Abstract
Transformed root cultures of Catharanthus roseus. Linn. (Apocynaceae) were supplied with precursors and organic compounds that interfere with precursor metabolism to understand more about alkaloid production. Alkaloid biosynthesis was found to be limited by the supply of loganin but not tryptamine during stationary growth phase. Moreover, multiple feedings of loganin increased the accumulation of alkaloids, supporting the importance of terpenoid pathway in alkaloid biosynthesis. Significant improvements in productivity were seen with phenobarbital and succinic acid, which provide further evidence for the regulatory role of G10H and of non-mevalonate pathway in alkaloid biosynthesis in C. roseus..
Introduction
Two convergent metabolic pathways supply the indole and the iridoid precursors for the biosynthesis of alkaloids in Catharanthus roseus. Linn. (Apocynaceae). The optimization of culture conditions such as medium composition including addition of biosynthetic precursor(s) to the medium may enhance secondary metabolite production where the productivity is limited by a lack of that particular precursor (Veeresham & Kokate, Citation1997). Contradictory effects on terepene indole alkaloids (TIA) accumulation have been obtained for the addition of tryptophan and tryptamine (Doller et al., Citation1976; Zenk et al., Citation1977; Krueger & Carew, Citation1978). In general, most of the cell and tissue cultures of C. roseus. have a limitation in the supply of terpenoid precursors, which can be overcome by the addition of either loganin or secologanin (Zenk et al., Citation1977; Naudascher et al., Citation1989aCitation1989b; Moreno et al., Citation1993; Whitmer et al., Citation1998; Morgan & Shanks, Citation2000). In a cell line overexpressing the strictosidine synthase (str.) gene, combined feeding of loganin and tryptamine enhanced greatly the flux into alkaloid pathway during late growth phase (Whitmer et al., Citation1998). Moreover, multiple feedings of precursors in such transgenic cell cultures could increase alkaloid levels significantly (Whitmer et al., Citation2002aCitation2002b).
On the basis of the knowledge of regulation of biosynthetic pathways, several organic compounds have been added in order to improve the availability of precursors for the production of alkaloids in cell cultures of C. roseus.. Bioregulators like phenobarbital, trans.-cinnamic acid, succinic acid, and malic acid have been found to enhance the flux into alkaloid pathway, thereby improving the yields (Simpson & Kelly, Citation1989; Contin et al., Citation1999; Zhao et al., Citation2000Citation2001). The synthesis and accumulation of secondary metabolites is closely related to tissue differentiation and, as a result, transformed root cultures have become a popular culture system in recent times for studies on biotechnological production. Such hairy root cultures possess additional advantages like genetic and biosynthetic stability and faster growth rate. Hence, it was believed interesting to study the effect of feeding loganin, alone or in combination with tryptamine, at different growth phases as well as the frequency of feeding precursors on alkaloid production in transformed root cultures of C. roseus.. Also, the addition of various organic compounds was undertaken for testing the feasibility of improving alkaloid yields.
Materials and Methods
Transformed root cultures
Transformed root cultures were initiated from leaf explants of C. roseus. var. nirmal. by infecting with Agrobacterium rhizogenes. A4 as described by Giri et al. (Citation2001). Transgenecity of the roots was confirmed by detecting mannopine by paper electrophoresis (Petit et al., Citation1983). The roots (50 mg fresh weight) were grown in 100-ml Erlenmeyer flasks containing 25 ml of 1/4 Murashige and Skoog (MS) medium (Murashige & Skoog, Citation1962) supplemented with sucrose (30 g/l) on a gyratory shaker (100 rpm) at 26°C in the dark.
Addition of precursors and organic compounds
Solutions of loganin (1 mg/ml), tryptamine hydrochloride (2 mg/ml), and succinic acid (60 mg/ml) were prepared in double-distilled water. Solutions of trans.-cinnamic acid (75 mg/ml) and phenobarbital (2 mg/ml) were prepared in 50% ethanol. The solutions were filter sterilized before addition to the cultures. Required volumes of stock solutions of loganin and tryptamine hydrochloride were added once or multiple times on days 13, 17, and 21 according to the design of the experiment to get a final concentration of 52 µM each. The organic compounds, tested at two concentrations each, were added to 21-day-old cultures.
