Abstract
In the present current, quercetin and its 3-O.-rutinoside, the ethyl acetate and ethanol extracts, and two new flavones from Casimiroa edulis. Llave et Lex (Rotaceae) were tested for their antioxidant activity via scavenging by the ABTS˙+ [2,2′-azinobis(3-ethylbenzothiazolone-6-sulfonic acid)]free radical. Among all the tested fractions and compounds, the ethanol extract exhibited the strongest antioxidant activity followed by EtOAc extract, giving 842 and 712 µM Trolox equivalents/g dry weight, respectively. Moderate activities were detected for the other tested materials. The two new flavones were identified as 5-methoxy-6-hydroxyflavone and its 6-O.-β.-D-glucoside. The structure elucidation was based on UV, electrospray ionization mass spectrometry (ESIMS), 1H and 13C NMR, proton-proton correlation spectroscopy (1H-1H COSY), distortionless enhancement by polarization transfer (DEPT), heteronuclear single quantum coherence (HSQC), and heteronuclear multiple bond correlations spectrum (HMBC).
Introduction
Plants are sources of natural antioxidants, and some of the compounds have significant antioxidative properties and health benefits (Exarchou et al., Citation2002). The antioxidative effect is mainly due to phenolic compounds such as flavonoids, phenolic acids, tannins, and phenolic diterpenes (Shahidi et al.,Citation1992; Chung et al., Citation1998; Pietta, Citation2000). Casimiroa edulis. Llave et Lex (Rotaceae) is a species indigenous to temperate zones of Mexico and Central America, and it is popularly called “zapote blanco,” which has been known since pre-Hispanic times for its interesting sedative-like effects and its use as a sleep inducer (Romero et al., Citation1983). The tree is cultivated in Egypt for its edible fruits. In folk medicine, a concoction of leaves and occasionally of seeds is administered for sedative action. The aqueous and alcohol extracts of leaves and seeds, respectively, showed anticonvulsant, hypnotic, antihypertension, diuretic, anti-inflammatory (Petit-Play et al., Citation1982), and sedative activities (Magos & Vidrio, Citation1991; Magos et al., Citation1995). Other pharmacological activities have been studied for the plant in Mexico and in the United States. (Aiko et al., Citation1998; Magos et al., Citation1999). Secondary metabolites of various plant parts of Casimiroa edulis. that have been previously reported include imidazole (Romero et al., Citation1983), quinolinone derivatives (Toube et al., Citation1967; Dreyer, Citation1968), zapotidine alkaloids (Kincl et al., Citation1958), furanoquinoline alkaloids together with 2-quinolone and 4-quinolones, edulein, edulitin, edulinine, casmiroin (Dryer, 1968; Enriques et al., Citation1984) (viz., casmiroedine, casimiroine, skimmianine, 1-methyl-2-phenyl-4-quinolone, eduline) (Power & Callan, Citation1911; Aebi, Citation1956; Raman et al., Citation1962), coumarins, furanocoumarins and scopoletin methyl ether (Dreyer, Citation1968; Kincl et al., Citation1958), isoimpinellin, casmiroin, skimmianine, edulein, the histamine derivatives such as N.,N.-dimethylhistamine (Dreyer, Citation1968),casmidine and casimiroedin (Enriques et al., Citation1984), as compounds of marked hypotensive activity. Flavonoids including 5,6,2′,6′-tetramethoxyflavone (known as zapotin) (Dreye & Bertelli, Citation1976; Garratt et al., Citation1967) and limonoids (Sondheimer et al., Citation1959; Murphy et al., Citation1968) all have been previously isolated from leaves. No previous work on fruits of C. edulis. has been reported except for the study of its provitamin content among the fruits of 33 different species in the U.S. market (Aebi, Citation1956) and the 5,6-dihydroxyflavone from the plant bark (Iriarte et al., Citation1956). In the current study, the leaves of C. edulis. were studied for their antioxidant activity and for determination of the active compounds.
Materials and Methods
Apparatus
Melting points were determined on a Kofler hot-stage apparatus (UK) and are uncorrected; mass spectra (electrospray negative ion) sample was dissolved in acetonitrile on a Micromass Quattro spectrometer (Germany). 1H and 13C NMR spectra, using external electronic referencing through the deuterium resonance frequency of the solvent, were determined at 600.17 or 150.91 MHz, respectively, with a JEOL ECA 600 spectrometer fitted with an auto 5 mm X/H probe. Carbon atom types were established in the 13C NMR spectrum by employing a combination of broad- and proton-decoupled and distortionless enhancement by polarization transfer (DEPT) experiments with 64 K data points over a spectrum width of 17,605 · 6 Hz. [1J.C–H] and 2J.C–H and 3J.C–H]. 1H-13C correlations were established by using Heteronuclear multiple quantum correlation (HMQC) and Heteronuclear multiple bound correlation (HMBC) pulse sequences, respectively. 1H-1H correlations were by double quantum filtered correlation spectroscopy (COSY).
