ABSTRACT
Background
Peripherin-2 (PRPH2) is a transmembrane glycoprotein crucial for the morphogenesis and stabilization of the photoreceptor outer segments. Variations in PRPH2 gene are associated with vision-threatening diseases.
Methods
Clinical manifestations and multimodal imaging were presented, as well as treatment history and six-year follow-up. In addition, genetic testing was performed to confirm the diagnosis.
Results
In this report, we present an extremely rare case of choroidal neovascularization (CNV) secondary to pattern dystrophy simulating fundus flavimaculatus (PDSFF). Multimodal imaging showed typical symmetric yellow flecks in posterior pole and choroidal neovascularization requiring timely treatment. A novel nonsense variant of c.552 C > G; p.Y184X in PRPH2 gene was detected. The patient received intravitreal anti-vascular endothelial growth factor (anti-VEGF) treatment and maintained a good vision after six years.
Conclusion
We described a novel PRPH2 variant (Y184X) associated with PDSFF, its multimodal imaging, and long-term prognosis. Intravitreal anti-VEGF treatment can offer excellent visual prognosis in patients with PDSFF-associated CNV.
Introduction
Peripherin-2 (PRPH2), or retinal degeneration slow (RDS), is a transmembrane glycoprotein crucial for the morphogenesis and stabilization of the photoreceptor outer segments. Variations in PRPH2 gene are associated with progressive vision loss such as pattern dystrophy (PD), central areolar choroidal dystrophy, retinitis pigmentosa (RP), and other forms of macular degeneration (MD). Here we present a novel nonsense variant of c.552 C > G (p.Y184X) in PRPH2 gene, resulting in a rare case of choroidal neovascularization (CNV) secondary to pattern dystrophy simulating fundus flavimaculatus (PDSFF). The patient had an excellent response to anti-vascular endothelial growth factor (anti-VEGF) treatment and maintained a good vision after six years.
Case report
A 50-year-old Asian male presented to our department with metamorphopsia in the right eye in 2014. His best corrected visual acuity (BCVA) was 1.0 in Decimal (logMAR: 0.0, Snellen: 20/20) for both eyes. Dilated fundus examination revealed bilateral yellow flecks in the posterior pole. Fundus photograph showed multiple yellow flecks in the macula extending beyond the vascular arcades. The flecks were mostly situated around the retinal vascular arcades, nasal and superior to the optic disc and in the macular area, where flecks were usually largest. A yellow confluent lesion in the fovea was observed, with hemorrhage over it (). The lesions were better defined by autofluorescent (AF) imaging, scattering hyperautofluorescence corresponding to flecks with adjacent zones of hypoautofluorescence corresponding to atrophic areas in the posterior pole (). Fundus fluorescein angiography (FFA) showed small areas of hyperfluorescent flecks bilaterally in the early and late phase of the examination, and unilateral leakage suggesting choroidal neovascularization (CNV) in the right eye (). Indocyanine green angiography (ICGA) showed hypofluorescence bilaterally corresponding to the flecks, and abnormal submacular choroidal vascular network with leakage in the right eye (). Optical coherence tomography (OCT) demonstrated subretinal hyperreflective material with surrounding subretinal fluid in the right eye, confirming the presence of CNV. In the left eye, OCT showed subretinal juxtafoveal hyperreflective vitelliform lesions corresponding to the yellow flecks, interruption of retinal pigment epithelium (RPE), and a small amount of cystoid edema ().
