Abstract
Based on sethnomedicine, Murraya koenigii. (L.) Spreng. is used as a stimulant, antidysentery, and for the management of diabetes mellitus. Twelve carbazole alkaloids were isolated from the stem, seed, and leaf of the plant growing in Nigeria and Sri Lanka. The methanol extracts were devoid of hypoglycemic activity, and some isolates decreased insulin secretion when they were subjected to both in vivo. and in vitro. (insulin secretion from INS-1 cells) antidiabetic tests. The cytotoxicity of the leaf and stem methanol extracts determined by the brine shrimp lethality bioassay were LC50 61.5 and 14.5 µg/ml, respectively. These extracts caused CNS depression in albino mice at the dose levels of 25–400 mg/kg. Also, they had an IC50 of 34.0 and 35.0 µg/ml at 24 h, respectively, against trichomonas. These results confirmed the use of the plant as an antidysentery caused by trichomonas but refute the antidiabetic and stimulant ethnomedical claims for the plant. The differences observed in their alkaloidal composition suggested probable influence of geographical location on the elaboration of carbazole alkaloids in the plant and differences in the localization of carbazole alkaloids in the plant parts.
Introduction
Murraya koenigii. (L.) Spreng. is a rutaceous, small tree grown widely in the tropics and subtropics including Nigeria (Stone, Citation1985). Ethnomedical uses include tonic, stimulant, bitters, stomachic, carminative, antidysentery, antidiarrhea, antiemetic, antiperiodic, anticough, febrifuge, and as an application over eruptions, bruises, and bites of venomous animals [Chakraborty et al., Citation1965; Das et al., Citation1965; Dastur, Citation1970; Gupta & Nigam, Citation1971; Atta-ur-Rahman et al., Citation1988; Gill, 1994 (personal communication)]. In Nigeria and Asia, it is commonly used as an antidiabetic and flavorant [Gupta & Nigam, Citation1971; Macleod & Pieris, Citation1982; Fiebig et al., Citation1985; Gill, 1994 (personal communication); Yadav et al., Citation2002]. Antimicrobial (Das et al., Citation1965; Chakrabarty et al., Citation1997; Nutan et al., Citation1998), cytotoxicity (Fiebig et al., Citation1985; Chakrabarty et al., Citation1997; Nutan et al., Citation1998), hypotensive, antitreponemal, spasmodic (Bhakuni et al., Citation1969; Kong et al., Citation1986), anti-inflammatory, antioxidant (Ramsewak et al., Citation1999; Tachibana et al., Citation2001), and anti-trypanocidal (Adewunmi et al., Citation2001) activities have been reported. The antidiabetic reports of the leaf have been controversial (Naraya & Sastry, Citation1975; Khan et al., Citation1995; Kar et al., Citation1999; Bawden et al., Citation2002; Yadav et al., Citation2002).
Diabetes mellitus, a life-threatening endocrine problem, is on the increase all over the world, especially in Nigeria. Until recently, it was regarded as the disease of the affluent in Africa (Karam, Citation1992; Amos et al., Citation1997). The on-going search for natural antidiabetic remedies is concentrated on plants used as such in ethnomedicine of various countries. We therefore investigated the antidiabetic, stimulant, and antidysentery claims of this medicinal plant and established the cytotoxicity of the leaf and stem MeOH extracts to the nauplii of Artemia salina. Leach. (brine shrimp).
Numerous mono and dimeric carbazole alkaloids have been isolated from the plant (Gupta & Nigam, Citation1971; Fiebig et al., Citation1985; Atta-ur-Rahman et al., Citation1988; Hegnauer, Citation1990; Chakraborty & Roy, Citation1991; Ito et al., Citation1993; Reisch et al., Citation1992Citation1994aCitationb; Bhattacharyya et al., Citation1994; Adebajo, Citation1997; Chakrabarty et al., Citation1997; Nutan et al., Citation1998). Report of geographical variation in the constituents of the leaf essential oils (Onayade & Adebajo, Citation2000) led us to compare the constituents of the stem and seeds of the plants growing in two locations in Sri Lanka (Reisch et al., Citation1994aCitationb) and Nigeria.
