Abstract
Background
Seasonal influenza vaccination coverage levels remain too low in many countries.
Objectives
This study aimed to evaluate the impact of a reminder letter from their general practitioner (GP) on patients’ influenza vaccination.
Methods
Eligible patients for this controlled non-randomised study were the vulnerable categories targeted by the 2019–2020 national health insurance fund (NHIF) vaccination campaign, on the lists of 14 GPs from three practices in Paris (France) and unvaccinated on January 2, 2020 (mid-campaign). The choice of practices and assigning five GPs to the intervention arm were made for convenience. At mid-campaign, GPs in the intervention arm sent a standardised letter reminding each eligible patient to be vaccinated. In the control arm, GPs worked as usual. The intervention effect, calculated from the NHIF databases, was estimated by the difference between the groups in their vaccination coverage at the end of the campaign, with a linear mixed model adjusted for age, sex, chronic disease (at the patient level) and medical practice (at the GP level).
Results
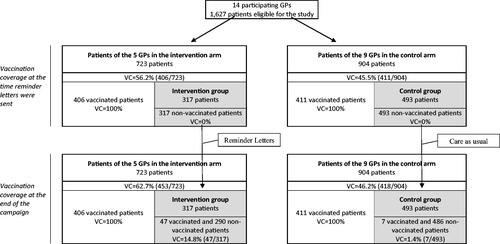
The vaccination coverage at the end of the campaign was 14.7% in the intervention group (n = 317) and 1.7% in the control group (n = 493): a difference of 13.1% points (95% confidence interval [9.0–17.2], number needed to send 7.7). At the campaign’s end, vaccination coverage among patients from the lists of GPs in the intervention arm was 62.7%, and 46.2% among patients from the control-arm GP lists.
Conclusion
Reminder letters could help increase influenza vaccination coverage.
KEY MESSAGES
At the campaign’s end, vaccination coverage was 14.7% in the intervention group and 1.7% in the control group.
Sending reminder letters to non-vaccinated patients signed by their GPs at mid-campaign increased vaccination coverage by more than 13% points.
A total of 7.7 letters needed to be sent to obtain one additional vaccination.
Introduction
Seasonal influenza is responsible for substantial morbidity, mortality and economic cost [Citation1,Citation2]. To limit this burden, many countries recommend annual vaccination for people at risk for its severe forms. In France, as part of the influenza vaccination campaign conducted by the national health insurance fund (NHIF), eligible adults (those older than 65 years or those with specific chronic diseases, cardiorespiratory in particular) receive a voucher by mail each year in October that entitles them to obtain the vaccine from a pharmacy and then be vaccinated by the professional of their choice (doctor, midwife, nurse, or pharmacist)—both free of charge.
As in many countries [Citation3], French vaccination coverage for influenza remains low (barely 47% for the 2018–19 campaign [Citation4])—below the WHO objective of 75%. Several reminder or recall systems have been tested to improve this insufficient vaccination coverage, producing gains of around eight percentage points [Citation5].
Receiving reminder letters from their general practitioners (GPs) might help increase vaccination coverage. These doctors are responsible for providing their patients with preventive care. Moreover, one of the reasons patients most frequently cite for getting vaccinated is their doctor’s recommendation [Citation6,Citation7]. GPs’ knowledge of their patients and patient’s trust in their GPs should enable the mobilisation of more than just those who forgot about the vaccination [Citation8]. Unlike other countries (USA, Canada, Denmark, Spain and New Zealand) [Citation5], this type of reminder has not yet been evaluated in France.
This study aimed to evaluate the impact of a patient reminder letter from their GP on influenza vaccination.
Methods
Study design
The effect of a patient reminder letter was evaluated in a controlled non-randomised study. This quasi-experimental survey took place during the 2019–2020 influenza vaccination campaign (from October to March) among patients on the lists of 14 GPs from the 13th arrondissement of Paris, France (a district with more than 183,000 inhabitants in southern Paris). Before the campaign, the research team proposed the study to the GPs in three multi-professional health centres whose members share an interest in quality of care and a specific public health vision of their work. All GPs agreed to participate. To launch the study very quickly at the end of 2019, when the NHIF sent the research team the lists of patients not vaccinated at mid-campaign (those requiring reminders), the GPs working then (not on holiday) were allocated to the intervention arm (i.e. 3 of 6 in the first medical practice, 1 of 5 in the second and 1 of 3 in the third) and the others (on holiday) to the control arm.