Extraction and analysis of alkaloids
Alkaloids were extracted from hairy roots and media according to Jung et al. (Citation1992). The roots were extracted three-times with methanol for 30 min in a bath sonicator at room temperature and concentrated under vacuum. The residue was dissolved in 20 ml of 1 N HCl and extracted with 20 ml ethyl acetate at a low pH. The acid solution was adjusted to pH 10 and extracted three times with an equal volume of ethyl acetate. The ethyl acetate solution was evaporated to dryness under vacuum and the residue was dissolved in 1 ml of methanol (HPLC grade) for further analysis. For extraction of alkaloids from the media, a known volume was acidified to pH 1.5 and extracted with ethyl acetate at low and high pH values as in the case of alkaloid extraction from hairy roots.
The solvent system consisting of chloroform:acetone: diethylamine (20:4:1, v/v/v) was used to separate the alkaloids, which were then visualized by spraying with Dragendorff's reagent (Asada & Shuler, Citation1989). The alkaloid extracts were quantified by HPLC as described by Rijhwani and Shanks (Citation1998). The HPLC system consisted of a solvent delivery module LC-10AT (Shimadzu Corporation, Kyoto, Japan), a rheodyne syringe loading sample injector model 7725i with a 20-µl injection loop (Rheodyne, L.P., California, USA), a Shimadzu model SPD-M10 Avp diode array detector with wavelength set at 254 nm and the spectra recording from 200 to 360 nm, coupled to a Shimadzu LC10 computing integrator. The analytical column used was Luna 18(2), 250 × 4.6 mm i.d., 5-µm particle size (Phenomenax, USA). The mobile phase consisted of a mixture of methanol:acetonitrile:5 mM diammonium hydrogen phosphate (pH 7.3) (32:32:36, v/v/v) degassed using bath sonicator. The chromatography was performed isocratically at a flow rate of 1 ml/min at room temperature.
Statistical analysis
Analysis of variance (ANOVA) and Tukey's test were carried out using GraphPad Prism 4 (GraphPad Software Inc., USA) for determining the significance of treatment effects. A p value of <0.05 was considered significant.
Results and Discussion
Effect of feeding biosynthetic precursors
The effect of feeding loganin alone or in combination with tryptamine on alkaloid accumulation in transformed root cultures of C. roseus. was studied at different growth phases. In this experiment, cultures were fed on days 13, 17, and 21 and harvested 4 days after treatment. No significant effect on the growth of hairy roots was observed with the addition of precursors when compared with control cultures. Also, there was no effect on the release of alkaloids from hairy roots into the media.
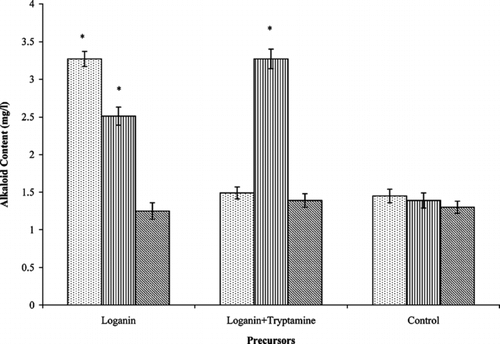
Feeding of loganin alone or in combination with tryptamine did not improve significantly the alkaloid levels during early and late exponential growth phases (data not shown). However, the feeding of precursors at early stationary phase resulted in significant improvements in the accumulation of ajmalicine and serpentine (). With loganin feeding, ajmalicine production (3.27 mg/l) was improved by 2.3-fold and there was 1.8-fold increase in the yield of serpentine (2.51 mg/l) when compared with control cultures (1.42 and 1.39 mg/l, respectively). However, ajmalicine accumulation was not affected with the combined feeding of loganin and tryptamine, but there was 2.4-fold improvement in serpentine production (3.27 mg/l) over the control. Catharanthine levels were unaffected by either of these treatments.
