.
- McLean S, Chandler D, Nurmatov U, et al. Telehealthcare for asthma: a Cochrane review. CMAJ. 2011;183:E733–742.
- Halterman JS, Fagnano M, Montes G, et al. The school-based preventive asthma care trial: results of a pilot study. J Pediatr. 2012;161:1109–1115.
- Zorc JJ, Chew A, Allen JL, et al. Beliefs and barriers to follow-up after an emergency department asthma visit: a randomized trial. Pediatrics. 2009;124:1135–1142.
- Evans R, Gergen PJ, Mitchell H, et al. A randomized clinical trial to reduce asthma morbidity among inner-city children: results of the National Cooperative Inner-City Asthma Study. J Pediatr. 1999;135:332–338.
- Morgan WJ, Crain EF, Gruchalla RS, et al. Results of a home- based environmental intervention among urban children with asthma. N Engl J Med. 2004;351:1068–1080.
- Teach SJ, Crain EF, Quint DM, et al. Improved asthma outcomes in a high-morbidity pediatric population: results of an emergency department-based randomized clinical trial. Arch Pediatr Adolesc Med. 2006;160:535–541.
- Kintner EK, Sikorskii A. Randomized clinical trial of a school-based academic and counseling program for older school-age students. Nurs Res. 2009;58:321–331.
- Halterman JS, Fagnano M, Tremblay PJ, et al. Prompting asthma intervention in Rochester—uniting parents and providers (PAIR-UP): a randomized trial. JAMA Pediatr. 2014;168:e141983.
- Halterman JS, Szilagyi PG, Fisher SG, et al. Randomized controlled trial to improve care for urban children with asthma: results of the School-Based Asthma Therapy trial. Arch Pediatr Adolesc Med. 2011;165:262–268.
- Klinnert MD, Liu AH, Pearson MR, et al. Outcome of a randomized multifaceted intervention with low-income families of wheezing infants. Arch Pediatr Adolesc Med. 2007;161:783–190.
- Klinnert MD, Liu AH, Pearson MR, et al. Short-term impact of a randomized multifaceted intervention for wheezing infants in low-income families. Arch Pediatr Adolesc Med. 2005;159:75–82.
- Fisher EB, Strunk RC, Highstein GR, et al. A randomized control-led evaluation of the effect of community health workers on hospitalization for asthma: the asthma coach. Arch Pediatr Adolesc Med. 2009;163:225–232.
- Krieger JW, Takaro TK, Song L, et al. The Seattle-King County Healthy Homes Project: a randomized, controlled trial of a community health worker intervention to decrease exposure to indoor asthma triggers. Am J Public Health. 2005;95:652–659.
- Allen J, Gay B, Crebolder H, et al. The European definitions of the key features of the discipline of general practice: the role of the GP and core competencies. Br J Gen Pract. 2002;52:526–527.
- Gold D, Adamkiewicz G, Arshad SH, et al. NIAID, NIEHS, NHLBI, MCAN Workshop Report: the indoor environment and childhood asthma: implications for home environmental intervention in asthma prevention and management. J Allergy Clinical Immunol. 2017;140:933–949.
- Jackson DJ, Sykes A, Mallia P, Johnston SL. Asthma exacerbations: origin, effect, and prevention. J Allergy Clinical Immunol. 2011;128:1165–1174.
- Busse WW, Lemanske RF, Gern JE. The role of viral respiratory infections in asthma and asthma exacerbations. Lancet. 2010;376:826–834.
- To T1, Daly C, Feldman R, et al. Results from a community-based program evaluating the effect of changing smoking status on asthma symptom control. BMC Public Health. 2012;12:293.
- Borys JM, Le Bodo Y, Jebb SA, et al. EPODE approach for childhood obesity prevention: methods, progress and international development: EPODE approach for obesity prevention. Obesity Reviews. 2012;13:299–315.
- Borys JM, Valdeyron L, Levy E, et al. EPODE – a model for reducing the incidence of obesity and weight-related comorbidities. Eur Endocrinol. 2013;9:116–20.
- Borys JM, Richard P, Ruault du Plessis H, et al. Tackling health inequities and reducing obesity prevalence: the EPODE community-based approach. Ann Nutr Metab. 2016;68(suppl 2):35–38.
- Wagner E. Chronic disease management: what will it take to improve care for chronic illness? Eff Clin Pract. 1998;1:2–4.
- Halterman JS, Borrelli B, Fisher S, et al. Improving care for urban children with asthma: design and methods of the School-Based Asthma Therapy (SBAT) Trial. J Asthma. 2008;45:279–286.
- Kennedy S, Bailey R, Jaffee K, et al. Effectiveness of evidence-based asthma interventions. Pediatrics. 2017;139(6):e20164221.