?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.Abstract
Polymeric forms of ionic liquids have many potential applications because of their ionic nature. Two ionic liquid monomers, 1-(4-vinylbenzyl)-3-methyl imidazolium hexafluorophosphate (VMIH) and 1-(4-vinylbenzyl)-4-(dimethylamino)-pyridinium hexafluorophosphate (VDPH), were synthesized through the quaternization of N-methylimidazole and 4-(dimethylamino) pyridine with 4-vinylbenzylchloride, respectively, and a subsequent anion exchange reaction with potassium hexafluorophosphate. The homopolymers of VMIH, VDPH, and its copolymers with methyl styrene (in various mole ratios) were synthesized by free radical polymerizations at 70 °C using α,α′-azobis(isobutyronitrile) as an initiator. Anionic drug molecule, naproxen (an anti-inflammatory drug), was effectively loaded into these positive charges polymers (PCP) and remained inside of the PCP under acidic environment (pH 2–6.5). The amount of loading of drug was increased with increasing positive charge densities resulting from the increasing number of ionic liquids groups. PCP as a controllable release of anionic drug molecules can be used as an oral delivery drug systems targeting at intestine. This drug can be remained trapped in the polymers when passing through the acidic and neutral environment and be released in intestine, where the environmental pH is close to basic.
Introduction
Intelligent delivery of drugs often needs advanced design of functional materials. Organ-specific drug delivery system is an important aspect of pharmacotherapy [Citation1]. Colon-related diseases such as irritable bowel syndrome, Crohn’s disease, and ulcerative colitis could be more effectively treated when the therapeutic drugs are delivered to the colon tissue [Citation2]. To reduce the nonspecific absorption along the delivery path and to deliver the active form of therapeutic drug to the lower section of the gastrointestinal tract (GIT), oral drugs should be formulated to be acid resistant to first pass through the stomach and to reduce nonspecific adsorption in upper section of the intestine before the drugs can effectively reach colon tissue. Although several approaches have been studied to deliver drugs to colon tissue, their efficiency in the delivery still encounters a lot of challenges. Our response to this challenge was to design a system with a controlled-release function that decreased not only the degradation, but also the nonspecific release of drug molecules in the GIT.
The interest of ionic liquids in polymer chemistry is very recent. Typical research has reported its use as green solvents for the synthesis of homopolymers [Citation3] and block copolymers [Citation4]. The polymer of ionic liquids may be a new class of polymer materials with exceptional properties such as thermal stability, mechanical properties, electrochemical activity, and CO2 absorption ability. This wide scope of applications offers good opportunities for the development of new kinds of ionic liquid-based polymers. Interestingly, functionalization of polymers having some of the characteristics of ionic liquids has been pursued as a way of developing high performance polymer electrolytes [Citation5] and gas separation membranes.
Yoshizawa and coworkers [Citation6] have polymerized novel ionic liquid monomers. Polymerized ionic liquids integrate a macromolecular structure with ionic liquid moieties. The properties of polymerized ionic liquids are strongly associated with both the polymer and ionic liquid structure, where the structure of the cation and anion (e.g. ion type and substitute groups on the cation) is known to determine the physicochemical properties of ionic liquids [Citation7,Citation8]. The effect of the ionic liquid moiety on polymer properties, such as ionic conductivity [Citation9-11], microwave absorption [Citation12], carbon dioxide sorption, and drug delivery has recently been investigated [Citation13].
4-Chloromethylstyrene (CMS), also called 4-vinylbenzylchloride (VBC), is a monomer that can be reacted with a series of reagent to produce polymer with functional groups [Citation14-16].
In this paper, we report a synthesis of positively charged polymers under basic conditions. The high positive charge density of ionic liquid groups generated a strong electrostatic attraction between the surface of polymers and the negative (acidic) groups of the drug molecules. Naproxen was employed as loading compound to examine the capability of controlled release in different environmental pH (2–8). The effects of ionic liquid concentrations, loading concentrations, and solution pH on the loading efficiency were investigated.
