ABSTRACT
We present a 6-year-old boy with unilateral retinoblastoma of the left eye. MRI showed an intraocular tumor that extended into the optic nerve beyond the lamina cribrosa. The affected eye was enucleated and the optic nerve resection margin proved to be free. Following protocol, this patient received six courses of adjuvant systemic chemotherapy. Unfortunately, after 5 months this patient returned with the leptomeningeal spread of the tumor and died quickly thereafter.
Histopathologic analysis of the enucleated eye and distal optic nerve revealed that the postlaminar tumor cells occupied the entire width of the optic nerve, extending all the way up to the pia mater, whereas, more often the tumor invasion is restricted to the center of the optic nerve. This was also visible on the MR images where contrast enhancement occupied the entire nerve width. A resection margin with tumor cells is recognized as a risk factor for metastasis, but perhaps the proximity of tumor cells to the leptomeninges should also be judged with caution as a potential increased risk for metastatic spread.
Introduction
One in every 17,000 children develops retinoblastoma, making it the most prevalent ocular pediatric cancer (Citation1). Survival after retinoblastoma is high with percentages well over 95% in developed countries (Citation2). In high-income countries, only a small percentage of patients develop trilateral retinoblastoma (Citation3, Citation4), metastases (Citation5, Citation6), or a second primary tumor (Citation7), in whom survival is considerably lower.
Several risk factors for developing metastases have been recognized, such as tumor invasion of the optic nerve beyond the lamina cribrosa, massive choroidal invasion, and invasion into the sclera and beyond (Citation8). The differences in the extent of postlaminar tumor invasion were not subdivided into further risk categories, only whether the resection margin was tumor free is considered important when assessing the potential of metastatic leptomeningeal tumor spread (Citation9). However, whether the tumor touches the outer margin of the optic nerve and pia mater potentially giving the tumor access to the CSF space is not often recognized as a separate increased risk factor for developing metastases. Even though previously this phenomenon was acknowledged by Finger et al. (Citation10) this usually constitutes patients with very extensive postlaminar tumor involvement of optic nerve, i.e., on MRI the optic nerve will often not only show contrast enhancement but will also be considerably thickened (Citation11). In this article, we present a patient with postlaminar optic nerve invasion limited in length, but close to the CSF space. The developments of CSF metastases in this case prompted the question whether more attention should be paid to optic nerve invasion width when assessing the risk of developing metastases?
Case report
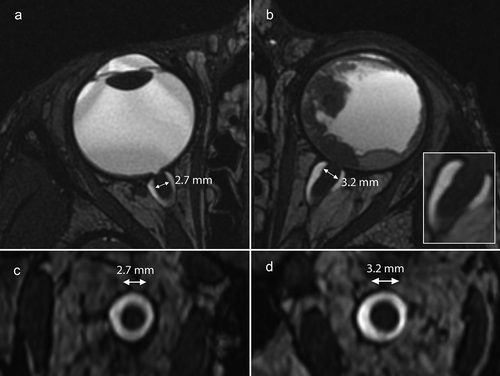
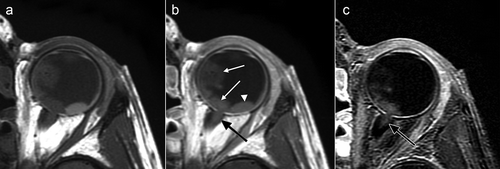
At the age of 6 years, a boy presented with a ‘red eye’ and an eye pressure of 44 mmHg in our clinic. He was clinically diagnosed with sporadic retinoblastoma with tumor cells extending into the anterior eye chamber. MRI showed a large tumor with a maximum diameter of about 20 mm with a (near) total retinal detachment (). Almost the entire retina was invaded by tumor. The distal part of the postlaminar optic nerve showed contrast enhancement in continuity with the intraocular mass suggesting postlaminar optic nerve invasion (length of postlaminar enhancement 2,9 mm).