Materials and Methods
Instrumentation and identification of compounds
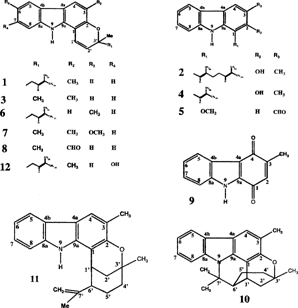
UVλmax nm (log ε.): MeOH. IR: KBr. MS: EI 70 eV; CI NH3. 1H- (200 MHz) and 13C- (50 MHz) NMR: CDCl3, (CDCl3 + CD3OD for 9, 10); TMS: internal standard. The values are given in δ (ppm) relative to TMS. CC: silica gel G60 (mesh 0.063–0.200 mm); PTLC: silica gel 60 F254 (0.25, 1 mm); m.p.: uncorrected. The compounds isolated were identified and confirmed by comparing their spectral data and m.p. with those in the literature for 1, 3, 5, 9, 10, 11 (Narasimhan et al., Citation1968; Furukawa et al., Citation1985); 2, 4 (Reisch et al., Citation1994b); 6 (Joshi et al., Citation1970; Kureel et al., Citation1970); 7 (Narasimhan et al., Citation1968); 8 (Chakraborty et al., Citation1971; Adesina et al., Citation1988); and 12 (Wu, Citation1991), and with some authentic samples. The OH and NH signals in the 1H NMR spectra were confirmed by D2O exchange. The carbon assignments were made from APT and 1H-13C (hetero-COSY) and the proton from 1H-1H (homo-COSY) correlations and decoupling experiments.
Plant material
The stem and seeds of M. koenigii. plants already identified in Nikaweratiya (MSPB 84C) and Marassana (MSPB 84D), Sri Lanka, were collected in May and September 1992, respectively (Reisch et al., Citation1994aCitationb). M. koenigii. plants growing on the campuses of University of Benin, Benin City, and Obafemi Awolowo University, Ile-Ife, Nigeria, were authenticated by Prof. S.L. Gill, and voucher specimen no. FHI 105244 was deposited in the herbarium of the Forestry Research Institute of Nigeria, Ibadan. Their stems and leaves were collected in May 1993 and June–July, 2002.
Isolation of constituents
A 2.43 g CHCl3 extract of the Marassana seed was subjected to repeated CC and PTLC following the method of Reisch et al. (Citation1994aCitationb) to give 1 (404.7 mg), 6 (11.3 mg), 3 (10.4 mg) and 7 (183.5 mg). About 1.5 g of the CHCl3 extract (12.1 g) of the Nikaweratiya stem was similarly purified to give 1 (53.6 mg), 2 (31.8 mg), 3 (8.2 mg), 4 (13.5 mg) and 5 (89.4 mg). A 2.9 g CHCl3 extract of the Nigerian stem (MKSC) was likewise purified by repeated CC and PTLC to give sequentially 1 (223.1 mg), 2 (8.0 mg), 3 (36.2 mg), 5 (51.7 mg), 9 (20.9 mg), and a 11.0 mg mixture further purified by repeated PTLC (petroleum ether-CHCl3, 5:5) to give 8 (6.9 mg). The 35 g CHCl3 Nigerian leaf extract was successively partitioned on VLC (220 g) using petroleum ether, CHCl3, EtOAc, and MeOH. The combined carbazole-rich CHCl3 and EtOAc fractions were bulked (17.6 g) and chromatographed on a column eluted with CHCl3 and EtOAc gradient mixtures. The 25 ml fractions collected were further purified by repeated CC and PTLC to give 1 (209.4 mg), 3 (30.1 mg), 6 (6.2 mg), 10 (33.1 mg), 11 (18.0 mg), and 12 (5.1 mg).
Animals
Swiss albino mice, weighing 20–24 g, and Swiss Wistar rats, weighing 230–400 g (either sex), were purchased from the Animal House, Department of Pharmacology, Faculty of Pharmacy (O.A.U.), Ile-Ife, Nigeria. The animals were housed and maintained under standard environmental conditions and were fed with rodent feed pellets (Bendel Feeds, Nigeria) with free access to water.
Antidiabetic testing
Preparation of extracts and pure isolates for antidiabetic testing
Air-dried, powdered leaf and stem of M. koenigii. were cold-extracted with MeOH for 3 × 48 h and concentrated in vacuo. to yield MKLM (15.2%) and MKSM (12.1%), respectively. The stem was similarly extracted with CHCl3 to give MKSC extract. These extracts were used for the in vivo. antidiabetic testing. Ten milligrams each of MKSC and the pure isolates 1–5, 7, 9 was dissolved in 1000 µl of DMSO on each day for the in vitro. antidiabetic tests. The final concentration of DMSO did not exceed 0.05% in all the experiments and did not show any effect even when the concentration was doubled (Blumentrath et al., Citation2001).
Glucose-lowering effects
Only MKLM and MKSM extracts were investigated for hypoglycemic activity in vivo. against normo- and alloxan-induced hyperglycemic rats following the method of Akhtar et al. (Citation2002). Rats were divided into 4 groups: normoglycemic, alloxan-induced hyperglycemic, positive (glibenclamide 5 mg/kg), and negative controls (normal saline). Two doses, 1 and 2 g/kg extract, were administered orally and blood glucose determined with the aid of a glucometer (Glucotrend®, Roche Diagnostic/GMBH, Mahnheim, Germany) at 0, 0.5, 1, 2, and 4 h.
