Abstract
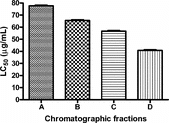
The anthelmintic effect of chromatographic fractions of leucaena leucocephala. (Lam.) de wit seed extract was investigated to determine the relative efficacy of the seed components as anthelmintic against gastrointestinal sheep nematodes. The fractions were obtained by thin-layer chromatographic (TLC) fingerprinting of eluates from gradient vacuum liquid chromatography (VLC) of the seed ethanol extract. These were evaluated for nematocidal activity by a larval development viability assay (LDVA) in vitro.. The effect of the test extracts on the transformation of L1 to L3 and the survival rate of infective larvae (L3), of strongyles of sheep, predominantly Haemonchus contortus. (Rudolphi), was used to determine relative bioactivity. Best-fit LC50 values were computed using global model of nonlinear regression curve-fitting. The composition of the fractions was identified by phytochemical screening. The extracts killed infective larvae of H. contortus. of sheep in a concentration-dependent manner. Best-fit LC50 values are 77.66, 65.56, 56.72, and 40.80 µg/ml for fractions A, B, C, and D, respectively (95% CI). The fractions are composed of alkaloids (A), alkaloids and tannins (B and C), and flavonoids and tannins (D). Fraction D was significantly more active than all the other fractions (p < 0.001, one-way ANOVA, Tukey's multiple comparison test). The most active fraction (D) contains polar polyphenols, thus providing a scientific justification for the use of aqueous extract in traditional practice. The polyphenol fraction of L. leucocephala. seed could find application in anthelmintic therapy in veterinary practice.
Introduction
The profitability of sheep breeding is low in most countries, and farmers look for convenient, safe, and efficient broad-spectrum drugs to control parasites. It is desirable to limit the cost of treatment in the parasite control initiatives in order to improve the overall profitability. Farmers in Nigeria commonly use fresh seeds of Leucaena leucocephala. (Lam.) de wit (Fabaceae) to deworm their animals. The seed is also reportedly useful in expelling ascaris worms (Adebowale, Citation1998).
In an earlier communication (Ademola & Idowu, 2005), we reported the anthelmintic effect of an aqueous extract of L. leucocephala. using an in vitro. larval development assay. It is felt, however, that direct aqueous extraction of the pounded fresh seeds as it is done in traditional practice carries a certain risk of mimosine toxicity. This is due to the fact that mimosine, a well-known toxic amino acid that is found in the seeds of L. leucocephala., is slightly soluble in water (The Merck Index, Citation2001). It is desirable, therefore, to assess the relative efficacy of the components of the seed, with the view of devising a mode of preparation of the active fraction without the risk of mimosine toxicity.
We report in this communication a comparative evaluation of the efficacy of chromatographic fractions of the seed ethanol extract against gastrointestinal sheep nematodes, identified as predominantly Heamonchus contortus. (Rudolphi).
Materials and Methods
Chemicals and reagents
Ethyl acetate, methanol, n.-hexane, ethanol (96%), chloroform, ammonia solution, ferric chloride, hydrochloric acid, concentrated sulfuric acid, potassium iodide, iodine, potassium iodate, vanillin crystals, mercuric chloride, picric acid, zinc powder, glacial acetic acid, 3,5-dinitrobenzoic acid, sodium hydroxide, acetic anhydride, propylene glycol (BDH, Poole, UK) precoated TLC aluminum sheets (0.2 mm), and silica gel 60 G (Merck, Darmstadt, Germany) were used.
Equipment
Ultraviolet lamp (254 and 365 nm), vacuum oven (Gallenkamp, Loughborough, UK), analytical balance H80 and Mettler Toledo PB 602 (Mettler, Leicester, UK) Soxhlet extraction apparatus, and vacuum liquid chromatograph apparatus were used.
Plant collection
The plant L. leucocephala. is a shrub that is freely available in Ibadan, southwestern Nigeria. The dry pods and seeds were collected in the morning around the University of Ibadan, Ibadan, Nigeria. The seeds and the plant were identified by Dr. A. O. Ayodele (Department of Botany and Microbiology, University of Ibadan). A voucher specimen was deposited at the herbarium of the Department of Botany and Microbiology, University of Ibadan.
Preparation of chromatographic fractions
Powdered seed (600 g) was defatted by continuous Soxhlet extraction with hexane for 24 h. The defatted marc was air-dried and then extracted by Soxhlet extraction with 96% ethanol. The crude ethanol extract was concentrated and dried in a vacuum oven at 60°C. Phytochemical screening was performed by standard procedures (Trease & Evans, 1983) on the ethanol extract to determine the various secondary metabolites in the extract.
