ABSTRACT
Background
Retinal retinoblastoma growth phenotypes can be endophytic, exophytic, diffuse infiltrating or anterior diffuse. Herein, we describe a novel tumor growth pattern in two patients.
Material and Methods
Imaging with spectral-domain optical coherence tomography (SD-OCT)
Results
Both cases were diagnosed with unilateral group D retinoblastoma treated with first-line or bridge intra-arterial chemotherapy (IAC). Case 1 had a new intravitreal/epiretinal relapse 3 months after brachytherapy and intravitreal chemotherapy. SD-OCT showed a disruption of the inner limiting membrane (INL) underneath a parapapillary epiretinal seed. The intravitreal/epiretinal disease completely regressed with intravitreal melphalan. Three months later, an isolated intraretinal growth was documented on SD-OCT at the site of previously INL disruption, which was treated by thermotherapy. He remained disease-free at 1-year follow-up with 0.6 visual acuity. Case 2 was seen 2 months after treatment interruption due to the COVID-19 pandemic. Fundus examination showed a massive intravitreal/epipapillary invasion completely obscuring the papilla. Salvage treatment of this seeing eye consisted of combined intra-arterial and intravitreal melphalan and topotecan injections. An infraclinical papillary regrowth 4 months later was treated with additional IAC. Six months later, enucleation was performed due to an infraclinical papillary relapse with suspicion of intralaminar invasion. Histopathology showed retrolaminar optic nerve invasion with tumor-free surgical section. The child received four cycles of adjuvant chemotherapy and remained disease-free at 1-year follow-up.
Conclusion
Epiretinal/epipapillary vitreous seeding can be the source of a secondary intraretinal/optic nerve head relapse. SD-OCT is instrumental to follow such cases. Enucleation remains the safest option if secondary optic nerve invasion is suspected.
Introduction
Retinoblastoma (rb) initially develops from a red/green cone precursor as an invisible translucent lesion before growing toward the inner or outer retina, or in both directions, characterizing the endophytic, exophytic, or mixed growth, respectively (Citation1). In a minority of cases (less than 3%), the tumor does not form a mass but appears as a thickening of the inner ganglion cell layer (diffuse infiltrating growth), or grow from an ectopic peripheral retinal cell rapidly invading the aqueous humor and/or the vitreous cavity (anterior diffuse growth). Besides these different retinal growth types, four distinct seeding patterns of the disease have been described, namely vitreous, retrohyaloid, subretinal, and aqueous seeding (Citation2).
It is well known that treated rb can recur at the same initial location from an incompletely regressed tumor, or present secondary invasion into a new ocular compartment (secondary seeding) and even exteriorize outside the eye as a result of disease progression. Thus, secondary vitreous seeding can occur following the disruption of the inner limiting membrane of the retina and hyaloid from a growing endophytic retinal tumor, while aqueous invasion can occur via a trans-hyaloidal, trans-oral, trans-ciliary, epiciliary, or supraciliary invasion. On the other hand, progression of a retinal tumor toward the optic nerve head can gradually lead to prelaminar invasion to then adopt an intra-neuronal growth. Such invasion mechanisms have been observed clinically, with the use of spectral-domain optic coherence tomography (SD-OCT), ultrasound biomicroscopy, or by histopathology (Citation3). The purpose of this study is to report two cases documented by SD-OCT of secondary intraretinal and papillary relapse, originating from a previously treated epiretinal and epipapillary vitreous seeding, respectively.
Case description
Case 1
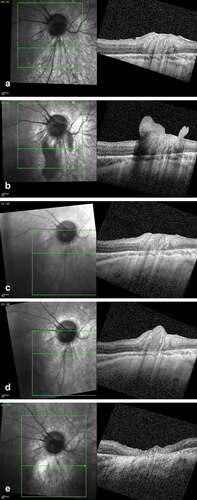
A 6.5-year-old-boy was diagnosed with a unilateral International Intraocular Retinoblastoma Classification (IIRC) (Citation4) group D rb in his right eye, presenting as a large exophytic tumor with subtotal retinal detachment and diffuse subretinal seeding. Initial treatment included three intra-arterial melphalan followed by three combined intra-arterial melphalan–topotecan for retinal and subretinal disease progression and two intravitreal melphalan for secondary vitreous disease. Eight months later, a main tumor relapse resistant to focal treatments was managed with plaque brachytherapy associated to two intravitreal melphalan given prophylactically to treat any potentially dislocated tumor into the vitreous during plaque surgery (). Despite that, dense vitreous and epiretinal relapse occurred 3 months later, managed with four combined intravitreal melphalan and topotecan injections (). Three months later, SD-OCT documented an inferior parapapillary relapse at the site of a previously regressed epiretinal seeding, partially invading the retina (). The lesion was treated to a flat scar with thermotherapy, which needed to be repeated 8 months later due to the detection by SD-OCT of an infraclinical relapse (). The child has remained disease-free at a 1-year follow-up, with a best corrected visual acuity after cataract surgery of 0.6.