The antioxidant assay kit was purchased from Randox Laboratories (Crumlin, Co. Antrim, United Kingdom), and the assay was performed using a Boehringer Mannheim Hitachi 717 automatic analyzer (Japan). Aluminum sheets 20 × 20 cm Silica gel G 60 F254 Merck KGaA 64271 (Darmstadt, Germany), was used for thin-layer chromatography. Silica gel 60 particle size (70–230 mesh) was used for column chromatography. Solvent systems (a) chloroform:ethanol (9:1), (b) ethyl acetate:methanol:water (30:5:4), (c) ethyl acetate:methanol:acetic acid:water (65:15:10:10) were used for developing the chromatoplates. Visualization of chromatograms was achieved under UV before and after exposure to ammonia vapor or by spraying with aluminum chloride (Stahl, Citation1969). All solvents used were of analytical grade.
Plant material
The leaves of Casimiroa edulis. Llave et Lex (Rotaceae) were collected from Dakahlia governorate, Egypt, in 2001. The plant identification was verified by the late Prof. N. El-Hadidi, Professor of Botany, Botany Department, Faculty of Science, Cairo University, and a voucher specimen has been deposited in the herbarium of the Desert Research Center, Cairo, Egypt. The plant sample was air-dried in the shade and then reduced to a fine powder that was packed in tightly closed containers and stored for phytochemical and biological studies.
Preparation of the plant extracts
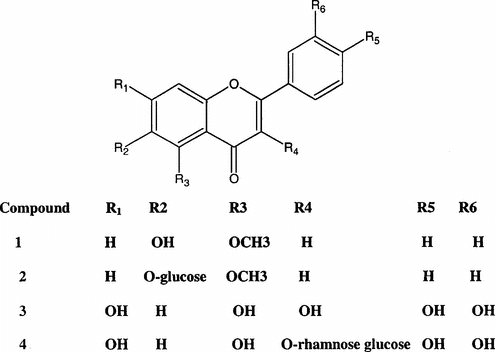
The leaves (2 kg) of C. edulis. were exhaustively extracted with 95% ethanol (EtOH), 3 × 5 l; the extract was filtered under vacuum at a temperature not exceeding 35°C, and concentrated in a rotary evaporator to yield a brownish syrup residue (150 g), which was partitioned between 10 l ethyl acetate (EtOAc) and 1 l H2O. The ethyl acetate fraction, after removal of the solvent and drying over Na2SO4, afforded 30 g of extracts that were subjected to column chromatography on silica gel (500 g) using CHCl3:MeOH (99:1 v/v). Fractions of 80 cm3 were collected; the combined fractions were evaporated to give 600, 400, and 1000 mg, respectively. The first fraction was discarded (chlorophyll and fatty matter), and the other two fractions were reapplied on a silica gel column using ethyl acetate with gradually increased amounts of methanol; purification involved preparative TLC. Final purification was done on Sephadex LH-20 using MeOH/H2O as eluent. Crystallization from chloroform yielded compounds 1–4 ().
Toxicological studies
LD50 of the tested extracts were determined (Finney, Citation1964), and for this purpose, albino mice (20–22 g) were divided into groups of five animals each. Preliminary experiments were done to determine the minimal dose that kills all mice and the maximal dose that fails to kill any animal. Several doses at equal intervals were administrated orally to a group of five mice. Animals were kept under observation for 24 h during which time symptoms of toxicity and rate of mortality in each group were recorded from which the LD50 therapeutic dose was calculated.
