Abstract
A total extract of Linum persicum Kotschy ex Boiss. was investigated for biological activity. The extract was not active against Gram-negative and Gram-positive bacteria. The extract exhibited significant activity against brine shrimp (Artemia salina). Cytotoxic activity of the extract was evaluated by the MTT assay on five different cell lines. The total extract showed the highest cytotoxicity against human chronic myeloid leukemia (K-562), T-cell lines (Jurkat), and lung carcinoma (A5). Three aryltetralin lignans, podophyllotoxin, 5-methoxypodophyllotoxin, and 5-methoxy podophyllotoxin acetate, were isolated from the methanol extract of the aerial parts of L. persicum, and the structures were confirmed by spectroscopic methods.
Introduction
Podophyllotoxins represent an important and unique position among natural products. Many studies in chemical identification, biological assessment, and defining their mechanism of action have been performed. It is starting material for the semisynthetic anticancer drugs etopside and teniposide, so considerable attention has focused on the availability of these aryltetralin lignans (Bohlin & Rosen, Citation1996). Podophyllotoxin is extracted from two important species, Podophyllum hexandrum. Royle. and Podophyllum peltatum. L., which grow in the wild, and because of the limited supply of podophyllum rhizomes, there is significant interest in systemic cultivation, biotechnological exploitation, or identification of other sources of these natural lignans (Imbert, Citation1998). Linum. is one of the genera, with some reports on the presence of podophyllotoxin and related compounds, such as L. album. Kotschy ex Boiss., L. flavum. L., L. capitatum. Kit., and L. catharticum. L. (Broomhead & Dewick, Citation1990; Belma, Citation1998; Smollny et al., Citation1998) Linum persicum. Kotschy ex Boiss. is an endemic plant, which grows in the wild in the Dena mountains in Iran (Rechinger, Citation1974). In our literature review, we did not find any reports on this species. The aim of the current project was to investigate the biological activity of the methanol extract of L. persicum.. Disk-diffusion method tests to study antimicrobial activity as well as brine shrimp lethality and five different cell lines to determine cytotoxic effects were employed. Also, three podophyllotoxin-related lignans were isolated from the extract.
Materials and Methods
General
1H NMR spectra were recorded in CDCl3 (USA) using a Varian Unity 400 MHz with TMS as internal standard. Electron Impact Ionization-Mass Spectrometry (EI-MS) measurements were carried out on a Hewlett-Packard HP6890. Optical rotation was measured using Polarimeter (USA), Perkin-Elmer 241. Silica gel 100 (0.063–0.2 mm) (Merck) was used for column chromatography, silica gel 60 for preparative thin-layer chromatography (TLC), and analytical TLC plates 60 F254 (Merck) for TLC.
Plant Materials
The aerial parts of Linum persicum. were collected from Dena Mountains at 2820-m altitude in June 2002. Dr. A. Jafari identified the plant. A voucher specimen (No. 5413) has been deposited in the herbarium of the Central Research of Natural Resource and Animal Husbandry, Yasuje, Iran.
Bioassays
Antimicrobial assay
The bacterial strains consisted of Gram-positive bacteria Staphylococcus aureus. (PTCC 1112), Bacillus subtilis. (PTCC 1023), and the Gram-negative bacteria Escherichia coli. (PTCC 1330) and Pseudomonas aeroginosa. (PTCC 1074), as well as the fungus Candida albicans. (PTCC 5027). They were obtained from the Persian Type Culture Collection, Iranian Research Organization for Science and Technology. The disk diffusion assay was used to study antimicrobial and antifungal activity (Taylor et al., Citation1995). The extracts were prepared at a concentration of 20 mg/ml in methanol. Paper disks with a diameter of 6 mm containing 25, 50, 75, or 100 µl of extract were deposited on the surface of the seeded nutrient agar (for antibacterial assay) and Sabouraud dextrose agar (for antifungal assay) in Petri dishes. The bacterial Petri dishes were incubated for 24 h at 37°C, and fungal Petri dishes were incubated for 24–48 h at 20°C. Three replicates were prepared for each dose.
Brine shrimp (Artemia salina Leach) lethality assay
The assay was performed with 10, 25, 50, 100, and 1000 μg/ml extract as previously outlined (Meyer et al., Citation1982). The number of live naupliis after 12 h was noted. The experiment was carried out in triplicate. LD50 values were determined from the best-fit line obtained by linear regression analysis.
