Abstract
Two dioxoaporphine alkaloids, N-methylouregidione (1) and ouregidione (2), and two oxoaporphine alkaloids, liriodenine (3) and oxostephanine (4), were isolated from the aerial part of Pseuduvaria setosa (King) J. Sinclair. All four alkaloids displayed antituberculosis activity against Mycobacterium tuberculosis, with 3 as the most active at minimum inhibitory concentration (MIC) of 12.5 µg/ml. Compound 3 was also the only alkaloid isolated from this plant that displayed antimalarial activity against Plasmodium falciparum with the 50% inhibitory concentration (IC50) of 2.8 µg/ml. Compounds 3 and 4 were strongly cytotoxic to both epidermoid carcinoma (KB) and breast cancer (BC) cell lines, whereas 2 was moderately active against BC cells. Both 1 and 2 were active against small cell lung cancer (NCI-H187) cell line and were able to stimulate lymphocyte proliferation with stimulation indices (SI) of more than 1.
Introduction
Several plants within the family Annonaceae have been demonstrated to contain chemical constituents, including alkaloids, terpenoids, and polyacetylenes, with interesting pharmacological activities. The genus Pseuduvaria. of this family is a small genus with ca. 17 species distributed in Southeast Asia (Mabberley, Citation1990). Pseuduvaria setosa. (King) J. Sinclair (syn.: Orophea setosa. King) is one of the three Pseuduvaria. species that can be found in southern Thailand (Smitinand, Citation2001). The root of this small rainforest tree has been used traditionally as a cure for coughs and externally as rubbing powder on the body to treat fever (Perry, Citation1980). Previous work on plants of this genus have revealed the presence of alkaloids in every Pseuduvaria. species investigated (Johns et al., Citation1970; Mahmood et al., Citation1986; Zhong et al., Citation1988). However, no information on the biological activities of these plants is currently available.
In our search for biologically active constituents from Thai annonaceous plants, we have subjected the ethanol extract of the aerial part of Pseuduvaria setosa. to in vitro. screening for anticancer activity against three cancer cell lines (KB, BC, and NCI-H187), antimalarial activity against Plasmodium falciparum., antituberculosis activity against Mycobacterium tuberculosis., and immunomodulating activity through its ability to stimulate or suppress lymphocyte proliferation. The extract was strongly active against small cell lung cancer cell line (NCI-H187) with an IC50 (50% inhibitory concentration) of 3.0 µg/ml and was moderately active against the malarial protozoa with an IC50 of 8.3 µg/ml. Subsequent extraction demonstrated these activities to be concentrated within the alkaloid-containing hexane and CHCl3 fractions. The current paper describes the isolation, characterization, and biological activities of four aporphine alkaloids isolated from the aerial part of Pseuduvaria setosa..
Materials and Methods
General experimental procedures
Melting points were measured on a Fisher-Johns apparatus (Fisher Scientific International, Hampton, USA). IR spectra, in KBr, were obtained on a Perkin-Elmer FT-IR 1760X spectrometer (Perkin-Elmer, Wellesley, USA). Mass spectra (EI-MS) were measured at 70 eV on a LCT-LCMS Micromass spectrometer (Waters, Milford, USA). NMR spectra in CDCl3 were recorded at 300 MHz on a Bruker Avance DPX-300 spectrometer (Bruker Analytik GMBH, Rheinstetten, Germany).
Plant material
The aerial part of Pseuduvaria setosa. (King) J. Sinclair (Annonaceae) was collected from Trang, Thailand, in March 2002 and identified by R. Suttisri. A voucher specimen (RS02031) was deposited for future reference in the herbarium of the Department of Pharmaceutical Botany, Faculty of Pharmaceutical Sciences, Chulalongkorn University, Thailand.
Isolation of alkaloids
The dried aerial part (220 g) of P. setosa. was cut into small pieces and repeatedly extracted with 95% EtOH. The filtered extract was evaporated in vacuo. at 45°C to give an EtOH extract (23.2 g), which was resuspended in a mixture of MeOH and water (300 ml), then successively extracted with hexane (300 ml × 5) and CHCl3 (300 ml × 5). Each fraction was evaporated under reduced pressure to yield hexane fraction (PS002, 6.2 g), CHCl3 fraction (PS003, 3.6 g) and aqueous fraction (PS004, 11.2 g), respectively. Both hexane and CHCl3 fractions, when subjected to in vitro. screening tests, displayed several biological activities and, therefore, were further fractionated by column chromatography.
