Abstract
We present the results of an in vitro. investigation of the inhibitory effect of the prenylated flavonoid, 5,7,4′-trihydroxy-6,8-diprenylisoflavone, isolated from Euchresta japonica. Benth (Luguminosae) and Artocarpus heterophyllus. Lam. (Moraceae), on lipid peroxidation by interaction of hemoglobin and hydrogen peroxide. This inhibitory effect was stronger than that of genistein, a non-prenylated isoflavone. The result demonstrates that prenylated flavonoid has more antioxidative effect than non-prenylated flavonoid.
Introduction
Lipid peroxidation (LPO) is induced by the interaction of hemoglobin and hydrogen. Such LPO is involved in atherosclerosis (Berliner & Heinecke, Citation1996) and liver disease (Jaeschke et al., Citation2002). It was recognized that some flavonoids have inhibitory effects on LPO by oxygen free radical (Heim et al., Citation2002). We showed that some isoflavones, such as biochanin A, daidzein, formononetin, and genistein, have inhibitory effects on lecithin peroxidation by the interaction of hemoglobin and hydrogen peroxide (Toda & Shirataki, Citation1999). There are no prenyl groups in the chemical structures of biochanin A, daidzein, formononetin, and genistein. Shirataki et al. (Citation1982) isolated prenylated flavonoid, 5,7,4′ -trihydroxy-6,8-diprenylisoflavone, from Euchresta japonica. Benth (Leguminosae) and Artocarpus heterophyllus. Lam. (Moraceae), which has been used as an antidote or an analgesic.
In this paper, we investigated the inhibitory effect of 5,7,4′ -trihydroxy-6,8-diprenylisoflavone on LPO, which was induced by the interaction of hemoglobin and hydrogen peroxide.
Materials and Methods
Reagents
Butylated hydroxytoluene (BHT), genistein, lecithin (from egg yolk), mannitol, thiobarbituric acid (TBA), α-tocopherol, and trichloroacetic acid (TCA) were obtained from Wako Pure Chemicals (Osaka, Japan).
Extraction and isolation of 5,7,4′-trihydroxy-6,8-diprenylisoflavone from Euchresta japonica.
The air-dried roots of Euchresta japonica. and Artocarpus heterophyllus. were collected in Mt. Ichifusa, Kumamoto prefecture, Japan, in April 1981 and identified by Dr. Yoshiaki Shirataki, Department of Pharmaceutical Science, Josai University, Japan. A voucher specimen was deposited in the Department of Pharmaceutical Science, Josai University, Japan, by Dr. Yoshiaki Shirataki. Cut root was extracted with methanol. The methanol extract was fractioned repeatedly by silica gel column chromatography, with benzene–ethyl acetate mixture as the eluent. They afforded 5,7,4′ -trihydroxy-6,8-diprenylisoflavone, identified on the basis of melting point and spectral comparison by Dr. Yoshiaki Shirataki, Department of Pharmaceutical Science, Josai University, Japan (Shirataki et al., Citation1982).
Inhibitory test on lecithin peroxidation
Lecithin peroxidation induced by the interaction of hemoglobin and hydrogen peroxide was performed by the following method. The test sample was added to the reaction mixture containing 2.5 mM lecithin, 15 µM hemoglobin, and 15 µM hydrogen peroxide in 50 mM Tris-HCl buffer (pH 7.4) in a total volume of 1 ml. The mixture was incubated at 37°C for 1 h. To the reaction mixture was added 2 ml of TBA reagent that contained 0.37% TBA, 15% TCA, 0.04% BHT, and 2% ethanol. This mixture was heated at 100°C for 15 min. The solution was centrifuged at 3000 rpm for 10 min. The absorbance of the supernatant at 535 nm was determined as malondialdehyde (MDA) (Burge & Aust, Citation1978). The inhibitory ratio of the test sample was evaluated by the following equation:
where MDab is absence of test sample and MDApr is presence of test sample.
Inhibitory ratios of each sample were tested with five experiments.
Statistical analysis
Values were expressed as the mean ± standard error of five experiments. The results were analyzed by the non-parametric analysis of variation (ANOVA) Scheffe f.-test.
Results and Discussion
shows that the inhibitory ratio of 5,7,4′ -trihydroxy-6,8-diprenylisoflavone is 80.7 ± 2.4% at a concentration of 250 µM and increased in a concentration-dependent manner.