Cytotoxicity evaluation
Five cell lines including Jurkat (T-cell lines), A5 (lung carcinoma), Hela (human cervix carcinoma), K-562 (human chronic myeloid leukemia), and Fen (human bladder epithelial carcinoma) were all obtained from the Iran Pasteur Institute (Tehran, Iran), except for the Fen cell line, which was kindly provided by Dr. A.M.E. Nouri, Department of Medical Oncology, The Royal London Hospital Trust (London, UK). Cells were cultured in RPMI-1640 (Sigma, St. Louis, MO, USA) supplemented with 10% fetal bovine serum (Gibco, Grand Island, NY, USA), 100 IU/ml penicillin, and 100 µg/ml streptomycin. Cells were cultured in 50 cm3 flasks (Nunc, Roskilde, Denmark) with 5 ml of culture medium in a humidified 5% CO2 incubator at 37°C.
An appropriate amount of the extract was mixed with DMSO (dimethyl sulfoxide). Then, with serial dilutions in RPMI-1640, seven concentrations (0.01, 0.05, 0.1, 1, 10, 100, and 500 μg/ml) were made. DMSO, as a negative control, was diluted with the same method. For a positive control, doxorubicin was mixed with PBS (phosphate-buffered saline) to make similar concentrations. MTT [3-(4,5-dimethylthiazole-2-yl)-2,5-diphenyl tetrazolium bromide] (Sigma), was dissolved in PBS at 5 mg/ml and then filtered to remove any undissolved particle. Each well of the microtiter plate was filled with 1 × 104 to 5 × 104 cells (depending on the cell line) in 90 µl culture medium. Then, 10 µl from the stock solutions of the extract and fractions, negative and positive controls (doxorubicin) was added in triplicate to the wells to reach final concentrations of 0.001, 0.005, 0.01, 0.1, 1, 10, and 50 μg/ml. Three wells containing only the same number of the cells was left in each plate. Plates were kept in a humidified incubator for 48 h. After the incubation period, the MTT assay was carried out by the procedure described previously (Jabbar et al., Citation1989).
Extraction and isolation
The aerial parts were air dried in shade, powdered, and defatted with petroleum ether for 4 h. A methanol extract was obtained by maceration of the plant in 3 × 1500 ml methanol at room temperature for 48 h. The methanol extract was filtered and concentrated under reduced pressure and yielded 4.3% (w/w). The extract was fractionated by column chromatography over silica gel (500 g) elution being performed with a gradient system of chloroform-methanol (100:0, 90:10, 75:25, 50:50). The second fraction (90:10) showed the lignan bands in analytical TLC plates by UV light or by spraying with H2SO4 (50%) or fast blue salt reagent. It was separated by preparative TLC using chloroform-methanol (90:10) and subsequently toluene-ethyl acetate (65:35). The bands were treated as follows:
Band 1 (RF 0.8) comp 1
This band was identified as (−)-5-methoxy podophyllotoxin acetate (Broomhead & Dewick, Citation1990); MS: 486 ([M]+, 100%), 456 (30), 427 (32), 381 (11), 312 (12), 259 (13), 215 (18), 164 (46), 153 (10); 1H NMR (400 MHz, CDCl3, TMS): δ.: 6.47 (2H, s, H-2′, 6′), 6.34 (1H, s, H-8), 6.12 (1H, d, J. = 7.9 Hz, H-4β.), 5.98 (1H, d, J. = 1.2 Hz, OCH2O), 5.96 (1H, d, J. = 1.2 Hz, OCH2O), 4.58 (1H, d, J. = 2.8 Hz, H-2), 4.44 (1H, dd, J. = 9.2 Hz, 6.8 Hz, H-3aα.), 4.20 (1H, dd, J. = 10.8, 6.0 Hz, H-3aβ.), 4.02 (3H, s, OMe-5), 3.82 (3H, s, OMe-4), 3.78 (6H, s, OMe-3′,5′), 2.80 (2H, m, H-2,3), 2.09 (3H, s, OAC).
Band 2 (RF 0.7) comp 2
This band was identified as (−)-5-methoxy podophyllotoxin (Broomhead & Dewick, Citation1990); MS: 444 ([M]+, 100%), 414 (8), 258 (8), 219 (9), 168 (20), 153 (10); 1H NMR (400 MHz, CDCl3, TMS): δ.: 6.44 (2H, s, H-2′,6′), 6.30 (1H, s, H-8), 5.96 (2H, s, OCH2O), 5.03 (1H, d, J. = 10.0 Hz, H-4β.), 4.65 (1H, dd, J. = 8.0, 7.2 Hz, H-3aα.), 4.54 (1H, d, J. = 4.4 Hz, H-1), 4.16 (3H, s, OMe-5), 4.06 (1H, s, OH), 4.04 (1H, dd, J. = 10.4, 9.2 Hz, H-3aβ.), 3.81 (3H, s, OMe-4), 3.77 (6H, s, OMe-3′,5′), 2.87 (1H, m, H-3), 2.75 (1H, dd, J. = 14.8, 4.4 Hz, H-2).