The hexane fraction was loaded onto a silica gel column and eluted with a gradient system of hexane: EtOAc from 9:1 to 0:1, then washed down with MeOH. Each 30-ml fraction was examined by TLC, and fractions with similar chromatographic patterns were combined to give nine fractions (PS201–PS209). Fraction PS207 was further purified on a silica gel column eluted with CHCl3:MeOH (20:1), followed by a Sephadex LH20 column eluted with CHCl3:MeOH (2:1), to yield N.-methylouregidione (1) (1.2 mg) ().
The CHCl3 fraction was subjected to silica gel column chromatography eluted with a gradient system of hexane, hexane:EtOAc, EtOAc:MeOH, and, finally, MeOH. Fractions of 30 ml each were collected and combined according to their TLC pattern into 21 fractions (PS301–PS321). Fraction PS306 was purified by gel filtration chromatography using two successive Sephadex LH20 columns eluted with CHCl3:MeOH (2:1) and MeOH, respectively, to give N.-methylouregidione (1) (4.9 mg). Fractions PS309, PS312, and PS315 were subjected to similar procedure to yield ouregidione (2) (7.9 mg), liriodenine (3) (6.7 mg), and oxostephanine (4) (1.5 mg), respectively.
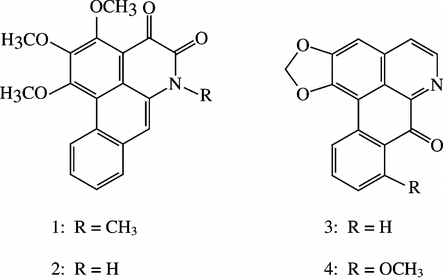
The structures of all four alkaloids from P. setosa. were determined based on their spectral data in comparison with literature values. Their structures are given in .
N-Methylouregidione (1,2,3-trimethoxy-4,5-dioxo-6a,7-dehydroaporphine; alkaloid 1.)
Yellow amorphous powder; IR (KBr) νmax 2923, 1662, 1634, 1389 cm−1; EI-MS m/z. 351 [M]+; 1H NMR (300 MHz, CDCl3) δ 9.45 (1H, m, H-11), 7.88 (1H, m, H-8), 7.63 (2H, m, H-9, H-10), 7.60 (1H, s, H-7), 4.16 (3H, s, 3-OCH3), 4.11 (3H, s, 2-OCH3), 4.07 (3H, s, 1-OCH3), and 3.84 (3H, s, N-CH3), consistent with the literature (Mahmood et al., Citation1986).
Ouregidione (alkaloid 2.)
Orange needle crystals; m.p. 248–250°C; IR (KBr) νmax 3359, 2922, 1661, 1633, 1510, 1389 cm−1; EI-MS m/z. 337 [M]+; 1H NMR (300 MHz, CDCl3) δ 11.45 (1H, s, NH-6), 9.47 (1H, m, H-11), 7.96 (1H, m, H-8), 7.80 (1H, s, H-7), 7.64 (2H, m, H-9, H-10), 4.20 (3H, s, 3-OCH3), 4.16 (3H, s, 2-OCH3) and 4.09 (3H, s, 1-OCH3), in agreement with reported data (Wijeratne et al., Citation1996).
Liriodenine (alkaloid 3.)
Yellow amorphous powder; IR (KBr) νmax 2920, 1652, 1577, 1418, 1310, 1262, 1228 cm−1; EI-MS m/z. 275 [M]+; 1H NMR (300 MHz, CDCl3) δ 8.84 (1H, d, J. = 5.0 Hz, H-5), 8.60 (1H, d, J. = 7.8 Hz, H-11), 8.55 (1H, d, J. = 7.8 Hz, H-8), 7.74 (1H, d, J. = 5.0 Hz, H-4), 7.72 (1H, t, J. = 7.8 Hz, H-10), 7.54 (1H, t, J. = 7.8 Hz, H-9), 7.14 (1H, s, H-3) and 6.35 (2H, s, –OCH2O–), in agreement with reported data (Wijeratne et al., Citation1996).
Oxostephanine (alkaloid 4.)
Orange amorphous powder; IR (KBr) νmax 2923, 1659, 1633, 1591, 1578, 1469, 1411, 1257 cm−1; EI-MS m/z. 305 [M]+; 1H NMR (300 MHz, CDCl3) δ 8.81 (1H, d, J. = 5.1 Hz, H-5), 8.28 (1H, d, J. = 8.3 Hz, H-11), 7.67 (1H, d, J. = 5.1 Hz, H-4), 7.62 (1H, t, J. = 8.3 Hz, H-10), 7.14 (1H, s, H-3), 7.07 (1H, d, J. = 8.3 Hz, H-9), 6.32 (2H, s, –OCH2O–) and 4.03 (3H, s, 8-OCH3), consistent with reported values (Watanabe et al., Citation1975).