Figure 1 Effect of feeding precursors on alkaloid production in Catharanthus roseus. var. nirmal. transformed root cultures at early stationary phase. Data with * are significantly different from control (p < 0.05). ![]()
The results indicate a limitation in the supply of terpenoid precursors in the synthesis and accumulation of ajmalicine and serpentine during stationary phase, the levels of which can be improved by feeding loganin alone or in combination with tryptamine. Morgan and Shanks (Citation2000) also found a limitation in the supply of terpenoid precursors in C. roseus. hairy root cultures during the stationary phase. Moreover, highest levels of alkaloids were obtained in transgenic cell lines after the combined addition of loganin and tryptamine (Whitmer et al., Citation2002aCitation2002b).
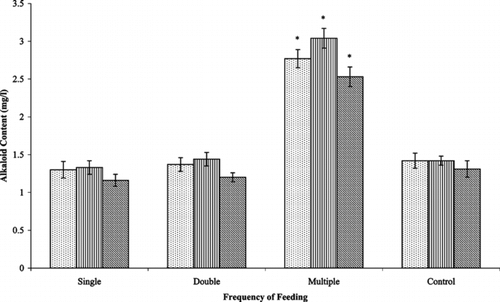
In order to determine the effect of multiple feeding of precursors on alkaloid accumulation, cultures were fed once (day 13), twice (day 13 and 17), and multiple times (days 13, 17, and 21) and harvested on day 25. Alkaloid accumulation was not affected with single and double feedings of loganin (data not shown). Similarly, single and multiple feedings of loganin and tryptamine combination did not increase the alkaloid levels (data not shown). But significant improvements in alkaloid levels were obtained with multiple feedings of loganin (). There were approximately two-fold improvements for the production of ajmalicine (2.77 mg/l) and serpentine (3.04 mg/l) over the control cultures (1.42 and 1.42 mg/l).An interesting effect was a significant increase in the content of catharanthine. Catharanthine level (2.53 mg/l) was improved by 1.9-fold over the control (1.31 mg/l). This is quite different from that observed with single feeding of loganin at early stationary phase in the previous experiment. Catharanthine accumulation may be inhibited by catabolism to other products (Whitmer et al., Citation2002aCitation2002b), and multiple feedings might have resulted in the maintenance of its levels.
Effect of organic compounds on indole alkaloid production
Phenobarbital, trans.-cinnamic acid, and succinic acid were added to transformed root cultures of C. roseus. at early stationary phase to see their effect on alkaloid accumulation. The cultures were exposed to these organic compounds for 4 days, which did not affect the growth of roots or the release of alkaloids into the media.
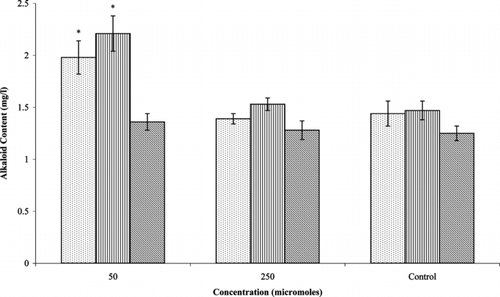
Treatment with phenobarbital increased the accumulation of ajmalicine and serpentine, but catharanthine levels were not affected much when compared with control cultures (). Significant effects were seen with the lowest concentration (50 µM). Ajmalicine production (1.98 mg/l) was improved by 1.4-fold over the control cultures (1.44 mg/l). There was 1.5-fold increase in the accumulation of serpentine (2.21 mg/l) when compared with control cultures (1.47 mg/l). Cytochrome P450 enzymes are widely involved in the biosynthesis of terpenoid indole alkaloids in C. roseus. (Meijer et al., Citation1993; Irmler et al., Citation2000). The cytochrome P450 monooxygenase, geraniol 10-hydroxylase (G10H), involved in an early step of the biosynthesis of secologanin, has been suggested as a potential site for regulation. Simpson and Kelly (Citation1989) employed cytochrome P450 inducers and inhibitors to study alkaloid production in cell cultures of C. roseus.. Phenobarbital was shown to induce the activity of G10H, thereby increasing the levels of ajmalicine (Contin et al., Citation1999). Similarly, Zhao et al. (Citation2001) found an improvement not only with the production of ajmalicine and serpentine in compact callus cluster cultures but also with catharanthine. The failure of phenobarbital treatment to improve catharanthine accumulation in the current study could be due to differences in the culture systems utilized, medium composition, or growth conditions.