Experimental
Materials
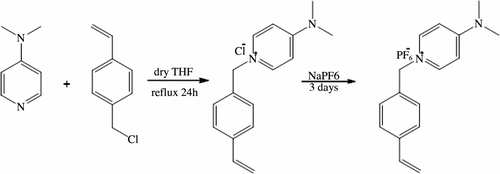
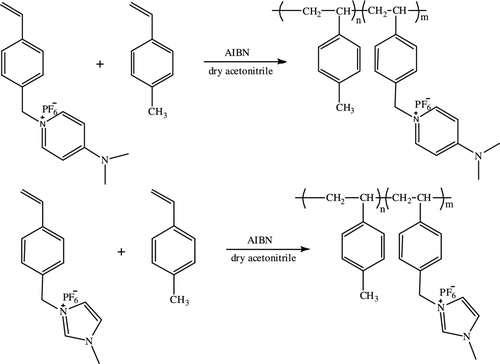
All synthesis procedure were carried out in an inert atmosphere. 4-(dimethylamino)-1-(4-vinylbenzyl) pyridinium hexafluorophosphate and homopolymers were prepared by the method described in the literature (Scheme ) [Citation17]. Acetonitrile, methanol, dichloromethane, tetrahydrofuran, N,N-dimethylamiono pyridine (DMAP), and 4-vinylbenzyl chloride were obtained from Merck. Sodium hexafluorophosphate and sodium tetrafluoroborate were purchased from Aldrich. Lithium bis (trifluoromethanesulfonyl) imide (LiTf2N) was purchased from across. Solvents were dried by standard methods. The initiator of α,α′-azobis(isobutyronitrile) (AIBN) was purified by crystallization from methanol.
Measurements
Infrared spectra were recorded with a 4600 Unicam FT-IR spectrophotometer. 1H NMR spectra were run on a Bruker 400 MHz spectrometer at room temperature. The amount of released naproxen was determined by a Philips PU 8620 UV spectrophotometer at the maximum adsorption of the free drug in aqueous buffered solutions using a 1-cm quartz cell.
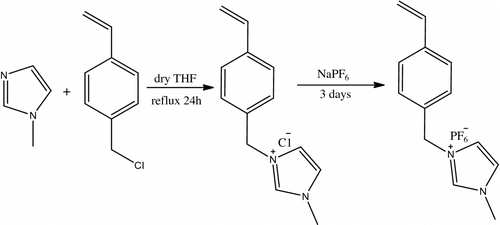
Synthesis of 1-methyl-3-(4-vinylbenzyl) imidazolium chloride
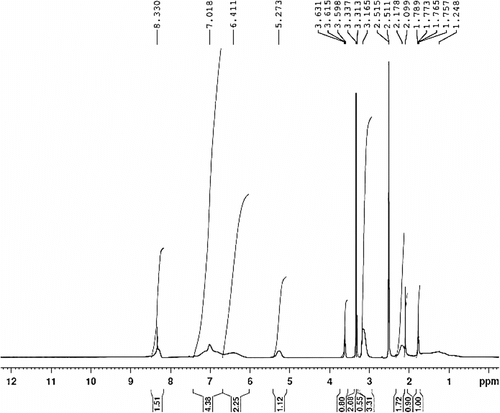
1-methyl-3-(4-vinylbenzyl) imidazolium chloride was synthesized by the method shown in Scheme . 1-Methyl imidazole (10 mmol) and 4-vinylbenzyl chloride (11 mmol) were added to a 50 ml two-necked round bottom flask containing dry tetrahydrofuran (THF) as solvent. After the mixture was heated to reflux with constant stirring for 24 h, the crude product was allowed to cool to room temperature, washed several times with an excess of THF, then dried under vacuum, and stored in refrigerator. 1H NMR (400 MHz, CDCl3 ppm): 9.19(1H, s, Im), 7.79(2H, d, Im), 7.62 (2H, d, Ph), 7.41 (2H, d, Ph), 6.41 (1H, m, CH2=CH), 5.91 (1H, d, CH 2=CH), 5.41 (2H, s, CH2), 5.33 (1H,d, CH 2=CH), 3.82 (3H, s, CH 3N). FT-IR (cm−1): 1628, 1571, 1513, 1449, 1161 (see Figures and ).