Antimalarial assay
In vitro. antimalarial activity was evaluated against Plasmodium falciparum. (K1, multidrug-resistant strain), employing the method described by Trager and Jensen (Citation1976). Quantitative assessment of antimalarial activity was performed in duplicate using the microculture radioisotope technique (Desjardins et al., Citation1979), of which the 50% inhibitory concentration (IC50) represents the concentration of the extract or alkaloid causing 50% reduction in the growth of P. falciparum. as indicated by the in vitro. uptake of [3H]-hypoxanthine by the parasite. Dihydroartemisinin was used as positive control. The IC50 values of more than 10 µg/ml were considered inactive.
Antitumor assay
Cytotoxicity of the plant extracts and alkaloids against three human cancer cell lines, epidermoid carcinoma (KB), breast cancer (BC), and small cell lung cancer (NCI-H187), was evaluated using the colorimetric method described by Skehan and co-workers (Citation1990). Ellipticine was employed as the reference substance. The IC50 values of more than 20 µg/ml were considered inactive.
Antituberculosis assay
Antituberculosis assay against Mycobacterium tuberculosis. H37Ra was performed in duplicate using the microplate Alamar blue assay (Collins & Franzblau, Citation1997). The reference compounds were isoniazid and kanamycin sulfate. The minimum inhibitory concentrations (MICs) of not more than 200 µg/ml were considered active.
Stimulation of lymphocyte proliferation assay
In vitro. cytotoxicity against splenic lymphocytes of plant extracts and pure compounds was determined by using the Alamar blue method (Collins & Franzblau, Citation1997). Then, immunomodulating activity of the extracts and alkaloids was determined according to Ahmed et al. (Citation1994) and Zhi-Jun et al. (Citation1997). Splenic cell suspension obtained from Wistar rat was adjusted to 2.5 × 106 cells/ml in complete RPMI-1640 medium. One hundred microliters of the suspension were placed in each well of sterile 96-well culture plate containing 10 µl of two-fold dilutions of 0.01–200 µg/ml of extract or alkaloid or 0.5% dimethylsulfoxide (DMSO) as the solvent control or 5–10 µg/ml of concanavalin A (Con A) as the positive control. Ninety microliters of complete RPMI-1640 medium were then added, and the plate was incubated at 37°C in a 5% CO2 incubator under humidified conditions for 48 h.
After the incubation period of 48 h, 20 µl of Alamar blue (Serotec, Oxford UK) were added to each well, and the plate was re-incubated for another 24 h. The absorbance at 570 nm (reduced form) and 600 nm (oxidized form) was then measured using a microplate reader (Anthos htII, Anthos Labtec, Salzbung, Austria). Specific optical density (OD), obtained by subtracting the absorbance of each sample at 600 nm from that at 570 nm, was used in the calculation of the stimulation index (SI) of lymphocyte proliferation:
The experiment was done in triplicate. The results are presented as mean±standard error of mean (SEM) of SI.
Results and Discussion
In vitro. antimalarial, anticancer, and antituberculosis activities, together with stimulation indices of lymphocyte proliferation, of the ethanol extract (PS001), hexane (PS002), CHCl3 (PS003), and aqueous (PS004) fractions of Pseuduvaria setosa. are presented in . Both the hexane and CHCl3 fractions could stimulate proliferation of the lymphocytes and were active against small cell lung cancer (NCI-H187) and Mycobacterium tuberculosis. H37Ra, although only the hexane fraction was active against Plasmodium falciparum..
Table 1 In vitro. biological activities of fractions isolated from the aerial part of Pseuduvaria setosa.
The dioxoaporphine alkaloid N.-methylouregidione (1) was isolated from the hexane fraction. As shown in , this alkaloid exhibited antituberculosis and moderate antitumor activities against small cell lung cancer. However, it was not active against the Plasmodium. protozoa, suggesting that other constituents of the hexane fraction might be responsible for its antimalarial activity. Alkaloid 1 has previously been reported as a constituent of another Pseuduvaria. species, P. macrophylla. (Mahmood et al., Citation1986). Total synthesis of this dioxoaporphine alkaloid has been performed (Hoarau et al., Citation2001).