Band 3 (RF 0.65) comp 3
This band was identified as (−)-podophyllotoxin (Broomhead & Dewick, Citation1990); MS: 414 ([M]+, 100%), 358 (40), 163 (25), 151 (89), 137 (43), 83 (49); 1H NMR (400 MHz, CDCl3, TMS): δ.: 7.14 (1H, s, H-5), 6.51 (1H, s, H-8), 6.38 (2H, s, H-2′,6′), 5.98 (1H, d, J. = 1.2 Hz, OCH2O), 5.96 (1H, d, J. = 1.2 Hz, OCH2O), 4.73 (1H, d, J. = 4.4 Hz, H-4β.), 4.63–4.59 (1H, m, H-3aα., H-1), 4.08 (1H, t, J. = 9 Hz, H-3aβ.), 3.91 (1H, s, OH), 3.81 (3H, s, OMe-4), 3.75 (6H, s, OMe-3′,5′).
Results and Discussion
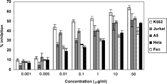
Plant materials of Linum persicum. were dried and extracted with methanol. The extract did not show any antibacterial and antifungal activity at concentrations of 0.5, 1, 1.5, and 2 mg/disk. One of the methods of studying the biological activity of natural products is the use of brine shrimp. It is a reliable, general bioassay with low cost for phytochemical screening and fractionation. The extract exhibited significant activity (LD50 = 28.6 µg/ml) against Artemia salina.. The good results of brine shrimp lethality led us to examine the cytotoxic activity of the extract on five different cell lines by the MTT assay. The data are shown in .
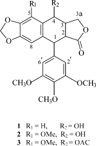
According to these results, the most cytotoxic effect was seen on the K-562 cell line (IC50 = 1.29). Significant activity was also observed against A5 (IC50 = 14.0) and Jurkat (IC50 = 22.6) cell lines. The extract was fractionated on silica gel by column chromatography. The second fraction, chloroform-methanol (90:10), showed the lignan bands in analytical TLC plates by spraying with H2SO4, which was further separated by preparative TLC. Three aryltetralin lignans, podophyllotoxin (1), 5-methoxy podophyllotoxin (2), and 5-methoxy podophyllotoxin acetate (3) were isolated (), and the structures of the compounds were confirmed by EI-MS and 1H NMR. The spectroscopic data were in good agreement with respective literature data (Broomhead et al., Citation1990).
Conclusions
The extract did not exhibit antibacterial and antifungal activity. There was a correlation observable between cytotoxicity and brine shrimp lethality. The extract was toxic against human chronic myeloid leukemia (K-562), T-cell lines (Jurkat), and lung carcinoma (A5). Linum. is one of the genera with several reports on the presence of podophyllotoxin and related compounds. These results led us to isolate three bioactive aryltetralin lignans from extract. Podophyllotoxin, 5-methoxypodophyllotoxin, and 5-methoxypodophyllotoxin acetate were identified in the extract and have been reported in L. flavum. previously (Broomhead & Dewick, Citation1990).
Acknowledgments
This work was supported by a grant from the Technology Council of Shiraz Province and the Research Council of Shiraz University of Medical Sciences.
References
- Belma K (1998): Aryltetralin lignans from Linum catharticum. L. Biochem.. Systematics Ecol. 26: 795–796.
- Bohlin L, Rosen B (1996): Podophyllotoxin derivatives: Drug discovery and development. Drug Discovery Today 1: 343–351. [CROSSREF], [CSA]
- Broomhead AJ, Dewick PM (1990): Aryltetralin lignans from Linum flavum. and Linum capitatum.. Phytochemistry 29: 3839–3844. [CROSSREF], [CSA]
- Imbert TF (1998): Discovery of podophyllotoxins. Biochimie 80: 207–222. [PUBMED], [INFOTRIEVE], [CROSSREF], [CSA]
- Jabbar SAB, Twentyman PR, Watson JV (1989): The MTT assay underestimates the growth inhibitory effects of interferons. Br J Cancer 60: 523–528. [PUBMED], [INFOTRIEVE], [CSA]
- Meyer BN, Ferrigini NR, Putnam JE, Jacobsen LB, Nichols DE, McLaughlin JL (1982): Brine shrimp: A convenient general bioassay for active plant constituents. Planta Med 45: 31–34. [PUBMED], [INFOTRIEVE], [CSA]
- Rechinger KH (1974): Linaceae.. In: Rechinger KH, ed., Flora Iranica. Graz, Druck und Verlagsanstalt, pp. 1–20.
- Smollny T, Wichers H, Kalenberg S, Shahsavari A, Petersen M, Alfermann AW (1998): Accumulation of podophyllotoxin and related lignans in cell suspension cultures of Linum album.. Phytochemistry 48: 975–979. [CROSSREF], [CSA]
- Taylor RSL, Manandhar NP, Towers GHN (1995): Screening of selected medicinal plants of Nepal for antimicrobial activities. J. Ethnopharmacol 546: 153–159. [CROSSREF], [CSA]