Abstract
A methanol extract of the Psophocarpus tetragonolobus. (L.) DC (Leguminosae) leaves was screened for antimicrobial activity against Pseudomonas aeruginosa.. Antimicrobial tests were carried out using the disk diffusion assay and broth dilution method against Pseudomonas aeruginosa.. The extract showed favorable antimicrobial activity against Pseudomonas aeruginosa. with a minimum inhibitory concentration (MIC) value of 2.55 mg/mL. Apart from the antibacterial effects, imaging using scanning electron microscopy (SEM) was done to determine the major alterations in the microstructure of the Pseudomonas aeruginosa.. The main abnormalities noted via SEM studies were alterations in morphology and complete collapse of the bacterial cells after 36 h of exposure to the extract. The effect of the extract on the growth profile of the bacteria was also examined. The extract changed the normal growth profile of Pseudomonas aeruginosa., thus confirming the bactericidal effect of the extract on Pseudomonas aeruginosa.. Furthermore, the extract was also tested against brine shrimp for toxicity. The extract exhibited no significant toxicity (LC50 = 1.30 mg/mL) against Artemia salina. L. (Artemiidae).
Introduction
Natural remedies have been applied worldwide for the treatment of many ailments since ancient times (Sokmen et al., Citation1999). Resistance of microbes to commonly used antibiotics has enhanced morbidity and mortality and has triggered the search for new drugs from natural products. Due to an increasing demand for biodiversity in screening programs seeking therapeutic drugs from natural products, interest, particularly in edible plants, has grown throughout the world.
Among the plants with healing properties in Malaysia, Psophocarpus tetragonolobus. (L.) DC (Leguminosae) has yet to gain importance and popularity. P. tetragonolobus. is a tropical plant found predominately in the rural areas of Papua New Guinea and Southeast Asia. It grows abundantly in hot, humid equatorial countries such as Malaysia, Indonesia, Thailand, the Philippines, India, Bangladesh, Myanmar, Sri Lanka, the West Indies, and South Florida (Zuraini et al., Citation2004). Because all the parts of this plant are edible, it was considered to be a “poor man's food.” However, because of a number of special properties including medicinal properties (Venketeswaran et al., Citation1990; Zuraini et al., Citation2004), the plants is now gaining the attention of researchers.
The high level of proteins in the P. tetragonolobus., namely, lectins, is used as a diagnostic tool because it binds certain blood cells and specialized transport cells. Apart from being a rich source of lectins, P. tetragonolobus. also contains erucic acid (an antitumor medication) and polyunsaturated fatty acids that can be used to treat acne and eczema (Farnsworth et al., Citation1985).
P. tetragonolobus. has been commonly used in traditional medicine for many years. The leaves are boiled to make a decoction, and it is used as a lotion to cure smallpox (Perry, Citation1980). In the Shan states, the roots are used as a poultice to treat vertigo (Burkill, Citation1935). In Indonesia, the leaf infusion is used as a wash for inflammation and suppurating sores, and the leaves are applied to cure boils. In New Guinea, on the other hand, the pod and edible tubers are considered roborant (Stopp, Citation1962). The leaves and the seeds are eaten to cure skin sores such as boils and ulcers (Perry, Citation1980).
There is still not much known about the P. tetragonolobus., and research must be conducted in many areas as the available data are deficient on the status, extent, and utilization of P. tetragonolobus.. Our previous research showed that the crude extract of P. tetragonolobus. leaves exhibited a good antimicrobial activity against Candida albicans. (Zuraini et al., Citation2004) and also good antioxidant activity (Yoga Latha et al., Citation2005). The current study was carried out to determine the antimicrobial activity of the crude extract of P. tetragonolobus. leaves against Pseudomonas aeruginosa. (Schroeter).
Toxicity screening models provide important preliminary data to select the natural remedies with potential antimicrobial properties (Solý's et al., Citation1993). As part of a permanent screening program in the search for natural products with antimicrobial properties, the current research also reports the toxicity of the crude extract of P. tetragonolobus. leaves, which has never been reported in literature. It is a convenient preliminary toxicity test because the brine shrimp is highly sensitive to a variety of chemical substances.