Figure 3 Effect of phenobarbital on alkaloid production in Catharanthus roseus. var. nirmal. transformed root cultures. Data with * are significantly different from control (p < 0.05). ![]()
The addition of trans.-cinnamic acid did not produce significant improvements on alkaloid accumulation in hairy root cultures of C. roseus.. Marginal increases in alkaloid levels were observed with the highest concentration (data not shown). trans.-Cinnamic acid inhibits phenylalanine ammonia lyase and thus blocks the carbon flow from shikimate into phenylpropanoid pathway. Significant improvements in indole alkaloid production were obtained in compact callus clusters after treatment with trans.-cinnamic acid, suggesting the considerable contribution of the indole branch in alkaloid biosynthesis in that culture system (Zhao et al., Citation2001). Similarly, Godoy-Hernandez et al. (2000) found a significant improvement in alkaloid production in osmotically stressed cell cultures of C. roseus. by the addition of trans-. cinnamic acid. Accumulation and excretion of phenolic compounds from plant cell cultures is generally seen when they are exposed to stress-inducing conditions like fungal homogenates and osmotic shock. In the current study, the hairy root cultures were not subjected to any stress and did not show any browning and, hence, trans.-cinnamic acid could be ineffective in improving the alkaloid production.
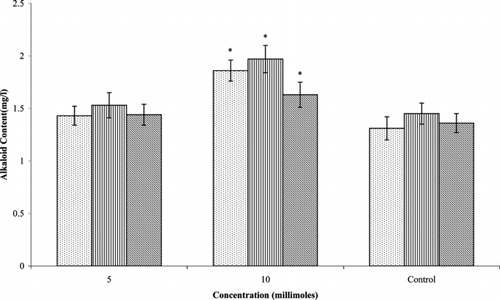
Significant effects of succinic acid treatment were seen on the accumulation of ajmalicine and serpentine with the highest concentration (10 mM). Production of ajmalicine (1.86 mg/l) and serpentine (1.97 mg/l) was improved by 1.4-fold each when compared with control cultures (1.31 and 1.45 mg/l, respectively) (). However, catharanthine contents (1.63 mg/l) were improved by 1.2-fold over the control cultures (1.36 mg/l). At the lowest concentration of succinic acid, however, alkaloid production was not affected. Similarly, alkaloid accumulation in cell cultures of C. roseus. was significantly improved by the addition of malic, succinic, and citric acids (Zhao et al., Citation2000Citation2001). All of these acids participate in the tricarboxylic acid cycle. Biosynthetic studies indicate that both acetate/mevalonate and triose phosphate/pyruvate pathways (non-mevalonate) are important for alkaloid production in C. roseus. (Hong et al., Citation2003). Zhao et al. (Citation2000Citation2001) suggested that exogenous malate and succinate may inhibit tricarboxylic acid cycle by a negative feedback control or they can be transformed to pyruvate. Therefore, addition of malate or succinate may stimulate indole alkaloid accumulation by increasing pyruvate pool or directing acetyl CoA and pyruvate flux to alkaloid pathway. A similar effect might have resulted in the increased accumulation of alkaloids in the current study.