Figure 1. Multimodal imaging of PDSFF at first visit and after six years. (a) Fundus photograph showed multiple yellow flecks in the macula extending beyond the vascular arcades in both eyes. (b) AF showed hyperautofluorescence with adjacent zones of hypoautofluorescence. (c) FFA showed small areas of hyperfluorescent flecks bilaterally, and unilateral leakage suggesting CNV in the right eye. (d) ICGA showed hypofluorescent flecks bilaterally, and abnormal submacular choroidal vascular network with leakage in the right eye. (e) Fundus photograph showed increased number of yellow flecks bilaterally and regression of the foveal lesion without hemorrhage in the right eye. (f) AF showed confluence of the flecks, and granular zones of hypoautofluorescence within the area of hyperautofluorescence. (g) FFA showed increased number of hyperfluorescent flecks bilaterally without leakage. (h) ICGA showed abnormal choroidal vascular network without leakage
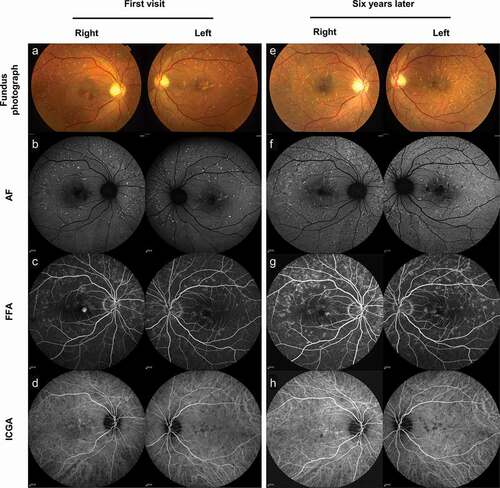
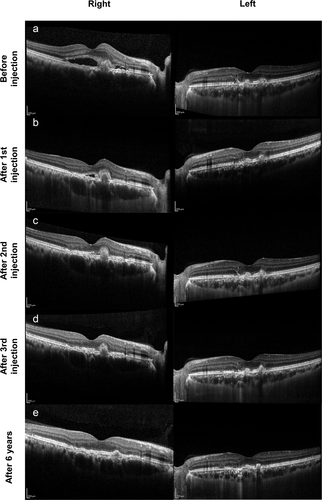
Figure 2. Follow-up of optical coherence tomography (OCT). (a) OCT demonstrated subretinal hyperreflective material with surrounding subretinal fluid in the right eye. In the left eye, OCT showed subretinal juxtafoveal hyperreflective vitelliform lesions corresponding to the yellow flecks, interruption of retinal pigment epithelium (RPE), and small cystoid edema. (b, c, d) OCT followed the changes in the right eye after each intravitreal injection. The left eye remained hyperreflectivity vitelliform lesions. (e) CNV and subretinal fluid in the right eye were completely regressed after 6 years
The patient underwent genetic testing (methods provided in supplementary materials and Supplementary Table S1) and found out a heterozygous variation of c.552 C > G (p.Y184X) in PRPH2 gene (Supplementary Table S2), a nonsense variation which had not been reported previously in literature.
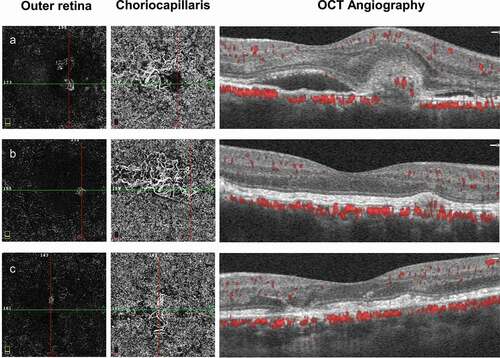
The patient received three intravitreal ranibizumab (Lucentis; Genentech, South San Francisco, CA) injections in the right eye for induction therapy and as-needed treatment afterward (nine injections in total, Supplementary Table S3). OCT was used to monitor the disease and need for further injections, demonstrating fibrosis and diminishment of the CNV lesion and absorption of subretinal fluid (). OCT Angiography (OCTA) was not utilized at the beginning of the treatment, but was utilized a few times between the injections. OCTA confirmed the existence of choroidal neovascularization in the outer retina layer, and enlarged and tangled vessels in the choriocapillaris layer in the right eye. After injection, regression of CNV and subretinal fluid was observed. Enlarged and tangled vessels in the choriocapillaris layer were also seen in the left eye without change overtime (). Six years later, OCT indicated completely regressed subretinal fluid and CNV. The patient maintained visual acuity of 1.0 in Decimal (logMAR: 0.0, Snellen: 20/20) without metamorphopsia in the right eye. Hyperreflective vitelliform lesions and interruption of retina RPE remained the same in the left eye, without macular edema ().