Materials and Methods
Microorganism
Pseudomonas aeruginosa. was used as the test organism and was obtained from the laboratory stock culture. The bacterium was cultured on nutrient agar slants at 37°C for 18 h. The stock culture was maintained on nutrient agar slants at 4°C.
P. tetragonolobus. sample
Fresh P. tetragonolobus. leaves were collected from Penang, Malaysia, in December 2004 and authenticated by the botanist of the School of Biological Sciences at Universiti Sains Malaysia, where a specimen was deposited in the herbarium.
Preparation of the crude extract
The leaves of P. tetragonolobus. were boiled in a Soxhlet with 300 mL of 80% methanol (v/v) for 4 h. The entire extract of P. tetragonolobus. leaves was evaporated to dryness in a rotary evaporator. The dried fractions were then redissolved in 10% DMSO (v/v) to yield the solution containing 100 mg of extract per milliliter solution.
Antimicrobial activity
The antimicrobial activity of the crude extract was determined following the method described by NCCLS (Citation2002) with slight modifications.
Disk diffusion technique
The test microbes were removed aseptically with an inoculating loop and transferred to a test tube containing 5.0 mL of sterile distilled water. Sufficient inoculum was added until the turbidity equaled 0.5 McFarland (108 CFU/mL) standard (bioMerieux, Marcy Petoile, France). One milliliter of the test tube suspension was added to the 15–20 mL of nutrient agar before setting aside the seeded agar plate (9 cm in diameter) to solidify for 15 min. Three Whatman filter paper no. 1 disks of 6-mm diameter were used to screen the antimicrobial activity. Each sterile disk was impregnated with 20 µL of the extract (corresponding with 100 mg/mL of crude extract), chloramphenicol (30 µg/mL, as positive control), or 10% DMSO (v/v) (as negative control), before it was placed on the surface of the seeded plates. The plates were incubated at 37°C overnight and examined for zones of growth inhibition.
Determination of minimum inhibitory concentrations
A 16-h culture was diluted with a sterile physiologic saline solution [PS; 0.85% (w/v) sodium chloride] with reference to the 0.5 McFarland standard to achieve inoculums of approximately 106 colony forming units (CFU) per milliliter. A serial dilution was carried out to give final concentrations between 1.275 and 200.00 mg crude extract per milliliter. The tubes were inoculated with 20 µL of the bacterial suspension per milliliter nutrient broth, homogenized, and incubated at 37°C. After incubation, 50 µL was withdrawn from each tube and inoculated on agar plates and incubated at 37°C for 24 h. The minimum inhibition concentration (MIC) value was determined as the lowest concentration of the crude extract in the broth medium that inhibited the visible growth of the test microorganism.
Scanning electron microscope observations
Scanning electron microscope observations were carried out on Pseudomonas aeruginosa. cells. One milliliter of the Pseudomonas aeruginosa. cells suspension at the concentration of 1 × 106 cells per milliliter was inoculated on a nutrient agar plate and then incubated at 37°C for 12 h. The extract (2 mL), at the concentration of 100 mg per mL, was then dropped onto the inoculated agar and was further incubated for another 36 h at the same incubation temperature. A 10% DMSO-treated culture was used as a control. A small block of bacteria containing agar was withdrawn from the inoculated plate at 0 and 36 h and fixed for scanning (Borgers et al., Citation1989).