Figure 4 Effect of succinic acid on alkaloid production in Catharanthus roseus. var. nirmal. transformed root cultures. Data with * are significantly different from control (p < 0.05). ![]()
Alkaloid production in hairy root cultures is thus limited by the supply of terpenoid precursors during stationary growth phase. Moreover, multiple feedings of loganin increased the accumulation of alkaloids, giving further support to the importance of the terpenoid pathway in alkaloid biosynthesis. Significant improvements in productivity were seen with phenobarbital and succinic acid, which provides further evidence to the regulatory role of G10H and of non-mevalonate pathway in alkaloid biosynthesis in C. roseus..
Acknowledgments
The authors are thankful to Dr. A.R. Kulkarni, CIMAP, Bangalore, for gifting C. roseus. var. nirmal. variety seeds; Dr. K.M. Oksman-Caldentey, VTT Biotechnology, Finland, and Dr. A. Giri, JNTU, Hyderabad, for providing A. rhizogenes. strains; and Prof. Y. Dessaux, National Centre for Scientific Research, France, and Prof. S.R. Jensen, Technical University of Denmark, Denmark, for the gift sample of mannopine and loganin, respectively. One of the authors (E.N.G.) is thankful to AICTE, New Delhi, India, for the research fellowship award.
References
- Asada M, Shuler ML (1989): Stimulation of ajmalicine production and excretion from Catharanthus roseus.: Effects of adsorption in situ., elicitors and alginate immobilization. Appl Microbiol Biotechnol 30: 475–481. [CSA]
- Contin A, Collu G, van der Heijden R, Verpoorte R (1999): The effects of phenobarbital and ketoconazole on the alkaloid biosynthesis in Catharanthus roseus. cell suspension cultures. Plant Physiol Biochem 37: 139–144. [CSA], [CROSSREF]
- Doller G, Alfermann A, Reinhard E (1976): Production of indole alkaloids in tissue cultures of Catharanthus roseus.. Planta Med 30: 14–20. [INFOTRIEVE], [CSA]
- Giri A, Ravindra ST, Dhingra V, Narasu ML (2001): Influence of different strains of Agrobacterium rhizogenes. on induction of hairy roots and artemisinin production in Artemisia annua.. Curr Sci 81: 379–382. [CSA]
- Godoy-Hernandez GC, Vazquez-Flota FA, Loyola-Vargas VM (2000): The exposure to trans.-cinnamic acid of osmotically stressed Catharanthus roseus. cells cultured in a 14-l bioreactor increases alkaloid accumulation. Biotechnol Lett 22: 921–925. [CSA], [CROSSREF]
- Hong S, Hughes EH, Shanks JV, San K, Gibson SI (2003): Role of the non-mevalonate pathway in indole alkaloid production by Catharanthus roseus. hairy roots. Biotechnol Prog 19: 1105–1108. [INFOTRIEVE], [CSA], [CROSSREF]
- Irmler S, Schroder G, St-Pierre B, Crouch NP, Hotze M, Schmidt J, Strack D, Matern U, Schroder J (2000): Indole alkaloid biosynthesis in Catharanthus roseus.: New enzyme activities and identification of cytochrome P450 CYP72A1 as secologanin synthase. Plant J 24: 797–804. [INFOTRIEVE], [CSA], [CROSSREF]
- Jung KH, Kwak SS, Kim SW, Lee H, Choi CY, Liu JR (1992): Improvement of the catharanthine productivity in hairy root cultures of Catharanthus roseus. by using monosaccharides as carbon source. Biotechnol Lett 14: 695–700. [CSA], [CROSSREF]
- Krueger RJ, Carew DP (1978): Catharanthus roseus. tissue culture: The effects of precursors on growth and alkaloid production. Lloydia 41: 327–331. [CSA]