Cell culture
INS-1 cells generously provided by Dr. C. Wollheim (Geneva, Switzerland) were grown in 24-wells for 5–6 days (half confluence: 1 to 2 × 106 cells/ml) in RPM1 medium supplemented with 10% (v/v) fetal calf serum, 100 U of penicillin/ml, and 0.1 mg of streptomycin/ml. Prior to the experiment, INS-1 cells in multiwells were washed three-times and then incubated in Krebs-Ringer buffer containing 10 mM HEPES and 0.5% bovine serum albumin (KRBH) at 5.6 mM glucose for 90 min (Bozdag et al., Citation2000; Blumentrath et al., Citation2001).
Insulin release
To measure insulin secretion, half-confluent cells in microwells were incubated for 90 min at 37°C in Krebs-Ringer buffer containing 10 mM HEPES and 0.5% bovine serum albumin. Insulin released into the medium was assayed with a radioimmunoassay using rat insulin as standard, (mono-125I-Tyr A 14)-porcine insulin and anti-insulin antibodies. Each compound had been checked for noninterference with the insulin radioimmunoassay. Glucose-mediated insulin release from the INS-1 cells in multiwells at glucose concentrations of 3.0 and 5.6 mM were used as standards. Insulin secretion at 5.6 mM glucose concentration was taken as 100%. Rat insulin was from Novo Nordisk (Bagsvaerd, Denmark), (mono-125I-Tyr A 14)-porcine insulin from Hoechst (Frankfurt, Germany), and anti-insulin antibodies from Linco (St. Louis MO, USA). The data were corrected for the effects of the DMSO used as part of the solvent (Bozdag et al., Citation2000; Blumentrath et al., Citation2001).
Effect on general locomotory behavior
The rearing behavioral profile of albino mice was assessed singly in a Plexiglass cage (45 × 25 × 25 cm). Mice were randomly divided into 6 groups of 5 animals each. Group 1 received normal saline (10 ml/kg, i.p.) as control, and groups 2–6 received MKLM and MKSM extracts (25, 50, 100, 200, 400 mg/kg, i.p.). Observations and scoring were made by counting the number of rearing for a period of 30 min in each group.
Phenobarbitone-induced sleeping time (PIST)
The effects of MKLM and MKSM extracts were examined on PIST on 7 groups of mice. Group 1 received normal saline (10 ml/kg, i.p.), groups 2–5 received MKLM and MKSM extracts (50, 100, 200, and 400 mg/kg, i.p.), group 6 received diazepam (2 mg/kg, i.p.) as positive control, while group 7 received the combination of the extract MKLM (400 mg/kg) or MKSM (200 mg/kg) + flumezanil (2 mg/kg, i.p.). Each animal in the groups was challenged with phenobarbitone (10 mg/kg) 30 min postadminisration of test agents. The time between loss and recovery of righting reflex was an indication for measuring the onset and duration of sleeping time (Assis et al., Citation2001).
Antitrichomonas test
Trichomonas gallinae. (Rivolta) Stabler isolated from the pigeon was dropped into a test tube of normal saline. The solution was distributed into test tubes of Ringer's egg-serum culture for enteric protozoan and incubated at 37°C for growth. Stock solutions of MKLM, MKSM extracts and metronidazole (Flagyl, Aventis Pharma, USA) in DMSO at the concentration of 20, 20, and 8 mg/ml, respectively, were made. Serial dilutions to 0, 6.25, 12.5, 25, 50, 100, 200, 400, 800, 1000 µg/ml for the extracts and 0, 2.5, 5.0, 10 µg/ml for metronidazole with the fluid nutrient solution were used as the test agents. Fifty microliters of each test agent and 150 µl of the nutrient solution were pipetted into the microwells and incubated in the steam incubator at 37°C for 24 and 48 h. The number of organisms per milliliter in each well for 0, 24, and 48 h were counted using a microscope. The experiments were done in triplicates (Narcasi & Secor, Citation1996).
Brine shrimp lethality test
The extracts MKLM and MKSM were tested for their lethality against nauplii of Artemisia salina. Leach using a standard method (Nutan et al., Citation1998).
Statistics
Statistical evaluation of whole means were carried out by one-way analysis-of-variance (ANOVA), followed by Duncan new multiple range test and Student's t.-test. Statistical significance was accepted at p ≤ 0.05.