Sodium acetate (13.608 g) was dissolved in 100 ml solution with distilled water (1 M). An aliquot of this solution (30 ml) was taken and diluted to 100 ml with distilled water to produce a second solution (0.3 M). Silica gel (thin-layer chromatography grade; 40 g) was weighed into a conical flask, and a solution of sodium acetate was added (1 M; 80 ml) and thoroughly mixed to make a slurry. The slurry was then poured into a large beaker and dried at 140°C for 2 h.
Sodium acetate treated silica gel (20 g) was packed dry in a short column for vacuum liquid chromatography (VLC), and ethanol extract (5 g) adsorbed on silica gel and dried was loaded on top of the column. Gradient column elution was performed with 50 ml of each mobile phase mixture in a series. The elution was performed under negative pressure supplied by a vacuum line and the column was allowed to dry before the next gradient mobile phase was applied. The mobile phase consists of several mixtures of hexane, ethyl acetate, and methanol, starting with 100% hexane and 10% increment in the next more polar component to obtain the series of mobile phase gradient. The final elution was performed with 100% methanol until the loaded sample was exhausted.
The eluates were monitored by thin-layer chromatography, using precoated silica gel plates previously treated by ascending development in 0.3 M sodium acetate and dried. The mobile phase consists of a mixture of methanol:ethyl acetate (70 + 30). The eluates were combined based on the similarity of TLC fingerprints, to obtain a lower number of fractions distinguished by the different Rf values of the components. Phytochemical screening was performed again on the final four fractions obtained, to determine their composition.
Evaluation of anthelmintic activity
A stock solution of each chromatographic fraction was prepared in distilled water or 20% propylene glycol in water as appropriate. Appropriate aliquots were taken to produce 10, 20, 30, 40, 50, 60, 70, 80, and 90 µg/ml in a final 2.5 ml volume of the culture medium. Two controls were set up by replacing the test extracts with distilled water and 20% propylene glycol in water, respectively.
Nematode egg recovery technique
The technique used was that previously described by Hubert and Kerboeuf (1992). Briefly, 10–15 g of sheep feces was suspended in water and cleared of organic debris by filtration through sieves (1 mm and 100 µm), the eggs being collected on a 20-µm sieve. The eggs were further cleared from organic debris by centrifugation in magnesium sulfate (density 1.10) for 5 min at 1000 × g.. The supernatant was filtered through 100- and 60-µm sieves, and the eggs were washed in water and collected on a 20-µm sieve. The concentration of eggs was estimated in 50-µl samples and adjusted to 1200 to 1300/ml.
Egg suspension
The concentration of eggs was estimated in 50-µl samples and adjusted to 100–120 eggs/ml. The egg suspension was diluted with the filtrate from the first step of egg extraction (described above) in order to provide rumen bacteria necessary for nematode larvae development. To avoid the proliferation of fungi, 5 µg of amphotericin B (Fungizone ND: Squibb, Hounslow, Middlesex, UK) were added per milliliter of egg suspension.
Nutritive medium
The nutritive medium was as described by Hubert and Kerboeuf (1992) and composed of Earle's balanced salt solution (Eurobio) plus yeast extract (Difco Laboratories, Livonia, MI, USA) in saline solution (1 g of yeast extract/90 ml of saline solution) in the proportion of 1:9 volume to volume.
Larval development test
The test was carried out in 5-ml tubes: 20 µl of nutritive medium was added to 80 µl of egg suspension containing approximately 100 eggs. The tubes were covered and put in an incubator at 27°C for 48 h. By then, the parasite had developed to first stage larvae, and the plant extract aliquots was added. The third-stage larvae were obtained 7 days later. At this time, the parasites were counted by separating the larvae into two classes, living third-stage larvae (L3) and dead larvae and living larvae of other stages. The test and appropriate control were performed in duplicate.
Determination of 50% lethal concentration andstatistical analysis
In the larval development assay, the LC50 was determined by computing the concentration of extract that gave a response halfway between the minimum and maximum responses in a concentration-response sigmoid curve. The larval development parameter (larval survival rate) is given by:
Determination of LC50 of a sigmoidal concentration-response (variable slope) curve was performed using GraphPad Prism version 4.01 for Windows (GraphPad, Sen Diego, CA, USA). The analysis of the family of data sets generated by four chromatographic fractions tested was performed by the global curve-fitting model of nonlinear regression analysis with top, hill slope, and bottom shared among the data sets. In addition, the bottom of the curve was constrained as > 0 and the top was constrained as < 1.0. A (global) best-fit value that applies to the family of data sets was computed for each of these shared parameters, while best-fit LC50 value (unshared parameter) was calculated with 95% confidence interval for each of the data sets (fractions). The relative bioactivity of the fractions was further assessed by comparing the best-fit LC50 value of the various fractions by one-way ANOVA and Tukey's multiple comparison test, which was performed using GraphPad Prism version 4.01 for Windows.