- Meijer AH, Verpoorte R, Hoge JHC (1993): Regulation of enzymes and genes involved in terpenoid indole alkaloid biosynthesis in Catharanthus roseus.. J Plant Res 3: 145–164. [CSA]
- Moreno PRH, van der Heijden R, Verpoorte R (1993): Effect of terpenoid precursor feeding and elicitation on the formation of indole alkaloids in cell suspension cultures of Catharanthus roseus.. Plant Cell Rep 12: 702–705. [CSA], [CROSSREF]
- Morgan JA, Shanks JV (2000): Determination of metabolic rate limitations by precursor feeding in Catharanthus roseus. hairy root cultures. J Biotechnol 79: 137–145. [INFOTRIEVE], [CSA], [CROSSREF]
- Murashige T, Skoog F (1962): A revised medium for rapid growth and bioassays with tobacco tissue cultures. Physiol Plant 15: 473–479. [CSA], [CROSSREF]
- Naudascher F, Doireau P, Guillot A, Thiersault M (1989a): Time-course studies on the use of loganin by Catharanthus roseus. cells cultured in vitro.. J Plant Physiol 135: 366–368. [CSA]
- Naudascher F, Doireau P, Guillot A, Thiersault M (1989b): Time-course studies on the use of secologanin by Catharanthus roseus. cells cultured in vitro.. J Plant Physiol 134: 608–612. [CSA]
- Petit A, David G, Dahl GA, Ellis JG, Guyon P, Casse-Delbart F, Tempe J (1983): Further extension of the opine concept: Plasmids in Agrobacterium rhizogenes. cooperate for opine degradation. Mol Gen Genet 190: 204–214. [CSA], [CROSSREF]
- Rijhwani SK, Shanks JV (1998): Effect of subculture cycle on growth and indole alkaloid production by Catharanthus roseus. hairy root cultures. Enzyme Microbiol Technol 22: 606–611. [CSA], [CROSSREF]
- Simpson AP, Kelly SL (1989): Cytochrome P450 inducer/inhibitor effects on cell cultures of Catharanthus roseus.. Plant Sci 60: 231–236. [CSA], [CROSSREF]
- Veeresham C, Kokate CK (1997): Taxol: A novel anticancer agent from cell culture of Taxus.. Ind Drugs 34: 354–359. [CSA]
- Whitmer S, Canel C, Hallard D, Goncalves C, Verpoorte R (1998): Influence of precursor availability on alkaloid accumulation by transgenic cell line of Catharanthus roseus.. Plant Physiol 116: 853–857. [INFOTRIEVE], [CSA], [CROSSREF]
- Whitmer S, van der Heijden R, Verpoorte R (2002a): Effect of precursor feeding on alkaloid accumulation by tryptophan decarboxylase over-expressing transgenic cell line T22 of Catharanthus roseus.. J Biotechnol 96: 193–203. [INFOTRIEVE], [CSA], [CROSSREF]
- Whitmer S, van der Heijden R, Verpoorte R (2002b): Effect of precursor feeding on alkaloid accumulation by strictosidine synthase over-expressing transgenic cell line S1 of Catharanthus roseus.. Plant Cell Tiss Org Cult 69: 85–93. [CSA], [CROSSREF]
- Zenk MH, El-Shagi H, Arens H, Stockigt J, Weiler E, Deus B (1977): Formation of the indole alkaloids ajmalicine and serpentine in cell suspension cultures of Catharanthus roseus., in Plant Tissue Culture and its Biotechnological Application.. In: Barz W, Reinhard E, Zenk MH, eds., Berlin-Heidelberg-New York, Springer, pp. 27–44.
- Zhao J, Hu Q, Guo YQ, Zhu WH (2001): Effects of stress factors, bioregulators and synthetic precursors on alkaloid production in compact callus cultures of Catharanthus roseus.. Appl Microbiol Biotechnol 55: 693–698. [INFOTRIEVE], [CSA], [CROSSREF]
- Zhao J, Zhu WH, Hu Q, He XW (2000): Improved alkaloid production in Catharanthus roseus. suspension cell cultures by various chemicals. Biotechnol Lett 22: 1221–1226. [CSA], [CROSSREF]