Antioxidant measurements
The alcohol and ethyl acetate extracts were centrifuged (2000 × g., 10 min) to remove insoluble material and the supernatants retained. Antioxidant activity in the supernatants in addition to the isolated compounds 1–4 were determined in vitro. () via scavenging of the ABTS˙+ [(2,2′ -azinobis(3-ethylbenzothiazolone-6-sulfonic acid)] radical, which was generated by a metmyoglobin/hydrogenperoxide system (Millere & Rice-Evans, Citation1997). Each plant extract (10 µl) was added separately to a 1-cm pathlength spectrophotometer cuvette (1 ml capacity) containing 20 mM phosphate-buffered saline, pH 7.4, 2.5 µM myoglobin, and 150 µM ABTS. The reaction was initiated by addition of 75 µM hydrogen peroxide, and the absorbance change at 734 nm was monitored at 30°C. A quantitative relationship exists between the absorbance at 734 nm measured after 6 min and the antioxidant property of the plant extract, as determined relative to Trolox (a water-soluble vitamin E analogue) antioxidant standards, and expressed in terms of mM Trolox equivalents (mMTE). Samples of the plant extracts for antioxidant activity were dried to constant weight (80°C for 20 h), and the final antioxidant activity was expressed in terms of mMTE/gram dried tissue. A two-point calibration was used. In the first stage of the assay, myoglobin was reacted with hydrogen peroxide to produce the ferrimyoglobin free radical, which was then incubated with a chromagen, 2,2-amino-di-(3-ethylbenzthiazole sophonate) to produce ATBSR+, a radical cation with a blue-green color measured at 600 nm. Antioxidants in the added plant aqueous extracts suppressed the blue-green color to a degree that was proportional to their concentration and antioxidant property. The absorbance of the resulting oxidized solution was compared with that of the calibrated standard, Trolox (6-hydroxy-2,5,7,8-tetramethylchroman-2-carboxylic acid). Results were expressed as µmol Trolox equivalents per gram plant dry weight.
Table 1 Antioxidant activity of different extracts and isolated compounds of Casimiroa edulis. Llave et Lex
6-Hydroxy 5-methoxyflavone(1)
(50 mg) yellow needle crystals, Rf = 0.78 (system a), m.p. 322°C. UV (MeOH), λmax, nm: 265, 310; (AlCl3): 266, 312; (AlCl3/HCl) 266, 310; (NaOAc) 267, 313; (NaOAc/H3BO3) 267, 313; (NaOMe) 308 and 329, 13C NMR (methanol-d.6 600 MHz): δ. ppm 62.46 (OCH3), 108.19 (C-3), 149.10 (C-5), 119.48 (C-10), 132.49 (C-1′), 127.39 (C-2′ and 6′), 130.26 (C-3′ and 5′), 148.57 (C-6), 133.1 (C-4′), 115.28 (C-8), 125.63 (C-7), 154.19 (C-9), 164.18 (C-2). 180. 29 (C-4).
5-Methoxyflavone 6-O-β-D-glucoside (2)
(100 mg) yellow needle crystals, Rf = 0.65 (system b), m.p. 290°C. UV (MeOH), λmax, nm: 267, 312; (AlCl3) 267, 313; (AlCl3/HCl) 268, 313; (NaOAc) 267, 313; (NaOAc/H3BO3) 267, 314; (NaOMe) 266, 311: 1H NMR (Methanol-d.6 600 MHz): δ ppm 7.98 (d, J. = 8.24 Hz, H-2′-6′), 7.72 (d, J. = 9.54 Hz, H-7), 7.54 (m, 3H, H-3′-5′-4′), 7.45 (d, J. = 9.27 Hz, H-8) 4.96 (d, J. = 7.56 anomeric glucoside proton), 3.9 (S, 3H, OCH3), 3–3.9 (m, rest of sugar protons) 13C NMR (Methanol-d.6 600 MHz): δ ppm 62.46 (OCH3), 108.19 (C-3), 115.28 (C-8), 125.63 (C-7), 119.48 (C-10), 132.49 (C-1′), 130.28 (C-3′ and 5′), 127.46 (C-2′ and 6′), 133.04 (C-4′), 149.10 (C-5), 149.28 (C-6), 154.19 (C-9), 164.18 (C-2), 180. 29 (C-4), 103.38 (C-1″) 62.5 (OCH3), 62.74 (C-6″), 78.4 (C-5″), 78.11 (C-″), 75.05 (C-2″), 71.34 (C-4″).
Results and Discussion
The known compounds 3–4 quercetin and its 3-O.-rutinoside were identified through their color reactions, UV spectra in methanol and with different shift reagents, ESIMS, 1H NMR with authentic samples, and published data (Mabry et al., Citation1970; Markham, Citation1982). The LD50 were found to be over 6 g/kg±0.4. This result revealed the safe use of the plant.