Figure 3. Follow-up of optical coherence tomography angiography (OCTA). (a) OCTA showed choroidal neovascularization in the outer retina layer, and enlarged and tangled vessels in the choriocapillaris layer in the right eye. (b) Regression of CNV in the outer retina layer, but remained in the choriocapillaris layer. (c) Enlarged and tangled vessels in the choriocapillaris layer in the left eye
Six years later, fundus photograph showed increased number of yellow flecks bilaterally and regression of the foveal lesion without hemorrhage in the right eye (). AF showed confluence of the flecks. Granular zones of hypoautofluorescence were seen within the area of hyperautofluorescence, which reflects the beginning of atrophy (). FFA showed increased number of hyperfluorescent flecks bilaterally without leakage (). ICGA showed abnormal submacular choroidal vascular network without leakage ().
Discussion
PRPH2, or RDS, is a transmembrane glycoprotein crucial for the morphogenesis and stabilization of the photoreceptor outer segments. More than 175 pathogenic variants in PRPH2 are associated with retinal dystrophies, with most having an autosomal dominant inheritance pattern (Citation1,Citation2). The variant of c.552 C > G in PRPH2 gene, first reported in our case, is a nonsense variation and was predicted lead to the substitution of the Tyr184 codon with a premature stop codon (p.Y1844X). This might result in either a shorter protein product or degradation of the mRNA by nonsense-mediated decay. The Tyr184 is highly conserved in orthologs and in paralogs of PRPH2, and the variation was predicted to be “likely pathogenic” by American college of medical genetics (ACMG) criteria (Supplementary Table S2) and “pathogenic” by Mutation Taster, Sorting Intolerant From Tolerant (SIFT), PolyPhen-2 and rare exome variant ensemble learner (REVEL), which is absent in multiple normal population database and had not been reported previously in literature. The variant located in the intradiscal (D2) loop of the PRPH2 protein. D2 loop is suggested to be involved in the protein folding and tetrameric subunit assembly formed by peripherin-2 and its nonglycosylated homolog rod outer segment membrane protein-1 (ROM1). These complexes subsequently assemble into covalently linked, larger hetero-octamers and peripherin-2 homo-oligomers for disc membrane packaging and stabilization. Variations in D2 loop may disturb the formation and stability of protein aggregates in the disc membranes, resulting in outer segment (OS) disorganization and clinically significant photoreceptor degeneration (Citation3,Citation4). The relationships between the clinical features and genetic variants are still unclear because the same genetic variant can affect rods and cones differently (Citation1,Citation5).
In our case, we suspect that the c.552 C > G variant caused FDSFF with typical findings, i.e., later onset, relatively good vision, and symmetric yellow flecks in posterior pole. The lesions were viewed more clearly on fundus autofluorescence imaging than other examinations. FFA demonstrated choroidal neovascularization requiring timely treatment. OCT followed up the changes of CNV and subretinal fluid after each injection, and at the same time demonstrated subretinal juxtafoveal hyperreflectivity vitelliform lesions corresponding to the yellow flecks. ICGA indicated that abnormal choroidal vascular network remained even after multiple anti-VEGF injections, but the vision was not affected. During the six-year follow-up, the patient developed increased number and confluence of lesions.