Eligible patients, intervention and control groups
Patients eligible for the study were adults residing in Paris, on a participating GP's patient list, and eligible for the 2019–2020 NHIF vaccination campaign.
Eligible patients not vaccinated at the end of 2019 (at mid-campaign) were in the intervention (control) group, depending on whether they were registered with a GP in the intervention (control) arm (grey areas in ).
Intervention
The NHIF identified the non-vaccinated patients at mid-campaign in its reimbursement database by the absence of data on voucher redemption. Affirmative redemption data attested to the patient’s vaccine collection at a pharmacy and its reimbursement. The NHIF then sent each participating GP in the intervention arm a list of their patients who were eligible for the study but had not been vaccinated.
On January 2, 2020, GPs in the intervention arm sent a standardised letter individually inviting their eligible patients not already vaccinated at mid-campaign (i.e. those in the intervention group) to be vaccinated against influenza. These letters urged the patient to get vaccinated and contained a summary of the disease burden, an explanation of how to use the vaccine voucher attached to the letter, and a reminder of the individual and collective benefits of vaccination (see Appendix). They were hand-signed by the GPs (who could also add a handwritten personalised salutation if they chose) and printed on practice letterhead.
No intervention occurred in the control arm: GPs worked as usual without sending any reminders.
Ethical and regulatory aspects
Before the 2019–2020 vaccination campaign, the NHIF sent a letter to patients eligible for the study to inform them that they would be participating in an influenza vaccination study in which their vaccination status would be shared with their GP and anonymised data about them with the team conducting the analysis. They were further informed that they could refuse to participate and be excluded from the study on a simple (written, email or telephone) request. The absence of any response in this situation is interpreted in France as lacking an objection. Patients did not sign an informed consent form. The National Data Protection Authority (Commission nationale de l’informatique et des libertés), responsible for these ethical issues and protecting individuals from illegal or inappropriate electronic data collection, confirmed that no additional steps were necessary to comply with current French statutes or regulations.
Data
The NHIF provided all the analysed data. The database was a list of eligible patients, including the following information: age, sex, presence of chronic disease (regardless of whether this disease constitutes a specific indication for vaccination), personal GP, and their dates of vaccine collection (if any) for the 2018–2019 and 2019–2020 campaigns (hereinafter referred to as the (Y − 1) and Y campaigns). The intervention took place only during campaign Y, but vaccination information from the campaign (Y − 1) was also needed for each eligible patient to analyse the temporal trend of vaccination coverage (see statistical analysis subsection). Patients were considered vaccinated when they redeemed the voucher and collected the vaccine at the pharmacy.
Sample size and detectable effect of the intervention
According to NHIF figures, the patient lists included 880 patients, 20% older than 65 years. With a presumed 70% participation rate, each participating GP would have had 123 eligible patients. Assuming that the vaccination coverage at mid-campaign was already very close to that at the end of the campaign and considering that vaccination coverage in Paris during the 2018–2019 campaign was 42% [Citation4], each participating GP had 71 (≈123 * 0.58) patients eligible for vaccination and unvaccinated at mid-campaign. Further, assuming at least five practitioners in each group (i.e. 356 patients), a power of 80%, an alpha-risk of 0.05, a two-sided test, we would be able to detect a difference between the control and intervention groups with vaccination coverage levels of 2 and 6% respectively, without taking the intraclass correlation coefficient into account.
Statistical analyses
The vaccination coverage among patients in the intervention group at the end of the campaign (noted VCintervention group) overestimates the intervention’s effect, as part of this vaccination coverage corresponded to the expected time trends for vaccination campaigns: even without intervention, some patients would have been vaccinated. If the parallel trend assumption is valid (explained below), this standard time trend can be estimated by the vaccination coverage in the control group at the end of the campaign (VCcontrol group). Thus, to estimate the effect of the reminder letter, we used the difference between the groups: VCintervention group − VCcontrol group.
This difference was estimated with a linear mixed model (with a random intercept to take into account the grouping of patients by GP) [Citation9], adjusted for patient characteristics (age in 10-year categories, sex, and presence of chronic disease) and the medical practice to which the GP belonged.
To ensure that the change over time of the vaccination coverage without intervention would have been the same in both groups (the parallel trend assumption [Citation10]), we repeated the same analyses for campaign Y − 1 (instead of Y). To show the effect of the intervention during campaign Y, we expected a difference between the intervention and control groups significantly different from zero; for campaign Y − 1, we expected a difference not significantly different from zero. Indeed, this result for Y − 1 justifies the use of the control group as representative of the trend expected without intervention.