Exchange of halide counteranion of monomers to hexafluorophosphate:
In this procedure, a solution of (50 mmol) of monomers in deionized water and an aqueous solution of hexafluorophosphate NaPF6 (55 mmol) in 100 ml flask were mixed for 3 days at room temperature to produce a new monomer. The obtained white solid was washed several times with an excess of deionized water and dried.
Silver nitrate testing indicated that no chloride was present. 19F NMR (400 MHz, DMSO ppm): −72(d, PF 6). FT-IR (cm−1): 1650, 1576, 1405, 1166, 843(P-F). 19F NMR (400 MHz, DMSO ppm): −73(d, PF 6). FT-IR (cm−1): 1650, 1576, 1405, 1166, 843(P-F).
Polymerization
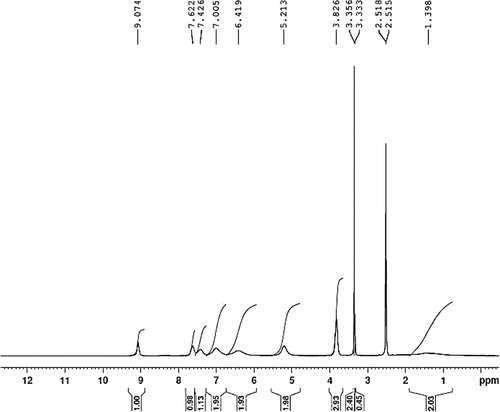
1-methyl-3-(4-vinylbenzyl) imidazolium hexafluorophosphate (0.1 g), AIBN (1 mol%) as a radical initiator, and 20 ml acetonitrile as a solvent were added to a 50 ml round-bottom flask. The system was purged with nitrogen gas. The reaction mixture was stirred at 65 °C for 72 h with a magnetic stirrer. The product was precipitated in cold methanol. Both of the two polymers were further purified by dissolving in acetonitrile, then precipitating in cold methanol, and dried under vacuum to give the purified polymer. 1H NMR (400 MHz, DMSO ppm): 1.10–2.1 (b, CH2–), 3.38 (N–CH 3), 5.2 (Ph-CH 2-Im), 6.4–7.01 (Ph), 7.4–7.6 (CH-Im), 9.07(N–CH–N). FT-IR (cm−1): 3030, 2926, 1616, 1577, 1457, 1165, 834.
Copolymerization
Copolymers synthesized by the method are shown in Scheme . The copolymerization of 4-(dimethylamino)-1-(4-vinylbenzyl) pyridinium hexafluorophosphate and 1-methyl-3-(4-vinylbenzyl) imidazolium hexafluorophosphate with different molar ratios of methyl styrene was done. Monomers dissolved in 15 mL of acetonitrile and were mixed with AIBN (1% molar) as a radical initiator, in a Pyrex glass ampoule. The ampoule was degassed, sealed under vacuum, and maintained at 70 °C in an oil bath, with stirring for about 72 h. Then the solutions were poured from ampoules into cooled methanol. The precipitates were collected and washed with methanol and dried under vacuum to give copolymers (P3…P8). The conditions for the production of copolymers are summarized in Table .
Table 1. The molar ratio of monomers.
1H NMR (DMSO, ppm) for P3, P4 and P5: 1.2–1.8 (CH 2 CH), 3.31(2 × CH 3), 3.63 (CH 3-Ph), 5.27 (CH 2-Ph), 6.4–7.2 (Ar CH), 8.3 (Ar 2 × CH–N). FT-IR (KBr, cm–1): 3027, 2925, 1652, 1573, 1440, 1166, 841.
1H NMR (DMSO, ppm) for P6, P7, and P8: 1.2–1.9 (CH 2 CH), 3.31 (N–CH 3), 3.82 (CH 3-Ph), 5.21 (CH 2-Ph), 6.4–7 (Ar CH), 7.42, 7.62 (CH-Im), 9.07 (N–CH–N). FT-IR (KBr, cm–1): 3171, 3124, 2925, 1614, 1577, 1455, 1164, 838.
Drug loading in polymers
0.1 g of each polymer was dispersed with stirring in 25 ml of solution containing 0.1 g of naproxen in acetone and mixed for 3 days at room temperature to suck up the total amount of the drug solution. The obtained yellow solid was washed several times with an excess of CH2Cl2 and dried.