Results
The seed is rich in fixed oil, and the hexane fraction gave a yield of 45.6 g (7.6% w/w), whereas the ethanol fraction gave a yield of 23.5 g (3.92% w/w). Phytochemical screening of the crude ethanol extract shows the presence of alkaloids, flavonoids, tannins (condensed and gallotannins), catechin, cardenolides, and carotenoids.
The chromatographic fractions contain compounds of widely varying polarities. Rf values ranged from 0.89 in the most nonpolar fraction (A) to 0.04 in the most polar fraction (D). Fraction A was shown to contain alkaloids, fractions B and C contain alkaloids and tannins, and fraction D contains flavonoids and tannins.
L. leucocephala. test extracts appear not to affect larval transformation from L1 to L3 after the incubation period, as more than 98% of the larvae in both tubes containing test extracts and control tubes were infective third stage type. However, the infective stage larvae were killed in a concentration-dependent manner by the chromatographic fractions (). The shared statistical parameters of the curvefitting analysis and the best-fit LC50 values for the chromatographic fractions are shown in . The best-fit LC50 values were calculated with reasonable precision (95% CI). shows the results of one-way ANOVA, and shows, Tukey's multiple comparison (post ANOVA) test, which shows that fraction D is significantly more active than all the other fractions (p < 0.001).
Figure 1 Concentration-response curve of chromatographic fractions of L. leucocephala. seed against infective larvae of H. contortus. using global model of nonlinear regression curve-fitting.
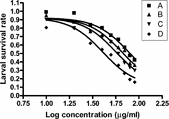
Table 1.. LC50 of L. leucocephala. test chromatographic fractions using global model of nonlinear regression curve-fitting.
Table 2.. Tukey's multiple comparison test comparing the LC50 values of chromatographic fractions of L. leucocephala seed..
Discussion
The larval development assay results suggest that L. leucocephala. seed extracts did not affect larval development from L1 to the infective stage (L3). However, the chromatographic fractions affected the survival of the L3 larvae in a concentration-dependent manner. The LC50 obtained for L. leucocephala. aqueous extract was 0.586 mg/ml (Ademola & Idowu, 2005). On the other hand, chromatographic fractions of L. leucocephala. are more potent. The LC50 value of 40 µg/ml obtained for the most active fraction is about 15-times more active than the crude aqueous extract.
The accuracy and precision of these values is described by the best-fit value and narrow 95% confidence interval. This demonstrates the advantage of the global nonlinear regression data analysis for the family of data sets generated by the various chromatographic fractions. Individual fitting of the data obtained from the fractions gave LC50 values with less precision and accuracy. The in vitro. anthelmintic activity of pure compounds is usually higher. LC50 values are typically in the nanogram/milliliter range. We recently reported the variation in the potency of various ivermectin injection brands by the larval development viability assay (LDVA), using the infective larvae of H. contortus., as described here. The LC50 values of different brands vary from 1.1 ng/ml to 17 ng/ml (Ademola et al., 2003). However, the problem of anthelmintic resistance by nematodes and increasing concern over the presence of drug residues in animal products, when pure compounds are administered, has led to a resurgence of interest in the use of phytomedicines, in the form of extracts containing a mixture of compounds (Athanasiadou et al., Citation2001).
Increase in the anthelmintic activity of the chromatographic fractions, relative to crude aqueous extract, is expected because they are more concentrated solutions of secondary metabolites in the seed. Phytochemical screening of the fractions gave information on their composition with respect to chemical class. It was observed that the seed is very rich in alkaloids, which were found in three of the four fractions. Each fraction represents a mixture of chemical compounds with similarity in chemical structure or other physicochemical properties. The seed of L. leucocephala. has been widely investigated, especially due to the toxicity of one of the alkaloids, mimosine [(β-3-hydroxy-4-pyridon-1-yl)-L-alanine]. Ingestion of mimosine results in hair loss, goiter, reproductive disorders, epithelial damage, reduced feed intake, and ultimately death in both nonruminants and ruminants (Crounse et al., 1962). These facts have limited its use as an alternative source of protein for livestock feed, despite its high nutritive value.