Growth profile of Pseudomonas aeruginosa. in the presence of the Psophocarpus tetragonolobus. leaves extract
In order to assess the bactericidal effect of the crude extract with MIC concentration over time, a growth profile curve was plotted. A 16-h culture was harvested by centrifugation, washed twice with PS, and resuspended in PS. The suspension was adjusted using the McFarland standard and was then further diluted in PS to achieve approximately 107 CFU mL−1. The crude extract was added to aliquots of 25 mL Mueller-Hinton broth (MHB) in 50 mL Erlenmeyer flasks in a shaking water bath at 37°C in an amount that would achieve a concentration of 0 (control) and 2.55 mg/mL (MIC concentration) after the addition of the inoculums. Later, a solution of 1 mL inoculums was added to all Erlenmeyer flasks. Immediately after the addition of the inoculums, a 1 mL portion was removed from each Erlenmeyer flask and the growth of Pseudomonas aeruginosa. was monitored by measuring the optical density (OD) with a spectrophotometer (GAT UV-9100, Ruili Co, China) at 540 nm. The growth of Pseudomonas aeruginosa. was measured every 4 h for 48 h using the above method.
Toxicity testing against the brine shrimp
Hatching shrimp
Brine shrimp eggs, Artemia salina., were hatched in artificial seawater prepared by dissolving 38 g of sea salt in 1 L of distilled water. After a 24-h incubation period at room temperature (22–29°C), the larvae were attracted to one side of the vessel with a light source and collected with a pipette. The larvae were separated from the eggs by aliquoting them three times in small beakers containing seawater.
Brine shrimp assay
The bioactivity of the extract was monitored by the brine shrimp lethality test (Meyer et al., Citation1982). Samples were dissolved in DMSO and diluted with artificial seawater. Two milliliters of seawater was placed in all the bijoux bottles. A two-fold dilution was carried out to obtain the concentration from 10 to 0.1525 mg/mL. The last bottle was filled with sea salt water and DMSO only, serving as a drug-free control. Suspensions of larvae, (100 µL) containing about 10–15 larvae were added into each bottle and incubated for 24 h. The bottles were then examined and the number of dead larvae in each bottle was counted. The total number of shrimp in each bottle was counted and recorded. The mean percentage mortality was plotted against the logarithm of concentrations, and the concentration that could kill 50% of the larvae (LC50) was determined from the graph.
Data analysis
The mean results of brine shrimp mortality against the logarithms of concentrations were plotted using the Microsoft Excel computer program, which also presents regression equations. The regression equations were used to calculate the LC50 value. Extracts giving LC50 values greater than 20 µg/mL are considered to be nontoxic (Geran et al., Citation1972).
Results
Antimicrobial activity
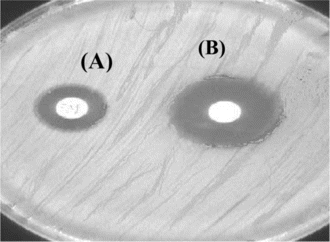
The results of antimicrobial activity of the crude extract against Pseudomonas aeruginosa. are given in . The extract exhibited a favorable activity against the bacteria tested. The zone of clearance produced by the commercial antibiotic disk was larger than those produced by the extract disk (). The agar dilution method recorded the MIC value of 2.55 mg/mL.
Table 1.. Antimicrobial activity (zone of inhibition and MIC)Footnotea. of crude methanol extract of the Psophocarpus tetragonolobus. leaves compared with commercial antibiotic chloramphenicol.
Scanning electron microscope observation
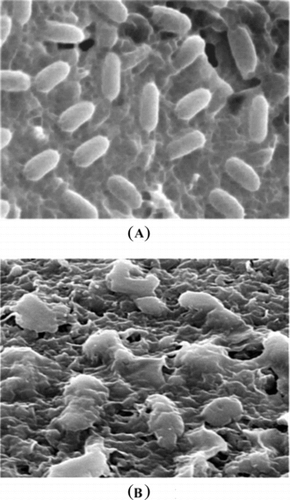
Cells treated with MIC concentration of the crude extract underwent considerable morphologic alterations in comparison with the control when observed by SEM (). shows the SEM photomicrographs of the untreated and extract-treated cells of Pseudomonas aeruginosa. at 36-h time of exposure. Untreated cells (A) appear as rods and smooth cells. After 36 h of exposure (B), complete collapsed cells were seen. It is believed that at this stage, the cells had lost their metabolic functions completely.