Figure 1. Precontrast (a), postcontrast (b), and subtraction (c). T1-weighted MR images of the left eye showing contrast enhancement of the tumor (white arrows in b) and distal optic nerve (black arrow in b). Enhancement of the optic nerve (black arrow in b and c) reached 3 mm from the lamina cribrosa into the optic nerve and reached the outer edges (width of the postlaminar enhancement was about 3.5 mm). Temporal from the tumor the retina has detached and shows T1-hyperintense subretinal fluid (white arrowhead in b), but no enhancement (no signal on the subtraction image, c).
With an ICRB group E classification, it was decided to enucleate the involved eye (Citation12). Histopathological analysis resulted in an IRSS stage I: substage N2C2S0 (N2 = postlaminar optic nerve invasion with a clear resection margin and no subarachnoidal invasion, C2 = massive choroidal invasion and S0 = no scleral invasion) (Citation9). There was a slight thickening of the distal optic nerve, which was not considered to be ‘overt’ extraocular disease.
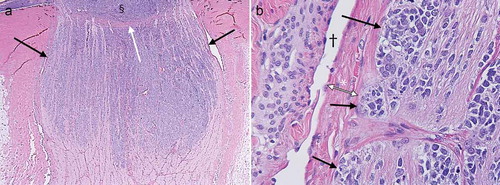
The enucleated specimen included 10 mm of the optic nerve with 3 mm of postlaminar invasion. Furthermore, tumor cells reaching the outer layers of the optic nerve were identified (). Meticulous histopathologic assessment of as many cut sections of the optic nerve as possible did not reveal any invasion of the subarachnoid space. Massive choroidal invasion close to the optic disc was present, which was not separately visible on MRI. Therefore, no further diagnostics were warranted at that time. Albeit a tumor-free resection margin, the postlaminar invasion prompted the decision to administer six courses of prophylactic chemotherapy (carboplatin, etoposide, and vincristine).
Figure 2. Histology slides showing an enlarged distal optic nerve with tumor cells (purple) occupying the entire width of the optic nerve (black arrows in a) corresponding with findings on MRI. Notice the lamina cribrosa bulging outwards (white arrow in a), probably from a high-ocular pressure (44 mmHg), and possibly partly due to mass effect from the intraocular tumor (§ in a). The tumor cells (black arrows in b) reach the outer layers of the optic nerve all the way up to the pia mater (* in b) with the CSF space represented by the void adjacent to the pia († in b).
Unfortunately 5 months after initial diagnosis this patient presented with a headache and vomiting in another hospital where he was treated for dehydration in the intensive care unit. However, there seemed to be no increased intracranial pressure and contrast-enhanced MRI of the brain was unremarkable. The symptoms resolved reasonably quickly and a subsequent brain MRI and lumbar puncture were scheduled to be performed. Quickly after discharge, however, this patient developed acute neurological symptoms and was readmitted to the intensive care unit of this hospital, this time the symptoms included an increased intracranial pressure. Contrast-enhanced MRI of the brain and spinal cord was performed and showed extensive leptomeningeal tumor spread confirmed by the presence of malignant cells in the CSF. In a multidisciplinary setting it was decided to administer high-dose chemotherapy, but, unfortunately a few days later – 6 months after diagnosis – this patient died from the complications of the CSF metastases.
Additional retrospective review of the initial MRI and enucleation specimen was performed. MR images correlated with the findings on histopathology: postlaminar invasion with tumor reaching the outer layers of the optic nerve and a slight thickening of the distal optic nerve on MRI up to 3.2 mm was noticed, versus 2.7 mm of the contralateral normal optic nerve, see .