Compound 2 gave 1 and glucose on acid hydrolysis, and 1 and 2 showed yellow color under UV that did not change upon exposure to ammonia vapors. The UV in methanol exhibited two weak bands at λ 310 and 265 nm, respectively, with a weak bathochromic shift in both bands upon addition of sodium methoxide (NaOCH3) for compound 1. The other flavonoid diagnostic reagents gave no shifts for both compounds, indicating the weakness of phenolic hydroxyls in both the new compounds 1 and 2. Both compounds display a distinguishing 1H NMR pattern, as it showed a group of signals around δ. ppm 7.55–7.53 (m, 3H), which was assigned to H-3′,5′, and 4′; however, owing to the small chemical shift differences, it was difficult to distinguish between these latter resonances on the basis of the 1H-1H COSY spectrum; in addition, the HMSQ experment (heteronuclear single quantum coherence) clearly demonstrated that the H-4′ resonance appeared downfield of the H-3′, 5′ resonance (Dagfinn et al., Citation1996). The 2′, 6′ protons were located as doublet, J. 8.24 Hz at δ 7.89. Two ortho. coupled aromatic protons were further located at δ. 7.7 and 7.45 J. 9.54 Hz and 9.27 Hz assigned for H-7 and 7–8, respectively (Dagfinn et al., Citation1996). H-3 gave a strong singlet at δ 6.75; a sharp singlet at δ 3.9 was also detected for both compounds, assigned for a methoxy group OCH3. In addition to all the mentioned signals, 2 also displays one signal for an anomeric protonat δ 4.9 (d, J. 7.5 Hz) indicating a β.-glucosyl moiety; furthermore, the remainder of the sugar protons were detected between δ 3–3.9. The 13C NMR of 2 along with the distortionless enhancement by polarization transfer (DEPT) experment allowed the identification of seven quaternary, one methine, one methyl, and thirteen methylene carbons. Electrospray ionozation mass spectrometry (ESIMS) in its negative mode exhibits m./z. 429.3 (M − 1), its positive mode gave 431.2 (M + 1) and 453.2 (M + Na). Thus, all these data established for 2 the molecular formula of C22H2209. HMSQ proved the suggested linkage of the β.-glucosyl moiety to C-6. HMBC (heteronuclear multiple bond correlations spectrum) allowed the confirmation of all the assigned protons and carbons as it showed long range coupling of H-8 with carbon 10, 6, and 9, also H-7 with carbon 6 and 9 and the OCH3with C-5. The structure of compound 1 was similarly confirmed.
Antioxidant activity (Rice-evans & Miller, Citation1994)
This study has revealed that the total antioxidant content ranged from 389 to 842 µmol Trolox equivalents/g dry weight. Alcohol and ethyl acetate extracts exhibited 842 and 712 µmol Trolox equivalents/g dry weight; compounds 3 and 4 were found to have the highest activity (640 and 772 µmol/g, respectively).
Acknowledgment
All spectra were recorded in the Chemistry and Forensic Medicine Department, School of Life Science, Bradford University, Bradford, United Kingdom. The research was supported at the University of Texas at Austin by the Robert A. Welch Foundation (grant F-130).
References
- Aebi A (1956): Uber die Isolierung von Casimiroa edulis. den samen von Casimiroa edulis. La Liave Lejarza. Helv Chem Acta 9: 145–1500.
- Aiko I, Shamon LA, Boyang Yu, Greenwood EM, Lee SK, Breenen RB, Mehta RG, Farnsworth NR, Fong HS, Pezzuto J, Kinghorn D (1998): Antimutagenic constituants of Casimiroa edulis. with potantial cancer chemopreventiv activity. J Agric Food Chem 46: 3509–3516. [CROSSREF], [CSA]
- Chung KT, Wong TY, Huang YW, Lin Y (1998): Tannins and human health: A review. Crit Rev Food Sci Nutr 38: 421–464. [INFOTRIEVE], [CROSSREF], [CSA]
- Dagfinn Aksens W, Standnes A, Andersen Øyvind M (1996): Complete assignment of the 1H and13C NMR spectra of flavone and it's A-ring hydroxy derivatives. Magn Reson Chem 34: 820–823. [CROSSREF], [CSA]
- Dreyer DL (1968): Citrus bitter principles IX Extractives of Casimiroa edulis. Llava et Lex. Structure of zapoterin. J Org Chem 3: 3477–3482. [CSA]
- Dreyer DL, Bertelli DJ (1976): The structure of zapotin. Tetrahedron 23: 4607–4612. [CROSSREF], [CSA]
- Enriques RG, Romero ML, Escobar LI, Joseph-Nathan P, Reynolds WF (1984): High-performance liquid chromatography study of Casimiroa edulis. II. Determination of furanocuomarins. J Chromatogr 287: 209–219. [CROSSREF], [CSA]
- Exarchou V, Nenadis N, Tsimidou M, Gerothanassis IP, Troganis A, Boskou D (2002): Antioxidant activities and phenolic composition of extracts from Greek oregano, Greek sage and summer savory. J Agric Food Chem 50: 5294–5299. [INFOTRIEVE], [CROSSREF], [CSA]
- Finney DJ (1964): Statistical Methods in Biological Assay. London, Charles, Griffin and Company, p. 97.