Table 2 In vitro. biological activities of aporphine alkaloids isolated from Pseuduvaria setosa.
Isolation of the CHCl3 fraction yielded four isoquinoline alkaloids: the dioxoaporphine 1 and ouregidione (2), and the oxoaporphine liriodenine (3) and oxostephanine (4). All four alkaloids were active against Mycobacterium tuberculosis. with 3 as the most active at MIC of 12.5 µg/ml. On the other hand, the ability of these pure compounds to stimulate or suppress lymphocyte proliferation appears to be related to whether they are dioxoaporphine or oxoaporphine alkaloids. At the nontoxic concentration to splenic cells of 12.5 µg/ml, both dioxoaporphines 1 and 2 were able to stimulate (with SI of more than 1) the immune response, whereas the oxoaporphines 3 and 4 tended to suppress lymphocyte proliferation (SI less than 1). In combination as the constituents of the CHCl3 fraction, these alkaloids produced the marginal lymphocyte stimulating effect of the fraction.
Alkaloids 3 and 4 were strongly cytotoxic to KB and BC cell lines, while 1 was nontoxic to both cells and 2 was moderately active against breast cancer cells. Liriodenine (3) has previously been demonstrated to be potently cytotoxic against human KB, A-549 lung carcinoma, HCT-8 colon tumor cells, as well as murine P-388 and L-1210 lymphocytic leukemia cells (Wu et al., Citation1988). Oxostephanine (4) was reported as selectively toxic to HeLa uterus carcinoma cells (Rodríguez et al., Citation1999), and was also shown to possess antibacterial (Ferdous et al., Citation1992) and anti-herpes virus activities (Montanha et al., Citation1995). Both dioxoaporphines 1 and 2 were active against human small cell lung cancer NCI-H187. However, N.-methyl substitution might diminish this activity because 2, without N.-methyl substitution, was seven times more active than 1. Ouregidione (2) has been shown to exhibit larvicidal activity against the mosquito Aedes aegypti. (Ee et al., Citation1999). This is the first report of its anticancer activity.
Of the four alkaloids isolated from Pseuduvaria setosa., only liriodenine (3) displayed antimalarial activity (IC50 = 2.8 µg/ml). This is in agreement with a previous study by del Rayo Camacho and co-workers (Citation2000), which found the oxoaporphine alkaloid to be active against both Plasmodium falciparum. and Leishmania donovani. promastigotes. Liriodenine was also demonstrated to act as a muscarinic receptor antagonist (Lin et al., Citation1994), to display antiarrhythmic and positive inotropic effects (Chang et al., Citation1996), and to potently inhibit mammalian topoisomerase II (Woo et al., Citation1997). The alkaloid has been found as a constituent of four out of six Pseuduvaria. species investigated to date, and its prevalence within this genus suggests these tropical plants to be notable sources for biologically active agents.
Acknowledgments
The authors thankfully acknowledge Bioassay Research Facility of BIOTEC, NSTDA, Thailand, for the evaluation of antituberculosis, antimalarial, and anticancer activities. This work was supported by grants from the Biodiversity Research and Training Program (BRT) and Thailand Research Fund.