All statistical analyses were carried out with Stata (version 17).
Results
None of the 1627 eligible patients expressed opposition to participating in the study. Their mean age was 66.4 years, and 45.5% were male. At the time the reminder letter was sent, 56.2% (=406/723) of the patients of GPs in the intervention arm had been vaccinated and 46.6% (=421/904) of those of GPs in the control arm (). Accordingly, 317 intervention and 493 control patients were not vaccinated at the end of 2019 and comprised the intervention and control groups. These groups did not differ in age, sex, chronic disease, or distribution among the three medical practices ().
Table 1. Patient characteristics.
At the campaign’s end (also the study end), the vaccination coverage in the intervention was 14.7% (95% confidence interval (CI) [11.6%, 17.9%]) and in the control group, 1.7% (95% CI [−1.0%, 4.3%]), for a difference of 13.1 percentage points between the two groups (p < .001, ). In other words, eight reminder letters (or more precisely 7.7; 95% CI [5.6, 10.5]) needed to be sent to obtain one additional vaccination (number required to remind = 1/(VCintervention group − VCcontrol group)).
Table 2. Vaccination coverage differences.
To ensure that during the previous campaign, vaccination coverage in both groups had had similar temporal trends (from the sending of the reminder letters to the end of the campaign—the parallel trend assumption), we conducted identical analyses on year (Y − 1) data.
The vaccination coverage at the end of the previous campaign in the intervention and control groups was respectively 1.0% (95% CI [0.2%, 1.9%]) and 0.6% (95% CI [−0.1%, 1.4%], ), a difference between the groups of 0.4% points (p = .51, ).
Figure 2. Flow chart during the previous campaign*. *There was no intervention during the previous campaign, i.e. campaign (Y − 1). The intervention group is the one that will receive the intervention but only during campaign Y. VC: vaccination coverage.
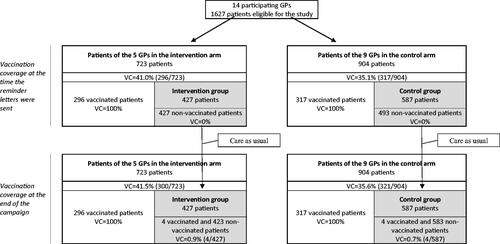
Table 3. Vaccination coverage differences during the previous campaignTable Footnotea.
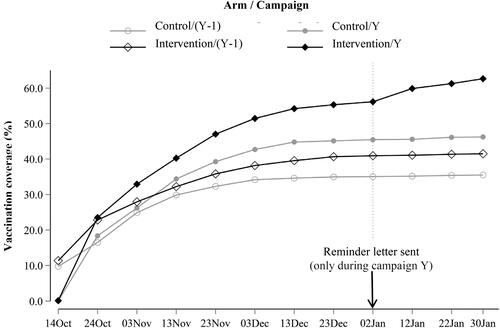
shows the change over time of vaccination coverage among eligible patients of physicians from the intervention and control arms during each of the two campaigns. After January 2, 2020, the date on which the reminder letters were sent, the vaccination coverage of patients of physicians from the intervention arm during campaign N increased further, which was not observed in the other three curves, where vaccination coverage levels remained constant. Even though the vaccination coverage of this group of patients was already the highest of the 4, it increased by 6.5 percentage points (62.7%–56.2%), while the other three increased by less than 0.7% points. Its vaccination coverage at the end of the campaign (62.7%) nonetheless remained below the 75% public health target.
Figure 3. Temporal trend of vaccination coverage, by arm and campaign. Reader guide: this figure shows the change over time of the vaccination coverage among the patients eligible for the NHIF vaccination campaign and belonging to the patient list of participating GPs. The black diamonds and the grey circles correspond to the intervention and control arms respectively. Full and empty shapes correspond to the campaigns Y (where the intervention took place) and Y − 1 (the previous one) respectively. The intervention effect is visible in the differences in the temporal evolution of the curves after January 2. The vaccination coverage of the intervention arm during campaign Y increases, while the other three vaccination coverage levels remain stable.
Discussion
Main findings
In this quasi-experimental study, sending a reminder letter to non-vaccinated patients signed by their personal GP at mid-campaign increased the influenza vaccination coverage by 13.1 percentage points.