- Garratt PJ, Scheinmann F, Sandheimer F (1967): Constituents of Casimiroa edulis. Llave et Lex. VIII. The structures of zapotin and zapotinin. Tetrahedron 23: 2413–2416. [CROSSREF], [CSA]
- Kincl FA, Romo J, Rosenkranz G, Sondheimer F. (1958): The constituents of Casimiroa edulis. Llave et Lex. Part I. The seeds. J Chem Soc 416: 4163–4169. [CSA]
- lriarte J, Kincl FA, Rosenkranz G, Sondheimer F, Syntex SA, Mexico DF, Mex (1956): The constituents of Casimiroa edulis.. J Chem Soc: 4170–4173. [CROSSREF], [CSA]
- Mabry TJ, Markham KR, Thomas MB (1970): The Systematic Identification of Flavonoids. New York, Heidelberg, Berlin, Springer-Verlag, p. 253.
- Magos GA.; Vidrio H (1991): Pharmacology of Casimiroa edulis.. Blood pressure and heart rate effects in anesthetized rats. Planta Med 7: 20–24. [CSA]
- Magos GA, Vidrio H, Enriquez R (1995): Pharmacology of Casimiroa edulis.. III. Relaxant and contractile effects in rat aortic ringa. J. Ethnopharmacol 47: 1–8. [INFOTRIEVE], [CROSSREF], [CSA]
- Magos GA, Vidrio H, Reynolds WF, Enriquez RG (1999): Pharmacology of Casimiroa edulis. IV Hypotensive effects of compounds isolated from methanol extracts in rats and Guinea pigs. J Ethnopharmacol 64: 35–44. [INFOTRIEVE], [CROSSREF], [CSA]
- Markham KR (1982): Techniques of Flavonoid Identification. London, Academic Press, p. 59.
- Miller NJ, Rice-Evans CA (1996): Spectroscopic determination of antioxidant activity. Redox Rep 2: 161–171. [CSA]
- Miller NJ, Rice-Evans CA (1997): Factors influencing the antioxidant activity determined by the ABTS + radical cation assay. Free Radical Res 26: 195–199. [CSA]
- Murphy JW, Toube T, Cross DA (1968): Spectra and stereochemistry. XXIX. The structure of zapoterin. Tetrahedron 49: 5153–5156. [CSA]
- Petit-Play G, Rideau M, Chenieux CJ (1982): Etude dde oquelquea Rutacees a alcaloides. II. Ruta Graveolens.: Revue otanique cimique et pharmacologique. (Etue particuliere dea acaloides quanternaires quinoleiques.) Plant Med Phytother 16: 55–72. [CSA]
- Pietta PG (2000): Flavonoids as antioxidants. J Nat Prod 63: 1035–1042. [INFOTRIEVE], [CROSSREF], [CSA]
- Power FB, Callan T (1911): The constituents of the seeds of Casimiroa edulis.. J Chem Soc 1993–2013. [CSA]
- Raman S, Reddy J, Lipscomb WN, Kapoor AL, Dijerassi C (1962): Structure of the alkaloid casimiroedine. Tetrahedron Lett 9: 357–360. [CSA]
- Rice-Evans C, Miller NJ (1994): Total antioxidant status in plasma and body fluids. Meth Enzymol 234: 279–293. [INFOTRIEVE], [CSA]
- Romero ML, Esobar LI, Lozoya X, Eniquez RG (1983): High-performance liquid chromatographical study of Casimiroa edulis.. J Chromatogr 281: 245–251. [CROSSREF], [CSA]
- Shahidi F, Janitha PK, Wanasundara PD (1992): Phenolic antioxidants. Crit Rev Food Sci Nutr 32: 67–103. [INFOTRIEVE], [CSA]
- Sondheimer F, Meisels A, Kinel FA, (1959): Constituents of Casimiroa edulis. Llave et Lex.V. Identity of casimirolid and obacunone. Org Chem 23: 2413–2416. [CSA]
- Stahl E (1969): Thin-Layer Chromatography, 2nd ed. London, George Allon and Unwirid, Berlin, Springer, p. 967.
- Toube TP, Murphy JW, Cross AAD (1967): The structures of edulitine and edulinine. XXIV. Spectra and stereochemistry. Tetrahedron 23: 2061–2065. [CROSSREF], [CSA]