Results
We report here the isolation and characterization of mahanimbine (1), mahanimbilol (2), girinimbine (3), girinimbilol (4), and murrayanine (5) from the stem of M. koenigii. growing in Nikaweratiya and 1, 3, isomahanimbine (6), and koenimbine (7) from the seed of the plant growing in Marassana. Also, 1, 2, 3, 5, murrayacine (8), and murrayaquinone-A (9) were isolated from the stem of the plant growing in Nigeria, while the leaf gave 1, 3, 6, murrayazoline (10), murrayazolidine (11), and mahanine (12). The distribution of these alkaloids among these parts is presented in . The leaf and stem MeOH extracts did not reduce the blood sugar level in the normo- and alloxan-induced hyperglycemic rats. The results of the insulin release activity of the extract and isolates (in vitro.) are presented in . The leaf (MKLM) and stem (MKSM) methanol extracts had LC50 61.5 and 14.5 and LC90 956.2 and 150.5 µg/ml values, respectively, against brine shrimp (). The MKLM and MKSM extracts also had IC50 values of 34.0, 35.0 and IC90 values of 48.0, 130.0 µg/ml, respectively, at 24 h, and IC50 values of 34.5, 36.0 and IC90 values of 48.0, 50.0 µg/ml, respectively, at 48 h against trichomonas (Tables and ). The activities of the extracts (25–400 mg/kg) on the behavior and sleep showed a CNS depressant effect, reducing the novelty induced rearing, a locomotory activity, and increasing sleeping time in mice (Tables and ). The unreported carbon data of 6, 10–11 are included in Tables and , the J. values of the protons of the ring A for 6, 9–11 in , and the side chains of these pyranocarbazoles are given in .
Table 1.. Distribution of the isolated carbazole alkaloids among three population of Murraya koenigii..
Table 2.. Effects of extract and isolated carbazole alkaloids of Murraya koenigii. on glucose-mediated insulin release from INS-1 cells.
Table 3.. Selective indices of the trichomonas activity of Murraya koenigii. leaf and stem methanol extracts.
Table 4.. The antitrichomonas activity of the leaf and stem methanol extracts.
Table 5.. Effects of Murraya koenigii. leaf and stem methanol extracts on novelty induced rearing in mice.
Table 6.. Effects of Murraya koenigii. leaf and stem methanol extracts on phenobarbitone-induced sleeping time in mice.
Table 7.. 1H (200 MHz) and 13C (50 MHz) NMR assignments of the main carbazole skeletons of 6, 9–11 (δ from TMS).
Table 8.. 1H (200 MHz) and 13C (50 MHz) NMR assignments of the side chains of the carbazoles 6, 10, 11 (δ from TMS).
Discussion
The MeOH extracts of the leaf and stem and the CHCl3 extract of the stem (MKSC) of M. koenigii. were inactive in reducing blood sugar levels in normo- and alloxan-induced hyperglycemic rats. Feeding of rats with 10% and 15% diet of the dried leaf, leaf ash, and 95% EtOH extract has been shown to be ineffective in lowering blood sugar in normal and glucose induced hyperglycemic rats of M. koenigii. (Kar et al., Citation1999; Yadav et al., Citation2002). Antihyperglycemic activity without any statistical significance has been reported on drug-induced mild and moderate diabetic rats fed with 10–15% diet of the leaf (Yadav et al., Citation2002). Conversely, hypoglycemic effect of the aqueous extracts of the leaf was reported on normal and alloxan-induced diabetic dogs (Naraya & Sastry, Citation1975). It is therefore possible that water-soluble compounds that are insoluble in organic solvents present in the plant may be responsible for the activity. However, consistent hypoglycemic action has been reported in vitro. (Khan et al., Citation1995; Bawden et al., Citation2002).
Because leaf hexane extracts have been shown to have significant inhibitory activity on the α-amylase enzyme (Bawden et al., Citation2002), we investigated the activity of the isolates obtained from the CHCl3 extracts of the leaf, stem, and seeds for specific insulin release ability in vitro.. They all decreased insulin release from the INS-1 cells compared to control (). The insulin released by MKSC, 1 and 4 at 100 µl were about double that of 10 µl, showing that they were able to stimulate insulin secretion at higher dose. There was no significant difference observed in the release of insulin at the two doses by 2, 3, 5, and 7, which meant that they did not have a dose-response relationship. Compounds 5 and 9 gave about the same release of insulin at 10 μl. They also showed a decrease in insulin release with increase in concentration. However, for 9 there was about a four-time reduction in the insulin secreted at 100 µl compared to that of 10 µl, which indicated that its inhibitory activity was dose-dependent. The greater inhibitory activity of 9 may be due to the presence of the 1,4-dioxo substitution on the carbazole ring C. The cytotoxicities of 5 and 9 are well-known (Fiebig et al., Citation1985; Itoigawa et al., Citation2000) and they chemically resemble each other. The 6-OMe substitution on the carbazole ring A in 7 did not have any significant effect on the activity of 3 at the two doses. The insulin release for 1 and 2 were the same at high dose, the presence of a free phenolic group on 2 probably doubled the insulin release of 1 at 10 µl. Conversely, 3 and 4 had the same insulin release at 10 µl, but the doubled insulin release of 4 over 3 at higher dose might be due to the presence of a free phenolic OH group on the former. Chemically, 1 and 2 are C23 carbazole alkaloids while 3 and 4 are C18. The replacement of a methyl group in 4 by the isopropyl-hex-1-ene rest in 2 doubled the insulin released at 10 µl while the same replacement in 3 gave doubled activity in 1 at 100 µl.