Determination of amount of drug entrapped
Polymer-drug adduct (20 mg) was dispersed in 20 mL of pH 8 buffered solution. The reaction mixture was maintained at 37 °C. After 24 h, the hydrolysis solution was sampled and neutralized with 1 M HCl and the solvent was evaporated in vacuo. The resulting crude product was treated with 10 mL of CH2Cl2 and heated. The suspension was then filtered and the CH2Cl2 solution tested using UV–vis spectroscopy. The difference between the amount of drug initially employed and the drug content in the washings is taken as an indication of the amount of drug entrapped.
In vitro release studies
Polymer-drug adduct (50 mg) was poured into 5 mL of different environmental pH (from 2 to 8). The mixture was introduced into a cellophane membrane dialysis bag. The bag was closed and transferred to a flask containing 25 mL of the same solution maintained at 37 °C. The external solution was continuously stirred and 3 mL samples were removed at selected intervals. The volume removed was replaced with fresh tampon solutions. Triplicate samples were used. The sample of hydrolyzate was analyzed by UV spectrophotometer, and the quantity of naproxen was determined using a standard calibration curve obtained under the same conditions.
Result and discussion
For preparing polymers and copolymers, first monomers were synthesized. N-methylimidazole and 4-(dimethylamino) pyridine were reacted with 4-vinylbenzyl chloride to produce 1-(4-vinylbenzyl)-3-methyl imidazolium hexafluorophosphate (VMIH) or 1-(4-vinylbenzyl)-4-(dimethylamino)-pyridinium hexafluorophosphate (VDPH) (Schemes and ). These monomers contain a chloride anion. Then the anion-exchange reactions were done with hexafluorophosphate anion, which has hydrophobic properties. The anion-exchange reaction was the key step. Usually, chloride ions may not be fully replaced. To promote the exchange reaction, excess potassium hexafluorophosphate was used in this work. The unreacted 4-(dimethylamino)-1-(4-vinylbenzyl) pyridinium chloride, 1-methyl-3-(4-vinylbenzyl) imidazolium chloride, and the excessive KPF6 were washed away by a large amount of water after the reaction. The obtained ionic liquid was tested with silver nitrate. No silver chloride precipitate was observed, and this indicated that the ionic liquids were free of chloride anion. The melting point was 89 °C for VMIH and 108 °C for VDPH.
The anion structure influenced the solubility of the polymer. Anion like hexafluorophosphate is hydrophobic, but the chloride anion is hydrophilic. Therefore, polymers that have hydrophilic anions such as Cl− are more soluble in polar solvents. In contrast, hydrophobic polymers are insoluble in water and ethanol, but soluble in acetone.
The average composition of the copolymer samples was determined from the corresponding 1H NMR spectroscopic analysis. NMR spectroscopic analysis has been established as a powerful tool for the determination of copolymer composition because of its simplicity, rapidity, and sensitivity. The assignment of the resonance peaks in the 1H NMR spectrum leads to the accurate evaluation of the content of each kind of monomeric unit incorporated into the copolymer chains. Table gives the mole composition of copolymers.
For example, for copolymer 3 (1:1), m and n are:
For copolymer 6 (1:1), m and n are:
Effects of the positive charge density on the release rate
Oral delivery of drugs is the preferred route of administration because it offers advantages over injection, which is the presently accepted route of therapeutic protein administration. To achieve successful colonic delivery, a drug needs to be protected from absorption of the environment of the upper GIT and then be abruptly released into the proximal colon, which is considered the optimum site for colon-targeted delivery of drugs.
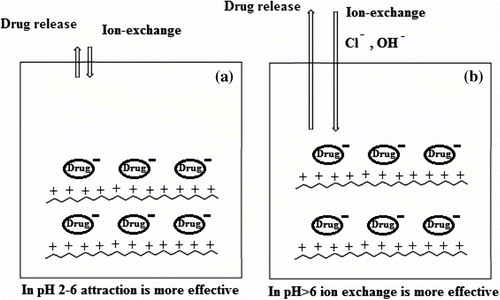
The degrees of hydrolysis of the network polymer containing naproxen in different environmental pH (2–8) at 37 °C are given in Table . As shown in Table , hydrolysis rate decreased at pH 2–6, but increased at pH > 7. The release of naproxen from polymers may be dependent on important mechanism: ion exchange. As shown in Scheme , positively charged polymers generated a strong electrostatic attraction between the surface of polymers and the negative (acidic) groups of the drug molecules. The negative charges of drug molecules could have strong attraction when placed with polymers in a weak acid and neutral solution, because in these pH hydrolysis rate decreased. In other hand, when the pH of the solution was changed to be basic, by increasing ionic strength in pH value (pH > 6), diffusion of the hydrolyzing agents on polymer is increased and the hydrolysis rate increased.