Growth profile of Pseudomonas aeruginosa. in the presence of the Psophocarpus tetragonolobus. leaves extract
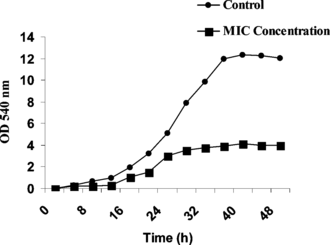
The growth profile of Pseudomonas aeruginosa. in MHB at MIC and 0 (control) concentrations is shown in . The MIC (2.55 mg/mL) concentration altered the normal growth profile for Pseudomonas aeruginosa. compared with the control (0 concentrations). This finding confirmed the bactericidal effect of the extract on Pseudomonas aeruginosa. at the MIC concentration.
Toxicity testing against brine shrimp
The result of the toxicity evaluation against the brine shrimp of the crude extract is shown in . The extract showed no significant toxicity against brine shrimp as the LC50 value was 1.30 mg/mL.
Table 2.. Toxicity of crude extract of Psophocarpus tetragonolobus. leaves against brine shrimp larvae (Artemia salina.).
Discussion and Conclusions
Plants have long been a very important source of drugs, and many plant species have been screened to see if they contain substances with therapeutic activity. Many long-standing medicinal plants were discovered by investigating the scientific basis of old folk remedies to determine the active ingredients in the concoction (Sokmen et al., Citation1999). The antibiotic discovery program was based on the ethnobotanical approach; screening plants that have been used by indigenous ethnic groups for treatment of various infectious diseases (Iwu et al., Citation1999). The identification and exploitation of screenings that focus on unique targets may offer new opportunities for drug discovery.
In this study, we particularly concentrated on Gram-negative bacteria Pseudomonas aeruginosa.. Pseudomonas aeruginosa. nowadays is considered as one of the leading pathogens in ICU pneumonia, AIDS primary bacteremia, drug user's endocarditis, exacerbations of cystic fibrosis, malignant external otitis, contact lens keratitis, and traumatic endophthalmitis (Hellen, Citation2000). Pseudomonas aeruginosa. is also a nosocomial pathogen, and the National Nosocomial Infection Surveillance System of the CDC reports that the overall incidence of P. aeruginosa. infection in U.S. hospitals between 1985 and 1991 was 4.0 per 100 discharges; making it the fourth most frequently isolated nosocomial pathogen (Rello et al., Citation1997). From this study, it appears that the extract exhibits a favorable antimicrobial activity against Pseudomonas aeruginosa.. This was confirmed by the alteration of the normal growth profile of Pseudomonas aeruginosa.. Furthermore, the SEM study showed that the extract could completely collapse the bacterial cells and inhibit the growth of the Pseudomonas aeruginosa., which orgenism can cause urinary and respiratory system infections (Tetsuro & Tetsuro, Citation2004), dermatitis, soft tissue infections, bacteremia (Kingsford & Raadsma, Citation1997), bone and joint infections, and gastrointestinal infections (Hellen, Citation2000).
Hence, Pseudomonas aeruginosa. infection could be treated by the extract, as the MIC for this bacterium was found to be only 2.55 mg/mL. In addition, it may be used as an antibacterial agent in known dosages, especially in rural communities where conventional drugs are unaffordable or unavailable and the health facilities are inaccessible.
The isolation of bioactive compounds from natural sources requires toxicity information on the constituent of interest (Cragg et al., Citation1993). It should be emphasized that toxic effects of the antibacterial agent on the host cells must be considered, as a substance may exhibit an apparent antimicrobial activity by virtue of its toxic effect on the cells. The results on brine shrimp assay indicate that the extract has a LC50 value greater than 20 µg/mL, which is the recommended cutoff point for detecting cytotoxic activity (Geran et al., Citation1972). This suggests that this plant may not be toxic. The results of the current study concur with the use of this plant by native people for food preparation. Further purification of the active compounds and in vivo. evaluation of antimicrobial activity against Pseudomonas aeruginosa. of the extract are therefore suggested for further studies.