All these results show that extract and isolates of M. koenigii. significantly inhibited the secretion of insulin from the INS-1 cells and should therefore in situ. decrease the production of insulin from the beta cells of the islets of Langerhans in the pancreas gland. They could therefore be used in cases of overproduction of insulin such as in cancer of the pancreas if their toxicity is proven to be limited to cancer cells.
The stem (MKSM, LC50 14.5) was more toxic to brine shrimp than leaf (MKLM, LC50 61.5). The isolates exhibited inhibition of insulin release by an unexplained effect on the INS-1 cells, as 1 and 4 increased stimulation of insulin production at higher dose while 9 reduced stimulation at higher dose (Tables and ). Therefore, the mechanism of insulin release inhibition of these isolates remains to be determined. Previously, toxicity of the leaf extract and fractions in brine shrimp has been reported (Nutan et al., Citation1998). However, this is the first report on this activity of the stem extract.
The leaf and stem MeOH extracts reduced locomotor (rearing) behavior dose-dependently and significantly when compared to the control. Similarly, these extracts increased phenobarbitone-induced sleeping time dose-dependently, significantly, and comparable with diazepam. The combination of the extracts and flumazenil (2 mg/kg) blocked the depressive effect and potentiation of sleeping time of these extracts (Tables and ). This is an indication that the extracts have a general depressant action on the CNS similar to that of diazepam. These behavioral effects are in agreement with studies that reveal agents that possess CNS depressant activities (Ming-Chin Lu, Citation1998).
Conversely, the leaf methanol extract had greater activity against trichomonas than the stem (Tables and ). At concentrations of 6.25 and 12.5 µg/ml of the two extracts, the protozoans still had the conditions to proliferate. Reasonable activity (25% death) was not observed with the two extracts until the dose of 25 µg/ml (). At 24 h, the leaf and stem have the IC50 values of 34.0 and 35.0 µg/ml, respectively, whereas at 48 h, the values for the leaf and stem were 34.5 and 36.0 µg/ml respectively (). This shows that long exposure of the parasites to the extracts did not have any significant effect on their activity. Although these results are weak compared to that of metronidazole, it justifies the ethnomedicinal use of the plant as an antidysentry agent, especially for dysentery caused by trichomonas.
The high concentrations needed to kill 90% populations may suggest the safety of these parts, especially the leaf (). With the selective indices of 1.8 and 19.9 for 50% and 90% antitrichomonas activity at 24 and 48 h, the leaf is shown to be of more therapeutic value than the stem.
Therefore, it should be safe for human consumption and for internal use in ethnomedicine. However, great caution must be exercised in prescribing or using the stem as a chewing stick and internally as stomachic, stimulant, for snakebite poisoning, or as antidysentery. These selective indices may suggest that the two extracts may be used for different pharmacological effects. The leaf is suggested to be more antiparasitic while the stem could be used as an anticancer agent (). Similarly, mahanimbine (1) has been reported to have a better potential as a trypanocidal agent, while murrayanine (5) was a better cytotoxic agent (Adewunmi et al., Citation2001). It may be important to note that 5 was found only in the stem in the current study and was not isolated from the seed and leaf (), which are the parts consumed as flavorants. High cytotoxicity of the CHCl3 extract of the root bark of M. koenigii. and 5 (4 µg/ml), as well as feeble cytotoxicity of 1 and 3 to KB cell line have been demonstrated (Fiebig et al., Citation1985). Varied cytotoxicities of 1, 3, 5, and 9 isolated from different plant sources against different cancer cell lines have been reported (Ahmad, Citation1999; Itoigawa et al., Citation2000; Cui et al., Citation2002).