References
- Ahmed SA, Gogal RM, Jr, Walsh JE (1994): A new rapid and simple non-radioactive assay to monitor and determine the proliferation of lymphocytes: An alternative to [3H]thymidine incorporation assay. J Immunol Methods 170: 211–224. [INFOTRIEVE], [CSA]
- Chang GJ, Wu MH, Wu YC, Su MJ (1996): Electrophysiological mechanisms for antiarrhythmic efficacy and positive inotropy of liriodenine, a natural aporphine alkaloid from Fissistigma glaucescens.. Br J Pharmacol 118: 1571–1583. [INFOTRIEVE], [CSA]
- Collins L, Franzblau SG (1997): Microplate Alamar blue assay versus BACTEC 460 system for high-throughput screening of compounds against Mycobacterium tuberculosis. and Mycobacterium avium.. Antimicrob Agents Chemother 41: 1004–1009. [INFOTRIEVE], [CSA]
- del Rayo Camacho M, Kirby GC, Warhurst DC, Croft SL, Phillipson JD (2000): Oxoaporphine alkaloids and quinones from Stephania dinklagei. and evaluation of their antiprotozoal activities. Planta Med 66: 478–480. [CROSSREF], [CSA]
- Desjardins RE, Canfield CJ, Haynes JD, Chulay JD (1979): Quantitative assessment of antimalarial activity in vitro by a semiautomated microdilution technique. Antimicrob Agents Chemother 16: 710–718. [INFOTRIEVE], [CSA]
- Ee GCL, Lee HL, Goh SH (1999): Larvicidal activity of Malaysian Goniothalamus. species. Nat Prod Lett 13: 137–142. [CSA]
- Ferdous AJ, Islam MO, Hasan CM, Islam SN (1992): In vitro antimicrobial activity of lanuginosine and oxostephanine. Fitoterapia 63: 549–550. [CSA]
- Hoarau C, Couture A, Deniau E, Grandclaudon P (2001): A new synthetic approach to dioxoaporphines – application to the synthesis of N.-methylouregidione. Eur J Org Chem 2559–2567. [CROSSREF], [CSA]
- Johns SR, Lamberton JA, Li CS, Sioumis AA (1970): Alkaloids from Pseuduvaria. species, Schefferomitra subaequalis., and Polyalthia nitidissima.; Isolation of a new alkaloid shown to be 1,2,9,10-tetramethoxynoraporphine. Aust J Chem 23: 423–426. [CSA]
- Lin CH, Yang CM, Ko FN, Wu YC, Teng CM (1994): Antimuscarinic action of liriodenine, isolated from Fissistigma glaucescens., in canine tracheal smooth muscle. Br J Pharmacol 113: 1464–1470. [INFOTRIEVE], [CSA]
- Mabberley DJ (1990): The Plant-book. Cambridge, Cambridge University Press, p. 483.
- Mahmood K, Chan KC, Park MH, Han YN, Han BH (1986): An aporphinoid alkaloid from Pseuduvaria macrophylla.. Phytochemistry 25: 1509–1510. [CROSSREF], [CSA]
- Montanha JA, Amoros M, Boustie J, Girre L (1995): Anti-herpes virus activity of aporphine alkaloids. Planta Med 61: 419–424. [INFOTRIEVE], [CSA]
- Perry LM (1980): Medicinal Plants of East and Southeast Asia: Attributed Properties and Uses. Cambridge, MA, MIT Press, p. 21.
- Rodríguez M, Hasegawa M, Méndez J, Pereira G, Arvelo F (1999): Bioactive oxoaporphine alkaloids from Guatteria calva.. Fitoterapia 70: 74–76. [CROSSREF], [CSA]
- Skehan P, Storeng R, Scudiero D, Monks A, McMahon J, Vistica D, Warren JT, Bokesch H, Kenney S, Boyd MR (1990): New colorimetric cytotoxicity assay for anticancer-drug screening. J Natl Cancer Inst 82: 1107–1111. [INFOTRIEVE], [CSA]
- Smitinand T (2001): Thai Plant Names: Revised Edition. Bangkok, Prachachon, pp. 434–435.
- Trager W, Jensen JB (1976): Human malaria parasites in continuous culture. Science 193: 673–675. [INFOTRIEVE], [CSA]
- Watanabe Y, Matsui M, Iibuchi M, Hiroe S (1975): Two oxoaporphine alkaloids of Stephania japonica.. Phytochemistry 14: 2522–2523. [CROSSREF], [CSA]
- Wijeratne EMK, Hatanaka Y, Kikuchi T, Tezuka Y, Gunatilaka AAL (1996): A dioxoaporphine and other alkaloids of two annonaceous plants of Sri Lanka. Phytochemistry 42: 1703–1706. [CROSSREF], [CSA]
- Woo SH, Reynolds MC, Sun NJ, Cassady JM, Snapka RM (1997): Inhibition of topoisomerase II by liriodenine. Biochem Pharmacol 54: 467–473. [INFOTRIEVE], [CROSSREF], [CSA]
- Wu YC, Lu ST, Chang JJ, Lee KH (1988): Cytotoxic aporphinoid alkaloids from Thalictrum sessile.. Phytochemistry 27: 1563–1564. [CROSSREF], [CSA]
- Zhi-Jun Y, Sriranganathan N, Vaught T, Arastu SK, Ahmed SA (1997): A dye-based lymphocyte proliferation assay that permits multiple immunological analyses: mRNA, cytogenetic, apoptosis, and immunophenotyping studies. J Immunol Methods 210: 25–39. [INFOTRIEVE], [CROSSREF], [CSA]
- Zhong SM, Zhao SS, Xie N (1988): Alkaloids from Pseuduvaria indochinensis.. Phytochemistry 27: 4004–4005. [CROSSREF], [CSA]