Discussion
This case prompted the question whether tumor extension up to the outer margin (pia mater) of the postlaminar optic nerve presents similar risk of metastases as a resection margin that is not tumor free. If retinoblastoma metastasizes, it is usually to the CSF space and often these patients had an overt invasion of the optic nerve and beyond (Citation6). Laurent et al. (Citation13) showed that minimally disseminated disease can be detected in the CSF of about one-third of patients with high-risk retinoblastoma. Of these patients, two-third (4/6) had postlaminar optic nerve invasion and one-third (2/6) had a tumor at the resection margin suggesting that CSF dissemination often occurs in patients with optic nerve invasion, but does not require a resection margin with tumor cells.
Even though previously Palma et al. and Dimaras et al. (Citation14, Citation15) have shown that metastatic retinoblastoma can be treated with high-dose chemotherapy and stem cell rescue, chances of surviving metastatic retinoblastoma are slim. After enucleation, the CSF of this patient was not examined for metastatic disease (which was not deemed necessary with an IRSS stage 1) and even though he did receive adjuvant systemic (conventional) chemotherapy this was insufficient to prevent (or treat) CSF metastases (Citation14). There might be a place for the addition of prophylactic intrathecal chemotherapy or higher doses of systemic chemotherapy in patients with an increased risk of CSF dissemination, e.g., after additional testing for minimally disseminated disease in the CSF proved to be positive (Citation16, Citation17).
This case showed slight invasion (lengthwise) of the postlaminar optic nerve, but histopathologically it extended to the outer layers of the optic nerve (as could also be seen on MRI). No tumor cells were present in the CSF space, although it is important to realize that even thorough histopathologic examination will not guarantee perfect detection. This patient developed leptomeningeal metastases despite a tumor-free resection margin, which might indicate that in fact tumor proximity to the CSF space poses an increased risk of developing CSF metastases. Retrospective MRI measurements of the optic nerve showed a relative thickening of the distal optic nerve of the affected eye. Maybe, this should be interpreted as an indication of tumor invasion into the outer layers of the optic nerve. This elicits the question whether more subtle postlaminar optic nerve invasion shows (measurable) optic nerve thickening as well or that differences represent normal variation and measurement errors. Future international studies with enough patients to address the topic of optic nerve thickening and its clinical relevance are warranted.
In an age when retinoblastoma patients are increasingly treated conservatively, clinical decision-making relies more and more on non-invasive biomarkers, such as reverse transcriptase-polymerase chain reaction for GD2 synthase mRNA or cell-free tumor DNA from the CSF to detect minimal residual disease (Citation13,Citation18–21). Detection of metastatic risk factors is often based on radiological imaging, but risk stratification leaves room for improvement (Citation22–24). Perhaps histological extension of the tumor up to the meninges and focal thickening of the nerve without evident subarachnoidal invasion should be considered a similar risk as microscopic transscleral invasion or an optic nerve cut-end with residual tumor cells (both IRSS stage II versus IRSS stage I for resection margin-free postlaminar invasion). Also, perhaps not only optic nerve invasion depth but also distal optic nerve thickness might help detect clinically significant postlaminar optic nerve invasion.
Knowledge about the proximity of the postlaminar tumor invasion to the leptomeninges might help further stratify patients into different categories for the risk to develop metastases. This may enhance risk estimation based on histologic assessment, but more important when detected by imaging it might aid a clinical decision in the absence of histologic assessment as eyes are increasingly treated conservatively. In the future, there might be a role for a sensitive test to rule out minimally disseminated disease in the CSF space in case of this finding. In case of minimally disseminated disease, these patients could probably benefit from a more aggressive intrathecal chemotherapy.
Declaration of interest
The authors report no conflicts of interest. The authors alone are responsible for the content and writing of this article.
References
- Moll AC, Kuik DJ, Bouter LM, Den Otter W, Bezemer PD, Koten JW, Imhof SM, Kuyt BP, Tan KE. Incidence and survival of retinoblastoma in The Netherlands: a register based study 1862-1995. Br J Ophthalmol. 1997;81(7):559–62. doi:10.1136/bjo.81.7.559.