The current isolation of carbazoles from the Nigerian leaf and stem represents the first report on these morphological parts collected outside Asia (). This is the first report of 6 in the seed. Carbazoles reported from the Nikaweratiyan seed (Reisch et al., Citation1994a) are different from those isolated from the Marassana seeds. The major alkaloid in the two seeds was 1, and the relative proportion of 3 in their seeds was the same. Alkaloid 7, which was the third most abundant in the Nikaweratiya seed, is being reported as the second most abundant in the Marassana seed. These and others given in may represent qualitative and quantitative differences in the alkaloidal composition of these two seeds, which were collected in the same month (September). There were also differences observed in the carbazoles of the Nigerian and Sri Lankan stems (), which were also collected in the same month (May) and season. Compounds 8 and 9 isolated from the stem of the Nigerian plant were absent in the Sri Lankan stems, and compound 4 of the Sri Lankan stems was not isolated from the Nigerian stem (). Therefore, our current work may suggest the possibility of geographical location as a hitherto unreported factor influencing the elaboration of carbazole alkaloids in M. koenigii.. Compounds 2, 4, and 5 of the Sri Lankan stems were not isolated from their seeds, and compounds 6 and 7 of the latter were not found in the former (). Moreover, the differences between the carbazoles isolated from the Nigerian leaf and stem may also indicate possible differences in the localization of carbazole alkaloids in these plant parts ().
Seasonal (Furukawa et al., Citation1985; Wu et al., Citation1996) and geographical variations (Imai et al., Citation1989) in the constituents of Murraya euchrestifolia. Hayata and Murraya paniculata. (L.) Jack, respectively, have been reported (Hegnauer, Citation1990). Such an analysis of M. koenigii. is lacking, however, because most phytochemical reports have been on plants growing in India (Hegnauer, Citation1990; Chakraborty & Roy, Citation1991; Reisch et al., Citation1992; Bhattacharyya et al., Citation1994). Isolation of five carbazoles from the Nigerian root (Adesina et al., Citation1988) is the only report on the plant growing outside the Asian continent. Some differences have also been reported in the constituents of the volatile oils of the plant collected in Nigeria and those reported from Sri Lankan, Malaysian, and Indian plants (Onayade & Adebajo, Citation2000).
The hitherto unreported carbon data of 6, 10–11 are included in . Most times, the H-6 to H-8 of unsubstituted ring A have been reported as an unresolved 3H multiplet, and the J. values were not given (Narasimhan et al., Citation1968; Joshi et al., Citation1970; Kureel et al., Citation1970; Bhattacharyya & Chowdhury, Citation1985; Ito et al., Citation1993; Bhattacharyya et al., Citation1994; Chakrabarty et al., Citation1997). The unreported J. values of the protons of the ring A for 6, 9–11 () and the side chains of these pyranocarbazoles () are hereby given. Spectral analyses revealed that the nonequivalent terminal 7′-Me groups at δH 1.57, (δC 17.6) and 1.65, (δC 25.7) were cis. (Z.) and trans. (E.), respectively, to the H-6′ vinylic proton of the isopropylidene side chain of the pyranocarbazoles ().
Acknowledgments
We thank the German Academic Exchange Programme (DAAD) for a fellowship to G.O. The technical assistance given by Mrs. Inge Kaiserling-Buddemeier, Ms. B. Tollkühn, and K. Bauer (Institute for Medicinal Chemistry, Department of Pharmacology, Westfälische Wilhelms-Universität) and Mrs. O.A. Sowemimo (Department of Pharmacognosy, Obafemi Awolowo University) is gratefully acknowledged. We are also grateful to Prof. L.S. Gill (Botany Department, University of Benin) for identification of the plant.
References
- Adebajo CA (1997): Isolation of Carbazole Alkaloids from Murraya koenigii (Linn.) Sprengor (Rutaceae). Ph.D. Thesis, Obafemi Awolowo University, Ile-Ife, Nigeria, pp. 450.
- Adesina SK, Olatunji OA, Bergenthal D, Reisch J (1988): New biogenetically-significant constituents of Clausena anisata. and Murraya koenigii.. Pharmazie 43: 221–222.
- Adewunmi CO, Agbedahunsi JM, Adebajo AC, Aladesanmi AJ, Murphy N, Wando J (2001): Ethno-veterinary medicine: screening of Nigerian medicinal plants for trypanocidal properties. J Ethnopharmacol 77: 19–24. [CROSSREF], [CSA]
- Ahmad K (1999): Chemical Constituents of Murraya koenigii and Their Biological Activity. Ph.D. Thesis, Universiti Putra, Malaysia, pp. 145.
- Akhtar MS, Khan MA, Mahk MT (2002): Hypoglycaemic activity of Alpinia galanga. rhizome and its extracts in rabbits. Fitoterapia 73: 623–628. [CROSSREF], [CSA]
- Amos AF, McCarty DJ, Zimmet P (1997): The rising global burden of diabetes and its complications: estimates and projections to the year 2010. Diabetic Medicine 14 (Suppl 5): S7–S85. [CROSSREF]
- Assis TS, Almeida RN, Barbosa-Filho JM, Medeiros IA (2001): CNS pharmacological effect of the total alkaloidal fraction from Albizia inopinata. leaves. Fitoterapia 72: 124–130. [CROSSREF], [CSA]
- Atta-ur-Rahman, Zaidi R, Firdous S (1988): NMR studies on mahanine. Fitoterapia 59: 494–495.