Strengths and limitations
This work’s first and main limitation is that the participating GPs were not randomised. Moreover, the fact that the vaccine was collected from the pharmacy does not mean it was administered. An alternative to using medico-administrative databases to determine the patient’s vaccination status would have been to interview the patients. This would have been tedious, subject to a social desirability bias and associated with an imprecision concerning the date of vaccination (particularly for the previous year) but it would probably have led to almost identical results insofar as declared and reimbursed consumptions are very well correlated [Citation11]. Questioning doctors was impossible because they do not always know that some of their patients have been vaccinated by another health professional (especially if they have not seen them since their vaccination). Other limitations are that no information about the non-delivery of reminder letters is available and contamination between patients, for example, during discussions in the waiting room, cannot be ruled out. The low vaccination coverage in the control group (1.4%) suggests that contamination was relatively low, but perhaps not null, as the vaccination coverage was only 0.7% the previous year ( and ). Finally, the secretarial work, that is, the printing out of the reminder letters from the lists of unvaccinated patients, was performed by clinical research assistants. The study also covered the cost of the mailings. This last point limits the study’s external validity, as French GPs are not familiar with direct mailings and have no specific funding dedicated to this type of action.
This study also has some strengths. A control group is very similar to the intervention group in terms of pre-intervention characteristics and the validation of the parallel trend assumption suggest a causal effect. We also demonstrated that real-time data between the NHIF and GPs are feasible. There were no medical exclusion criteria such as altered mental status, and no eligible patient refused to participate.
Comparison with existing literature
Our results are consistent with previous interventions in terms of increasing vaccination coverage [Citation12]. This is the first intervention of this kind in France, where only one reminder intervention, in emergency departments and using text messages, has been reported and showed an increase of 12% points [Citation13].
Implications for research and practice
The logical next step to this study is a randomised controlled trial in which postal mail is replaced by email or text message. This appears more feasible in terms of secretarial work and cost but would require the adaptation of some software since not all programmes can do direct mailing campaigns. Our results may support the hypotheses of researchers who wish to carry out such a trial. However, these designers should be aware that the elderly, the main target of the vaccination campaign, may be less comfortable with electronic health communications than with paper mail and that a digital message may have less impact than the handwritten signature and stamp of the doctor. On this last point, two French randomised trials on similar topics show that the involvement of patients’ GPs in encouraging prevention is insufficient. The presentation of pamphlets and posters in GPs’ waiting rooms had no effect on influenza vaccination coverage [Citation14]. Similarly, adding the GP’s signature to that of several institutions on a standard letter inviting patients to take a faecal occult blood test had no impact on patient’s frequency of taking the test in the Paris colorectal screening programme [Citation15].
The medico-administrative databases of the NHIF are primarily used for the financial management of care (reimbursements to insured persons and payments to professionals). For nearly 10 years, they have been used for research purposes during one-off or cohort studies [Citation16]. This study shows that it is possible to use these databases for clinical purposes. Their use for prevention could be developed, particularly in vaccination and cancer screening, where public health objectives are not being met. The universal feature of the NHIF offers the possibility of generalising reminder interventions in France, where these do not currently exist. In both cases, the GPs’ involvement was quite minimal.
Conclusion
In Paris, a reminder letter from each patient’s personal GP inviting unvaccinated patients to get vaccinated at mid-campaign appears to increase influenza vaccination markedly. Nevertheless, this finding needs to be confirmed by a randomised study, and the letter’s effect on the overall vaccination coverage level was not sufficient to meet the desired public health objective.
Acknowledgements
We thank all the GPs, patients and staff of the Caisse Primaire d’Assurance Maladie (CPAM) de Paris who participated in the study.
Disclosure statement
The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
Additional information
Funding
References
- GBD 2017 Influenza Collaborators. Mortality, morbidity, and hospitalisations due to influenza lower respiratory tract infections, 2017: an analysis for the global burden of disease study 2017. Lancet Respir Med. 2019;7(1):69–89. doi: 10.1016/S2213-2600(18)30496-X.
- Putri WCWS, Muscatello DJ, Stockwell MS, et al. Economic burden of seasonal influenza in the United States. Vaccine. 2018;36(27):3960–3966. doi: 10.1016/j.vaccine.2018.05.057.
- Jorgensen P, Mereckiene J, Cotter S, et al. How close are countries of the WHO european region to achieving the goal of vaccinating 75% of key risk groups against influenza? Results from national surveys on seasonal influenza vaccination programmes, 2008/2009 to 2014/2015. Vaccine. 2018;36(4):442–452. doi: 10.1016/j.vaccine.2017.12.019.