Table 2. Percent of naproxen release.
Conclusions
In this work, we synthesized new monomers and polymers of styrene with an ionic liquid in the side chain. New controllable drug release system has been designed by incorporating positive charges in the framework of polymers, so that anionic molecules can be efficiently adsorbed inside of the polymers with minimal release under week acidic pH value. By increasing ionic strength in pH value (pH > 6), diffusion of the hydrolyzing agents on polymer is increased and the hydrolysis rate increased. Based on the difference in hydrolysis rate at week acidic pH value and pH ≈ 7, these PCP appear to be candidates for colon-specific anionic drug delivery.
Correction Statement
This article has been republished with minor changes. These changes do not impact the academic content of the article.
Acknowledgments
The office of research vice chancellor Azarbaijan University of Tarbiat Moallem has supported this work.
References
- Van den Mooter G Kinget R Oral colon-specific drug delivery: a review Drug Delivery 1995 2 81 93
- MacFarlane GT Cummings JH MacFarlane S Gibson GR Influence of retention time on degradation of pancreatic enzymes by human colonic bacteria grown in a 3-stage continuous culture system Journal of Applied Bacteriology 1989 67 521 527
- Carmichael AJ Haddleton DM Bon SAF Seddon KR Copper(1) mediated living radical polymerisation in an ionic liquid Chemical Communications 2000 1237 1238
- Zhang H Hong K Mays JW Synthesis of block copolymers of styrene and methyl methacrylate by conventional free radical polymerization in room temperature ionic liquid Macromolecules 2002 35 5738 5741
- Yoshizawa M Ohno H Synthesis of molten salt-type polymer brush and effect of brush structure on the ionic conductivity Electrochimica Acta 2001 46 1723 1728
- Yoshizawa M Ogihura W Ohno H Novel polymer electrolytes prepared by copolymerization of ionic liquid monomers Polymers for Advanced Technologies 2002 13 589 594
- Brennecke JF Maginn EJ Ionic liquids: Innovative fluids for chemical processing AIChE Journal 2001 47 2384 2389
- Wasserscheid P Welton T Ionic liquids in synthesis Wiley-VCH Weinheim 2003
- Yoshizawa M Ohno H Molecular brush having molten salt domain for fast ion conduction Chemistry Letters 1999 28 889 890
- Ohno H Ito K Room-temperature molten salt polymers as a matrix for fast ion conduction Chemistry Letters 1998 27751
- Matsumi N Sugai K Miyake M Ohno H Polymerized ionic liquid via hydroboration polymerization as single ion conductive polymer electrolytes Macromolecules 2006 39 6924 6927
- Tang J Radosz M Shen Y Poly(ionic liquid)s as transparent microwave absorbing materials Macromolecules 2008 41 493 496
- Tang J Tang H Sun W Radosz M Shen Y Poly(ionic liquid)s as new materials for CO2 absorption Journal of Polymer Science Part A: Polymer Chemistry 2005 43 5477 5489
- Assadi MG Hosseinzadeh F Synthesis of some silyl mono- and polystyrenes with new properties Designed Monomers Polymers 2010 13 181 191
- Mahkam M Assadi MG Golipour N pH-sensitive hydrogel containing acetaminophen silyl ethers for colon-specific drug delivery Designed Monomers Polymers 2006 9 607 615
- Assadi MG Golipour N Synthesis and characterization of new monomer and polymers of hindered silyl styrene Designed Monomers Polymers 2007 10 79 89
- Assadi MG Herizchi R Ranjbar F Synthesis and characterization of some quaternized styrene monomers and polymers Designed Monomers Polymers 2011 14 303 313