- Bawden K, Quant J, Raman A (2002): An investigation of the inhibitory effects of plant extracts on starch α-amylase assay. J Pharm Pharmacol 54 (Suppl): S1. [CROSSREF]
- Bhakuni DS, Dhar ML, Dhar MM, Dhawan BN, Mehrotra BN (1969): Screening of Indian plants for biological activity. Part II. Indian J Exp Biol 7: 250–262.
- Bhattacharyya P, Chowdhury BK (1985): 2-Methoxy-3-methylcarbazole from Murraya koenigii.. Indian J Chem 24B: 452.
- Bhattacharyya P, Maiti AK, Basu K, Chowdhury BK (1994): Carbazole alkaloids from Murraya koenigii.. Phytochemistry 35: 1085–1086. [CROSSREF]
- Blumentrath J, Neye H, Verspohl EJ (2001): Effects of retinoids and thiazolidinediones on proliferation, insulin release, insulin mRNA, GLUT 2 transporter protein and mRNA of INS-1 cells. Cell Biochem Funct 19: 159–169. [CROSSREF], [CSA]
- Bozdag O, Verspohl EJ, Ertan R (2000): Synthesis and hypoglycaemic activity of some new flavone derivatives. Arzneimittelforschung/Drug. Res 50: 539–543. [CSA]
- Chakrabarty M, Nath AC, Khasnobis S, Chakrabarty M, Konda Y, Harigaya Y, Komiyama K (1997): Carbazole alkaloids from Murraya koenigii.. Phytochemistry 46: 751–755. [CROSSREF], [CSA]
- Chakraborty DP, Roy S (1991): Carbazole alkaloids. In: Herz W, Kirby GW, Steglich W, Tamm Ch, eds., Progress in the Chemistry of Organic Natural Products, Vol. 57. New York, Springer-Verlag, pp. 71–152.
- Chakraborty DP, Barma BK, Bose PK (1965): On the constitution of murrayanine, a carbazole derivative isolated from Murraya koenigii. Spreng. Tetrahedron 21: 681–685. [CROSSREF]
- Chakraborty DP, Das KC, Chowdhury BK (1971): Structure of murrayacine. J Org Chem 36: 725–727. [CROSSREF]
- Cui CB, Yan SY, Cai B, Yao XS (2002): Carbazole alkaloids as new cell cycle inhibitor and apoptosis inducers from Clausena dunniana. Levl. J Asian Nat Prod Res 4: 233–241. [CROSSREF], [CSA]
- Das KC, Chakraborty DP, Bose PK (1965): Antifungal activity of some constituents of Murraya koenigii. Spreng. Experentia 21: 340. [CSA]
- Dastur FNI (1970): In: Medicinal Plants of India and Pakistan, 3rd ed. D. B. Taraporevala Sons & Co. Private Ltd, Bombay, pp. 115–116.
- Fiebig M, Pezzuto JM, Soejarto DD, Kinghorn AD (1985): Koenoline, a further cytotoxic carbazole alkaloid from Murraya koenigii.. Phytochemistry 24: 3041–3043. [CROSSREF]
- Furukawa H, Wu TS, Ohta T, Kuoh CS (1985): Chemical constituents of Murraya euchrestifolia. Hayata. Structures of novel carbazolequinones and other new carbazole alkaloids. Chem Pharm Bull 33: 4132–4138.
- Gupta GL, Nigam SS (1971): Chemical examination of the leaves of Murraya koenigii.. Planta Med 19: 83–86.
- Hegnauer R (1990): In: Chemotaxonomie der Pflanzen, Vol. 9. Basel, Boston, Berlin, Birkhäuser-Verlag, pp. 443–464.
- Imai F, Itoh K, Kishibuchi N, Kinoshita T, Sankawa U (1989): Constituents of the root bark of Murraya paniculata. collected in Indonesia. Chem Pharm Bull 37: 119–123.
- Ito C, Thoyama Y, Omura M, Kajiura I, Furukawa H (1993): Alkaloidal constituents of Murraya koenigii.. Isolation and structural elucidation of novel binary carbazolequinones and carbazole alkaloids. Chem Pharm Bull 41: 2096–2100.