Table 1 Inhibitory effect of 5,7,4′-trihydroxy-6,8-diprenylisoflavone on LPO by interaction of ferrous ion and hydrogen peroxide in vitro.
The inhibitory ratios of genistein as a non-prenylated isoflavone, mannitol as hydroxy radical scavenger, and α-tocopherol as an antioxidant, are 67.9 ± 0.5%, 25.7 ± 4.3%, and 98.2 ± 5.3% at a concentration of 250 µM ().
The 50% inhibitory concentration (IC50) of 5,7,4′-trihydroxy-6,8-diprenylisoflavone, genistein, and α-tocopherol are 0.12, 3.62, and 3.07 µM, resepectively.
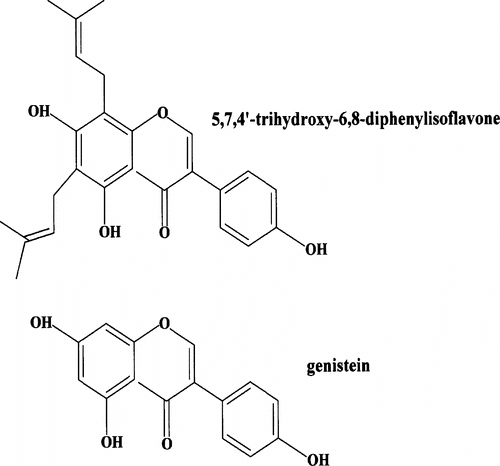
Genistein is 5,7,4′ -trihydroxyisoflavone. 5,7,4′-Trihydroxy-6,8-diprenylisoflavone has two hydroxy groups at 6,8-position (). Isoflavonoids have been shown to have antioxidative effects (Harper et al., Citation1999). It has been suggested that genistein may have antioxidant activity on LPO (Ferretti et al., Citation2004). Prenylated flavonones, cycloheterophullin, and artonins A and B, isolated from Artocarpus heterophyllus. Lam., have powerful antioxidant properties (Ko et al., Citation1998). Genistein has been demonstrated to have antioxidative effects on oxidative stress such as LPO by oxygen free radical (Toda & Shirataki, Citation1999). The current results demonstrate that a prenylated flavonoid has more antioxidative effect than non-prenylated flavonoids.
Our results are consistent with this previous observation. Further studies on the relationship between herbs containing prenylated isoflavonoids and antioxidative effects are in progress in our laboratory.
References
- Berliner JA, Heinecke JW (1996): The role of oxidized lipoprotein in atherogenesis. Free Radic Biol Med 20: 707–727. [INFOTRIEVE], [CSA]
- Burge JA, Aust SD (1978): Microsomal lipid peroxidation. Methods Enzymol 52: 302–310. [CSA]
- Ferretti G, Bacchetti T, Menanno F, Curatola G (2004): Effects of genistein against copper-induced lipid peroxidation of human high density lipoprotein (HDL). Atherosclerosis 172: 22–61. [CROSSREF], [CSA]
- Harper A, Kerr DJ, Gescher A, Chipman JK (1999): Antioxidant effects of isoflavonoids and lignans, and protectior against DNA oxidation. Free Radic Res 31: 149–160. [INFOTRIEVE], [CSA]
- Heim KE, Tagliaferro AR, Bobilya DJ (2002): Flavonoid antioxidants: Chemistry, metabolism and structure-activity relationships. J Nutr Biochem 13: 572–584. [INFOTRIEVE], [CROSSREF], [CSA]
- Jaeschke H, Gores GJ, Cederbaum AI, Hinson JA, Pessayre D, Lemaster JJ (2002): Mechanism of hepatotoxicity. Toxicol Sci 65: 166–176. [INFOTRIEVE], [CROSSREF], [CSA]
- Ko FN, Cheng ZJ, Lin CN, Teng CM (1998): Scavenging and antioxidant properties of prenylflavones isolated from Artocarpus.. Free Radic Biol Med 25: 160–168. [INFOTRIEVE], [CROSSREF], [CSA]
- Shirataki Y, Manaka A, Yokoe I, Komatsu M (1982): Two prenylflavones from Euchresta japonica.. Phytochemistry 21: 2959–2963. [CROSSREF], [CSA]
- Toda S, Shirataki Y (1999): Inhibitory effects of isoflavones on lipid peroxidation by reactive oxygen species. Phytother Res 13: 163–165. [INFOTRIEVE], [CROSSREF], [CSA]