- Santé Publique France. Données départementales de couverture vaccinale grippe par saison et dans chaque groupe d’âge (de la saison 2016–2017 à la saison 2020–2021). n.d. [cited 2022 June 23]. Available from: https://www.santepubliquefrance.fr/determinants-de-sante/vaccination/articles/donnees-departementales-de-couverture-vaccinale-grippe-par-saison-et-dans-chaque-groupe-d-age
- Vann JCJ, Jacobson RM, Coyne-Beasley T, et al. Patient reminder and recall interventions to improve immunization rates. Cochrane Database Syst Rev. 2018;1(1):CD003941. doi: 10.1002/14651858.CD003941.pub3.
- Kan T, Zhang J. Factors influencing seasonal influenza vaccination behaviour among elderly people: a systematic review. Public Health. 2018;156:67–78. doi: 10.1016/j.puhe.2017.12.007.
- Schmid P, Rauber D, Betsch C, et al. Barriers of influenza vaccination intention and behavior – a systematic review of influenza vaccine hesitancy, 2005–2016. PLOS One. 2017;12(1):e0170550. doi: 10.1371/journal.pone.0170550.
- Chi R-C, Neuzil KM. The association of sociodemographic factors and patient attitudes on influenza vaccination rates in older persons. Am J Med Sci. 2004;327(3):113–117. doi: 10.1097/00000441-200403000-00001.
- Raudenbush S, Bryk A. Hierarchical linear models. Applications and data analysis methods. London (UK): Sage Publications; 2002.
- Ryan AM, Burgess JF, Dimick JB. Why we should not be indifferent to specification choices for difference-in-differences. Health Serv Res. 2015;50(4):1211–1235. doi: 10.1111/1475-6773.12270.
- Noize P, Bazin F, Dufouil C, et al. Comparison of health insurance claims and patient interviews in assessing drug use: data from the Three-City (3C) study. Pharmacoepidemiol Drug Saf. 2009;18(4):310–319. doi: 10.1002/pds.1717.
- Thomas RE, Lorenzetti DL. Interventions to increase influenza vaccination rates of those 60 years and older in the community. Cochrane Database Syst Rev. 2018;5(5):CD005188. doi: 10.1002/14651858.CD005188.pub4.
- Tubiana S, Labarere J, Levraut J, et al. Effectiveness of a multifaceted informational-based and text message reminders on pneumococcal and influenza vaccinations in hospital emergency departments: a cluster-randomized controlled trial. Vaccines. 2021;9(9):962. doi: 10.3390/vaccines9090962.
- Berkhout C, Willefert-Bouche A, Chazard E, et al. Randomized controlled trial on promoting influenza vaccination in general practice waiting rooms. PLOS One. 2018;13(2):e0192155. doi: 10.1371/journal.pone.0192155.
- Barthe J, Perrodeau E, Gilberg S, et al. Impact of a doctor’s invitation on participation in colorectal cancer screening: a cluster randomized trial. Am J Med. 2015;128(9):1024.e1–1024.e7. doi: 10.1016/j.amjmed.2015.03.026.
- Zins M, Bonenfant S, Carton M, et al. The CONSTANCES cohort: an open epidemiological laboratory. BMC Public Health. 2010;10(1):479. doi: 10.1186/1471-2458-10-479.
Appendix:
Personalized reminder letter by the general practitioner
Medical Offices
Address
75013 PARIS
DR FIRST NAME LAST NAME
Paris, January 2, 2020
Purpose: Protect yourself against seasonal flu
Dear Ms/Dear Mr LAST NAME,
It appears that you have not yet been vaccinated against influenza.
As your general practitioner, I strongly advise you to get this vaccine. Nearly 3 million people have the flu each year and more than 10,000 deaths are attributed to it. The vaccine does not always enable patients to avoid the disease but it considerably reduces the risk of serious and even fatal complications.
In case you have not received the free voucher for this vaccine, I am sending you a new one, signed and stamped, which your pharmacist will complete and allows you to obtain the vaccine free of charge. You can easily choose to be vaccinated by a nurse, a pharmacist, a midwife, or a physician.
Getting vaccinated is very simple and is the first thing to do to protect yourself against influenza. This procedure is vital for your health and protects your family and friends, especially those who are vulnerable, including pregnant women and young children.
I am at your disposition for a conversation on this subject important for your health.
Very truly yours,
Dr First Name Last Name
Your primary care physician