- Itoigawa M, Kashiwada Y, Ito C, Furukawa H, Tachibana Y, Bastow KF, Lee KH (2000): Anti-tumor agents. 203. Carbazole alkaloid murrayaquinone-A and related synthetic carbazolequinones as cytotoxic agents. J Nat Prod 63: 893–897. [CROSSREF], [CSA]
- Joshi BS, Kamat VN, Gawad DH (1970): On the structures of girinimbine, mahanimbine, isomahanimbine, koenimbidine and murrayacine. Tetrahedron 26: 1475–1482. [CROSSREF]
- Kar A, Choudhary BK, Bandyopadhyay NG (1999): Preliminary studies on the inorganic constituents of some indigenous hypoglycaemic herbs on oral glucose tolerance test. J Ethnopharmacol 64: 179–184. [CROSSREF], [CSA]
- Karam JH (1992): Pancreatic hormones and antidiabetic drugs. In: Katzung BT, eds., Basic and Clinical Pharmacology, 5th ed. Appleton and Lange, Connecticut, USA, pp. 586.
- Khan BA, Abraham A, Leelamma S (1995): Hypoglycemic action of Murraya koenigii. (curry leaf) and Brassica juncea. (mustard): mechanism of action. Indian J Biochem Biophys 32: 106–108. [CSA]
- Kong YC, Ng KH, But PPH, Li Q, Yu SX, Zhang HT, Cheng KF, Soejarto DD, Kan WS, Waterman PG (1986): Sources of the anti-implantation alkaloid yuehchukene in the genus Murraya.. J Ethnopharmacol 15: 195–200. [CROSSREF]
- Kureel SP, Kapil RS, Popli SP (1970): Two novel alkaloids from Murraya koenigii. Spreng.: mahanimbicine and bicyclomahanimbicine. Chem Ind: 29: 958.
- Macleod AJ, Pieris NM (1982): Analysis of the volatile oils of Murraya koenigii. and Pandanus latifolius.. Phytochemistry 21: 1653–1657. [CROSSREF], [CSA]
- Ming-Chin Lu (1998): Studies on the sedative effect. Cistanche deserticola.. J Ethnopharmacol 59: 161–165. [CROSSREF], [CSA]
- Narasimhan NS, Paradkar MV, Chitguppi VP (1968): Structures of mahanimbin and koenimbin. Tetrahedron Lett 53: 5501–5504. [CROSSREF]
- Naraya K, Sastry KNV (1975): The hypoglycaemic effects of Murraya koenigii. (Spreng.) in normal and alloxan diabetic dogs. Mysore J Agric Sci 9: 132–136.
- Narcisi EM, Secor WE (1996): In-vitro effect of tinidazole and furazolidone on metronidazole resistant Trichomonas vaginalis.. Antimicrob Agents Chemother 40: 1121–1125. [CSA]
- Nutan MTH, Hasnat A, Rashid MA (1998): Antibacterial and cytotoxic activities of Murraya koenigii.. Fitoterapia 69: 173–175.
- Onayade OA, Adebajo AC (2000): Composition of the leaf volatile oil of Murraya koenigii. growing in Nigeria. J Herbs Spices Medicinal Plants 7: 59–66. [CSA]
- Ramsewak RS, Nair MG, Strasburg GM, DeWitt DL, Nitiss JL (1999): Biologically active carbazole alkaloids from Murraya koenigii.. J Agric Food Chem 47: 444–447. [CROSSREF], [CSA]
- Reisch J, Goj O, Wickramasinghe A, Bandara Herath HMT, Henkel G (1992): Carbazole alkaloids from seeds of Murraya koenigii.. Phytochemistry 31: 2877–2879. [CROSSREF]
- Reisch J, Adebajo AC, Aladesanmi AJ, Adesina KS, Bergenthal D, Meve U (1994a): Chemotypes of Murraya koenigii. growing in Sri Lanka. Planta Med 60: 295–296.
- Reisch J, Adebajo AC, Kumar V, Aladesanmi AJ (1994b): Two carbazole alkaloids from Murraya koenigii.. Phytochemistry 36: 1073–1076. [CROSSREF]
- Stone BC (1985): Rutaceae In: Dassanayake MD, Fosberg FR, eds., A Revised Handbook of the Flora of Ceylon, Vol. 5., Calcutta, Sri Lanka, Oxford & IBH Publishing Co., pp. 435–439.
- Tachibana Y, Kikuzaki H, Lajis NH, Nakatani N (2001): Antioxidative activity of carbazoles from Murraya koenigii. leaves. J Agric Food Chem 49: 5589–5594. [CROSSREF], [CSA]
- Wu TS (1991): Murrayamine-A, -B, -C and (+)-mahanine, carbazole alkaloids from Murraya euchrestifolia.. Phytochemistry 30: 1048–1051. [CROSSREF]
- Wu TS, Wang ML, Wu PL (1996): Seasonal variations of carbazole alkaloids in Murraya euchrestifolia.. Phytochemistry 43: 785–789. [CROSSREF]
- Yadav S, Vats V, Dhunnoo Y, Grover JK (2002): Hypoglycemic and antihyperglycemic activity of Murraya koenigii. leaves in diabetic rats. J Ethnopharmacol 82: 111–116. [CROSSREF], [CSA]