- Dimaras H, Kimani K, Dimba EA, Gronsdahl P, White A, Chan HS, Gallie BL. Retinoblastoma. Lancet. 2012;379(9824):1436–46. doi:10.1016/S0140-6736(11)61137-9.
- de Jong MC, Kors WA, de Graaf P, Castelijns JA, Kivelä T, Moll AC. Trilateral retinoblastoma: a systematic review and meta-analysis. Lancet Oncol. 2014;15(10):1157–67. doi:10.1016/S1470-2045(14)70336-5.
- de Jong MC, Kors WA, de Graaf P, Castelijns JA, Moll AC, Kivelä T. The Incidence of Trilateral Retinoblastoma: A Systematic Review and Meta-Analysis. Am J Ophthalmol. 2015;160(6):1116–26 e5. doi:10.1016/j.ajo.2015.09.009.
- Lu JE, Francis JH, Dunkel IJ, Shields CL, Yu MD, Berry JL, Kogachi K, Skalet AH, Miller AK, Santapuram PR, Daniels AB. Metastases and death rates after primary enucleation of unilateral retinoblastoma in the USA 2007-2017. Br J Ophthalmol. 2019;103(9):1272–77. doi:10.1136/bjophthalmol-2018-312915.
- Hu H, Zhang W, Wang Y, Huang D, Shi J, Li B, Zhang Y, Zhou Y. Characterization, treatment and prognosis of retinoblastoma with central nervous system metastasis. BMC Ophthalmol. 2018;18(1):107. doi:10.1186/s12886-018-0772-8.
- Marees T, Moll AC, Imhof SM, de Boer MR, Ringens PJ, van Leeuwen FE. Risk of second malignancies in survivors of retinoblastoma: more than 40 years of follow-up. J Natl Cancer Inst. 2008;100(24):1771–79. doi:10.1093/jnci/djn394.
- Kaliki S, Shields CL, Rojanaporn D, Al-Dahmash S, McLaughlin JP, Shields JA, Eagle RC Jr. High-risk retinoblastoma based on international classification of retinoblastoma: analysis of 519 enucleated eyes. Ophthalmology. 2013;120(5):997–1003. doi:10.1016/j.ophtha.2012.10.044.
- Chantada G, Doz F, Antoneli CB, Grundy R, Clare Stannard FF, Dunkel IJ, Grabowski E, Leal-Leal C, Rodríguez-Galindo C, Schvartzman E, et al. A proposal for an international retinoblastoma staging system. Pediatr Blood Cancer. 2006;47(6):801–05. doi:10.1002/pbc.20606.
- Finger PT, Harbour JW, Karcioglu ZA. Risk factors for metastasis in retinoblastoma. Surv Ophthalmol. 2002;47(1):1–16. doi:10.1016/S0039-6257(01)00279-X.
- Chawla B, Chaurasia S, Sharma S, Pattebahadur R, Hasan F, Seth R, Kashyap S, Sen S. Magnetic resonance imaging for tumor restaging after chemotherapy in retinoblastoma with optic nerve invasion. Ophthalmic Genet. 2018;39(5):584–88. doi:10.1080/13816810.2018.1502790.
- Shields CL, Mashayekhi A, Au AK, Czyz C, Leahey A, Meadows AT, et al. The international classification of retinoblastoma predicts chemoreduction success. Ophthalmology. 2006;113(12):2276–80. doi:10.1016/j.ophtha.2006.06.018.
- Laurent VE, Sampor C, Solernou V, Rossi J, Gabri M, Eandi-Eberle S, de Davila MT, Alonso DF, Chantada GL. Detection of minimally disseminated disease in the cerebrospinal fluid of children with high-risk retinoblastoma by reverse transcriptase-polymerase chain reaction for GD2 synthase mRNA. Eur J Cancer. 2013;49(13):2892–99. doi:10.1016/j.ejca.2013.04.021.