References
- Borgers M, Van De Ven MA, Van Cutsen J (1989): Structural degeneration of Aspergillus fumigatus. after exposure to saperconazole. J Med Vet Mycol 27: 381–389.
- Burkill IH (1935): Dictionary of the Economic Products of the Malay Peninsula. Edited by Ministry of Agriculture (Malaysia), 2nd ed. London, Crown Agents for the Colonies, p. 2402.
- Cragg GM, Boyd MR, Cardellina II JH, Grever MR, Schepartz S, Snader KM, Suffness M (1993): The search for new pharmaceutical crops: Drug discovery and development at the National Cancer Institute. In: Janick J, Simon JE, eds., New Crops. New York, Wiley, pp. 161–167.
- Farnsworth NR, Akerele O, Bingel AS, Soejarto DD, Guo ZG (1985): Medicinal plants in therapy. Bull WHO 63: 965–981.
- Geran RI, Greenberg HM, McDonald M, Abbott BJ (1972): Protocols for screening chemical agents and natural products against animal tumors and other biological systems. Cancer Chemother Rep 33: 1–17.
- Hellen G (2000): Therapeutic guidelines for Pseudomonas aeruginosa. infections. Int J Antimicrob Agents 16: 103–106.
- Iwu MW, Duncan AR, Okunji CO (1999): New antimicrobials of plant origin. In: Janick J, ed., Perspectives on New Crops and New Uses. Alexandria, VA, ASHS Press, pp. 457–462.
- Kingsford NM, Raadsma HW (1997): The occurrence of Pseudomonas aeruginosa. in fleece washings from sheep affected and unaffected with fleece rot. Vet Microbiol 54: 275–285.
- Meyer BN, Ferrigini RN, Jacobsen LB, Nicholas DE, McLaughlin JL (1982): Brine shrimp: A convenient general bioassay for active plant constituent. Planta Med 45: 31–35.
- NCCLS National Committee for Clinical Laboratory Standards (2002): Methods for Dilution Antimicrobial Susceptibility Tests for Bacteria that Grow Aerobically, 3rd ed. Approved standard. NCCLS document M100-S12. Wayne, PA, NCCLS.
- Perry LM (1980): Medicinal Plants of East and Southeast Asia. Cambridge, MA, The MIT Press, p. 231.
- Rello J, Rue M, Jubert P, Muses G, Sonora R, Valles J, Niederman M (1997): Survival in patients with nosocomial pneumonia: Impact of the severity of illness and the etiologic agent. Crit Care Med 25: 1862–1867.
- Sokmen A, Jones BM, Erturk M (1999): The in vitro. antibacterial activity of Turkish medicinal plants. J Ethnopharmacol 67: 79–86.
- Solý's PN, Wright CW, Anderson MA, Gupta MP, Phillipson JD (1993): A microwell cytotoxicity assay using Artemia salina. (brine shrimp). Planta Med 59: 250–252.
- Stopp K (1962): The medicinal used by the Mt. Hagen people (mbowamb) in New Guinea. Econ Bot 17: 16–22.
- Tetsuro M, Tetsuro M (2004): Newer carbapenems for urinary tract infections. Int J Antimicrob Agents 24: 35–38.
- Venketeswaran S, Dias MADL, Weyers UV (1990): The winged bean: A potential protein crop. In: Janick J, Simons JE, eds., Advance in New Crops. Portland, OR, Timber Press, p. 445.
- Yoga Latha L, Sasidharan S, Zuraini Z, Saravanan D, Suryani S, Sangetha S, Siti Aishah MB (2005): Antioxidant properties of Psophocarpus tetragonolobus.. J Trop Med Plants 6: 173–177.
- Zuraini Z, Yoga Latha L, Suryani S, Sasidharan S (2004): Anticandida albicans. activity of crude extract of the local plant, winged beans leaf. The Planter Kuala Lumpur 80(943): 653–657.