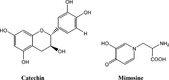
Our results further show that the most active chromatographic fractions do not contain alkaloids but polyphenols, namely flavonoids and tannins, which are well illustrated structurally by the building block of condensed tannins, catechin (). Polyphenols are polar compounds; as such, they exhibit reasonable water solubility. They are the most polar class of compounds in the seed as well, constituting the last fraction that was eluted by methanol from the crude ethanol extract. This finding provides a scientific basis for the preferred use of aqueous extract in traditional veterinary practice. However, because polyphenols are also aromatic compounds, their solubility in water is limited by the hydrophobic aromatic ring moiety in their structures, which explains why fractions obtained by extracting with organic solvent (methanol) are much more active than the aqueous extract. The absence of alkaloids in the most active fraction means the most potent anthelmintic principles of the seed can easily be obtained for veterinary use without the risk of mimosine toxicity. This approach appears safer than the traditional approach that simply extracts the whole seed. Administering the crude aqueous extract to animals indeed carries a certain risk of mimosine toxicity. Mimosine, due to polar fragments in its chemical structure (), will also exhibit a degree of solubility in water. It is reported to be slightly soluble in water and much less soluble in methanol and ethanol (The Merck Index, Citation2001). Selective isolation of the polyphenols in L. leucocephala. seed, as described here, is therefore a promising mode of preparing this potential anthelmintic phytomedicine for veterinary use, without the undesirable risk of mimosine toxicity.
Some other plants reportedly possessing anthelmintic activity include Alstonia boonia, Nauclea latifolia. (Asuzu & Njoku, Citation1996), Hedysarum coronarium. (Linn) (Molan et al., 2000) and Khaya senegalensis. (DESR) (Ademola et al., Citation2004). A commercially available herbal extract, Quebracho, which is rich in condensed tannins (Athanasiadou et al., Citation2001), has also being reported to have direct anthelmintic effects on strongyles of sheep.
Conclusions
Further investigations on the anthelmintic tannins and flavonoids are ongoing in our laboratories, to elucidate the chemical structures of the active principles. These findings also warrant investigation of the safety and toxicity of the polyphenols by in vivo. studies in sheep, with the view of developing a phytomedicine for veterinary use from the seeds of L. leucocephala..
References
- Adebowale EA (1998): Proceedings of a workshop on indigenous knowledge in agriculture and rural development. Organized by the South West Zonal farming systems research and extension. Ibadan, Nigeria, IAR&T, Obafemi Awolowo University, Moor Plantation.
- Ademola IO, Fagbemi BO, Idowu SO (2003): Comparative in vitro. studies on the efficacy of ivermectin against gastrointestinal sheep nematode. Trop J Pharm Res 2: 235–238.
- Ademola IO, Fagbemi BO, Idowu SO (2004): Evaluation of the anthelmintic activity of Khaya senegalensis. extract against gastrointestinal nematode of sheep: in vitro. and in vivo. studies. Vet Parasitol 122: 151–164. [PUBMED], [INFOTRIEVE], [CSA], [CROSSREF]
- Ademola IO, Idowu SO (2004): Anthelmintic activity of Leucaena leucocephala. seed extract on Haemonchus contortus. infective larvae. The Veterinary Record (2005). [CSA]
- Asuzu IU, Njoku CJ (1996): The anthelmintic effect of Alstonia boonia. and Nauclea latifolia. leaf aqueous extracts on Trichostrongylus infective larvae. Fitoterapia 3: 220–222. [CSA]
- Athanasiadou S, Kyriazakis I, Jackson F, Coop RL (2001): Direct anthelmintic effect of condensed tannins toward different nematodes of sheep, in vitro. and in vivo. studies. Vet Parasitol 99: 205–219. [PUBMED], [INFOTRIEVE], [CSA], [CROSSREF]
- Crounse RG, Maxuel JD, Blank H (1962): Inhibition of hair growth by mimosine. Nature 194: 694–695. [PUBMED], [INFOTRIEVE], [CSA]
- Hubert J, Kerboeuf D (1992): A microlarval development assay for the detection of anthelmintic resistance in sheep nematode. Vet Rec 130: 442–446. [PUBMED], [INFOTRIEVE], [CSA]
- Molan AL, Alexander RA, Brookes IM, Mcnabb WC (2000): Effect of an extract from sulla (Hedysarum coronarium.) containing condensed tannins on the migration of three sheep gastrointestinal nematodes in vitro.. Proc N Z Soc Anim Prod 60: 21–25. [CSA]
- The Merck Index, Encyclopedia of Chemicals, Drugs and Biologicals (2001): 13th ed. Merck & Co, Cambridge Soft Corporation, Cambridge, MA, USA, p. 6221.
- Trease CR, Evans WC (1983): In: Maryadele J O' Neil, Anne Smith, Patricia E. Heckelman, and Susan Budavari, eds., Textbook of Pharmacognosy, 12th ed. London, Balliere Tyndall.