- Palma J, Sasso DF, Dufort G, Koop K, Sampor C, Diez B, Richard L, Castillo L, Chantada GL. Successful treatment of metastatic retinoblastoma with high-dose chemotherapy and autologous stem cell rescue in South America. Bone Marrow Transplant. 2012;47(4):522–27. doi:10.1038/bmt.2011.108.
- Dimaras H, Heon E, Budning A, Doyle JJ, Halliday W, Gallie BL, Chan HS. Retinoblastoma CSF metastasis cured by multimodality chemotherapy without radiation. Ophthalmic Genet. 2009;30(3):121–26. doi:10.1080/13816810902988780.
- Rodriguez A, Zugbi S, Requejo F, Deu A, Sampor C, Sgroi M, Bosaleh A, Fandiño A, Schaiquevich P, Chantada G. Combined high-dose intra-arterial and intrathecal chemotherapy for the treatment of a case of extraocular retinoblastoma. Pediatr Blood Cancer. 2018;65(12):e27385. doi:10.1002/pbc.27385.
- Chantada GL, Guitter MR, Fandino AC, Raslawski EC, de Davila MT, Vaiani E, Scopinaro MJ. Treatment results in patients with retinoblastoma and invasion to the cut end of the optic nerve. Pediatr Blood Cancer. 2009;52(2):218–22. doi:10.1002/pbc.21735.
- Aschero R, Torbidoni A, Sampor C, Laurent V, Zugbi S, Winter U, Lubieniecki F, Alonso D, Schaiquevich P, Chantada GL. Minimally disseminated disease and outcome in overt orbital retinoblastoma. Pediatr Blood Cancer. 2019;66(6):e27662. doi:10.1002/pbc.27662.
- Dimaras H, Rushlow D, Halliday W, Doyle JJ, Babyn P, Abella EM, Williams J, Héon E, Gallie BL, Chan HS. Using RB1 mutations to assess minimal residual disease in metastatic retinoblastoma. Transl Res. 2010;156(2):91–97. doi:10.1016/j.trsl.2010.05.009.
- Laurent VE, Torbidoni AV, Sampor C, Ottaviani D, Vazquez V, Gabri MR, de Davila MT, Ramirez-Ortiz MA, Alonso CN, Rossi J, Alonso DF. Minimal disseminated disease in nonmetastatic retinoblastoma with high-risk pathologic features and association with disease-free survival. JAMA Ophthalmol. 2016;134(12):1374–79. doi:10.1001/jamaophthalmol.2016.4158.
- Mattox AK, Yan H, Bettegowda C. The potential of cerebrospinal fluid-based liquid biopsy approaches in CNS tumors. Neuro Oncol. 2019;21:1509–18. doi:10.1093/neuonc/noz156.
- de Jong MC, de Graaf P, Brisse HJ, Galluzzi P, Goricke SL, Moll AC, Munier FL, Popovic MB, Moulin AP, Binaghi S, Castelijns JA. The potential of 3T high-resolution magnetic resonance imaging for diagnosis, staging, and follow-up of retinoblastoma. Surv Ophthalmol. 2015;60(4):346–55. doi:10.1016/j.survophthal.2015.01.002.
- de Jong MC, de Graaf P, Noij DP, Goricke S, Maeder P, Galluzzi P, Brisse HJ, Moll AC, Castelijns JA, European Retinoblastoma Imaging Collaboration. Diagnostic performance of magnetic resonance imaging and computed tomography for advanced retinoblastoma: a systematic review and meta-analysis. Ophthalmology. 2014;121(5):1109–18. doi:10.1016/j.ophtha.2013.11.021.
- de Jong MC, van der Meer FJ, Goricke SL, Brisse HJ, Galluzzi P, Maeder P, Cerase, A. Diagnostic accuracy of intraocular tumor size measured with MR imaging in the prediction of postlaminar optic nerve invasion and massive choroidal invasion of retinoblastoma. Radiology. 2016;279(3):817–26. doi:10.1148/radiol.2015151213.