Figure 1. SD-OCT imaging of a retinal retinoblastoma relapse following epiretinal seed implantation. (a) Scan over the inferior parapapillary region at the end of the first-line treatment showing an intact retina. (b) Same region at time of the vitreous relapse showing a large epiretinal seed anchored and locally disrupting the inner limiting layer. (c) Complete regression of the epiretinal seed after four combined intravitreal melphalan and topotecan. (d) Isolated intra-retinal relapse 8 months later originating from the site of the inner limiting layer invasion at time of the vitreous seeding. (e) Flat retinal scar post thermotherapy.
Case 2
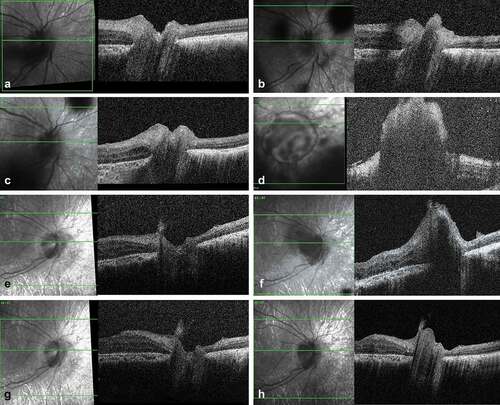
A young boy diagnosed with a unilateral IIRC group D rb in his right eye at the age of 4.5 years was lost to follow-up for 2 months due to COVID-19 travel restrictions while being treated with intravitreal melphalan for isolated massive vitreous and epipapillary relapse, this occurring 1 month after plaque removal for primary tumor recurrence (). Previous treatments included one bridge systemic chemotherapy and three intra-arterial combined with seven intravitreal melphalan given together with focal treatments as first-line therapy for retinal and vitreous disease, followed by two intracameral melphalan for posterior chamber relapse and two intra-arterial combined melphalan–topotecan and three intravitreal melphalan injections for diffuse subretinal and vitreous relapse. When travel could be resumed, fundus examination showed increased vitreous seeding with a 3 mm diameter papillary seed obscuring the optic nerve head, that totally regressed with two combined intra-arterial melphalan–topotecan injections and four combined intravitreal melphalan–topotecan injections (). Four months later, SD-OCT showed a prelaminary localized papillary relapse that was treated with two further intra-arterial combined melphalan–topotecan injections (). Five months later, uneventful lensectomy with intracapsular lens implantation without posterior capsulotomy was performed. Cytopathology analysis of the lensectomy material did not show any active retinoblastoma. One month after the surgery, SD-OCT of the optic nerve head showed an isolated infra-clinical papillary relapse with suspicion of pre-laminar and intralaminar invasion (). As the optic nerve invasion was confirmed on 3.5 T MRI, the eye was enucleated without delay. Histopathology showed a 2.2 mm intralaminar and retrolaminar optic nerve invasion with a 38 µm disease-free margin from the subarachnoid space and tumor-free section. The child was given four cycles of adjuvant chemotherapy for metastasis prophylaxis and has remained disease-free at a 1-year follow-up.
Figure 2. SD-OCT imaging of an optic nerve retinoblastoma relapse following an epipapillary seed implantation. (a) Scan of the optic disc at the end of the first-line treatment showing an intact optic nerve head. (b) Same imaging at time of the vitreous relapse showing an epipapillary seed filling the optic nerve cup. (c) Regression of the epipapillary seed after two intravitreal melphalan. (d) Massive relapse masking the optic nerve 2 months later, with no demarcation line between the anchored seed and the papilla. (e) Regression of the relapse after 2 intraarterial and 4 intravitreal injections with combined topotecan and melphalan. (d) New epipapillary growth with suspicion of prelaminar invasion four months later. (g) Regression of the relapse after two cycles of combined intra-arterial melphalan and topotecan. (h) Infraclinical optic nerve head recurrence 6 months later motivating secondary enucleation.