Pattern dystrophy simulating fundus flavimaculatus (PDSFF) is characterized by yellowish flecks over posterior pole and extending beyond the arcades. With the progression of the disease, multiple chorioretinal atrophic patches develop over posterior pole. PDSFF usually progresses slowly, and good vision is maintained until late‑stage of the disease. In case of CNV secondary to PDSFF, the vision could be affected accompanied with metamorphopsia, which is extremely rare, with only three cases in published literature (Citation6–8). To the best of our knowledge, CNV in the setting of pattern dystrophy has not been reported previously in a patient of Asian descent. Previous cases of CNV in the setting of PDSFF have demonstrated similarly excellent acuity outcomes and treatment modalities have included a PDT, ranibizumab, or bevacizumab, or a combination of the treatment modalities ().
Table 1. Treatment and visual outcomes in literature
In summary, we described a new PRPH2 variant (Y184X) associated with PDSFF, its multimodal imaging, and long-term prognosis, which may contribute to further genetic counseling and management of vision-threatening complications due to this variant. Intravitreal anti-VEGF treatment can offer excellent visual prognosis in patients with PDSFF-associated CNV.
Declaration of interest
The authors report no conflicts of interest. The authors alone are responsible for the content and writing of this article.
Supplementary Material
Download PDF (395 KB)Supplementary material
Supplemental data for this article can be accessed on the publisher’s website.
Additional information
Funding
References
- Coco-Martin RM, Sanchez-Tocino HT, Desco C, Usategui-Martín R, Tellería JJ. PRPH2-related retinal diseases: broadening the clinical spectrum and describing a new mutation. Genes. 2020;11(7):773. doi:https://doi.org/10.3390/genes11070773.
- Duncan JL, Talcott KE, Ratnam K, Sundquist SM, Lucero AS, Day S, Zhang Y, Roorda A. Cone structure in retinal degeneration associated with mutations in the peripherin/RDS gene. Invest Ophthalmol Vis Sci. 2011;52(3):1557–66. doi:https://doi.org/10.1167/iovs.10-6549.
- Chakraborty D, Strayve DG, Makia MS, Conley SM, Kakahel M, Al-Ubaidi MR, Naash MI. Novel molecular mechanisms for Prph2-associated pattern dystrophy. FASEB J. 2020;34(1):1211–30. doi:https://doi.org/10.1096/fj.201901888R.
- Zulliger R, Conley SM, Mwoyosvi ML, Al-Ubaidi MR, Naash MI. Oligomerization of Prph2 and Rom1 is essential for photoreceptor outer segment formation. Hum Mol Genet. 2018;27(20):3507–18. doi:https://doi.org/10.1093/hmg/ddy240.
- Boon CJ, van Schooneveld MJ, Den Hollander AI, van Lith-Verhoeven JJ, Zonneveld-Vrieling MN, Theelen T, Cremers FP, Hoyng CB, Klevering BJ. Mutations in the peripherin/RDS gene are an important cause of multifocal pattern dystrophy simulating STGD1/fundus flavimaculatus. Br J Ophthalmol. 2007;91(11):1504–11. doi:https://doi.org/10.1136/bjo.2007.115659.
- Battaglia Parodi M, Da Pozzo S, Ravalico G. Photodynamic therapy for choroidal neovascularization associated with pattern dystrophy. Retina (Philadelphia, Pa). 2003;23(2):171–76. doi:https://doi.org/10.1097/00006982-200304000-00006.
- Nangia P, Shah D, Saurabh K, Roy R. Efficacy of anti-VEGF in the treatment of choroidal neovascular membrane secondary to pattern dystrophy simulating fundus flavimaculatus. GMS Ophthalmol Cases. 2019;9:Doc21. doi:https://doi.org/10.3205/oc000110.
- Lee CS, Leys M, Family A. Affected by novel C213W mutation in PRPH2: long-term follow-up of CNV secondary to pattern dystrophy. Ophthalmic Surg Lasers Imaging Retina. 2020;51(6):354–62. doi:https://doi.org/10.3928/23258160-20200603-06.