Discussion
The role of SD-OCT in rb management for the early diagnosis of tumor or relapses, as well as for the treatment monitoring, has been reported by various authors (Citation5–13). Herein, SD-OCT allowed us to describe a novel form of retinal tumor growth carrying features distinct form the classic endophytic, exophytic, or non-exophytic/non-endophytic ones. In both cases, the secondary retinal/papillary growth pattern was the result of a particularly aggressive retinal tumor clones resistant to numerous previous treatments that survived their passage in the inhospitable and highly selective vitreous environment to finally sow the retinal/optic nerve surface on which they anchored with potentially new acquired invasive properties. In case #1, intravitreal chemotherapy achieved complete regression of the epiretinal seeding, but had no effect on the associated secondary intraretinal invasion that required focal treatment to completely regress. Similarly, in case #2, intravitreal chemotherapy decapitated the epipapillary seed, but optic nerve head invasion had already occurred as demonstrated by the prelaminar invasion on SD-OCT. The disease agressivity and resistance was further revealed by the explosive tumor growth both into the vitreous and the optic nerve head during the 2-month treatment interruption due to the COVID-19 pandemics, and later by two further papillary relapses that occurred despite the use of intra-arterial chemotherapy. Previously, our group reported two cases of rb implantation on the optic nerve head successfully treated with intra-arterial chemotherapy, one with suspicion of intralaminar invasion and the other just anchored on the papillary surface without invading it (Citation9). Both patients remained tumor-free with no further treatments at an updated follow-up of 93 and 94 months, respectively. However, considering the above, conservative management of cases with suspicion of epipapillary/prelaminary optic nerve head involvement should only be considered if close monitoring is guaranteed with OCT and 1.5 or 3 T MRI. Otherwise or if OCT shows papillary invasion despite previous given intra-arterial chemotherapy, enucleation should be performed without delay.
Disclosure statement
The authors report no conflicts of interest. The authors alone are responsible for the content and writing of this article.
Additional information
Funding
References
- Xu XL, Singh HP, Wang L, Qi DL, Poulos BK, Abramson DH, Jhanwar SC, Cobrinik D. Rb suppresses human cone-precursor-derived retinoblastoma tumours. Nature. 2014;514(7522):385–88. doi:10.1038/nature13813.
- Munier FL. Classification and management of seeds in retinoblastoma. Ellsworth Lecture Ghent August 24th 2013. Ophthalmic Genet. 2014;35(4):193–207. doi:10.3109/13816810.2014.973045.
- Munier FL, Beck-Popovic M, Chantada GL, Cobrinik D, Kivela TT, Lohmann D, Maeder P, Moll AC, Carcaboso AM, Moulin A, et al. Conservative management of retinoblastoma: challenging orthodoxy without compromising the state of metastatic grace. “Alive, with good vision and no comorbidity”. Prog Retin Eye Res. 2019;73:100764.
- Linn Murphree A. Intraocular retinoblastoma: the case for a new group classification. Ophthalmol Clin North Am. 2005;18(1):41–53, viii. doi:10.1016/j.ohc.2004.11.003.
- Berry JL, Cobrinik D, Kim JW. Detection and intraretinal localization of an ‘invisible’ retinoblastoma using optical coherence tomography. Ocul Oncol Pathol. 2016;2(3):148–52. doi:10.1159/000442167.
- Soliman SE, VandenHoven C, MacKeen LD, Heon E, Gallie BL. Optical coherence tomography-guided decisions in retinoblastoma management. Ophthalmology. 2017;124(6):859–72. doi:10.1016/j.ophtha.2017.01.052.
- Stathopoulos C, Moulin A, Gaillard MC, Beck-Popovic M, Puccinelli F, Munier FL. Conservative treatment of diffuse infiltrating retinoblastoma: optical coherence tomography-assisted diagnosis and follow-up in three consecutive cases. Br J Ophthalmol. 2019;103(6):826–30. doi:10.1136/bjophthalmol-2018-312546.
- Stathopoulos C, Gaillard MC, Puccinelli F, Maeder P, Hadjistilianou D, Beck-Popovic M, Munier FL. Successful conservative treatment of massive choroidal relapse in 2 retinoblastoma patients monitored by ultrasound biomicroscopy and/or spectral domain optic coherence tomography. Ophthalmic Genet. 2018;39(2):242–46. doi:10.1080/13816810.2017.1393826.
- Fabian ID, Puccinelli F, Gaillard MC, Beck-Popovic M, Munier FL. Diagnosis and management of secondary epipapillary retinoblastoma. Br J Ophthalmol. 2017;101(10):1412–18. doi:10.1136/bjophthalmol-2016-309899.
- Gaillard MC, Houghton S, Stathopoulos C, Munier FL. OCT-guided management of subclinical recurrent retinoblastoma. Ophthalmic Genet. 2018;39(3):338–43. doi:10.1080/13816810.2018.1436183.
- Seider MI, Grewal DS, Mruthyunjaya P. Portable optical coherence tomography detection or confirmation of ophthalmoscopically invisible or indeterminate active retinoblastoma. Ophthalmic Surg Lasers Imaging Retina. 2016;47(10):965–68. doi:10.3928/23258160-20161004-12.
- Hasanreisoglu M, Dolz-Marco R, Ferenczy SR, Shields JA, Shields CL. Spectral domain optical coherence tomography reveals hidden fovea beneath extensive vitreous seeding from retinoblastoma. Retina. 2015;35(7):1486–87. doi:10.1097/IAE.0000000000000477.
- Saktanasate J, Vongkulsiri S, Khoo CT. Invisible retinoblastoma. JAMA Ophthalmol. 2015;133(7):e151123. doi:10.1001/jamaophthalmol.2015.1123.
