Abstract
Antimicrobial, antiprotozoal, and toxic activities of crude extracts obtained from six cnidarian species [Cassiopea xamachana. (R.R. Bigelow, 1892), Carybdea marsupialis. (Linné, 1758), Linuche unguiculata. (Swartz, 1788), Bartholomea annulata. (Leseur, 1817), Lebrunia danae. (Duchassaing and Michelotti, 1860), and Stichodactyla helianthus. (Ellis, 1768)] from the Mexican Caribbean Sea were studied. The extract obtained from Linuche unguiculata. was found to be the most active against the yeast Candida albicans. and the protozoan Giardia lamblia. with 24 mm of inhibition zone diameter and an IC50 of 63.2 µg mL−1, respectively. Additionally, in an effort to assess the effects caused by the treatment of cnidarian's toxins in vertebrates, we used tilapias (Oreochromis niloticus.) as a laboratory model. The results showed that only 44 mg kg−1 of jellyfish (Carybdea marsupialis.) toxin were necessary to cause significant mortality in fish. Tilapias treated with the Stichodactyla. toxin demonstrated hemolytic damage and cellular abnormalities.
Introduction
The research to obtain bioactive natural products worldwide has been focused mainly on plants. Nevertheless, marine invertebrates represent an important source of natural products with diverse properties. Most of the studies conducted to evaluate natural products from marine invertebrates have been performed principally with porifera (sponges) (Belarbi et al., Citation2003), anthozoans (coral), and mollusca (bivalves) (Faulkner, Citation1998; Harvey, Citation2002). However, cnidarians constitute an important potential source of new biologically active compounds (Radwan et al., Citation2000; Bosmans et al., Citation2002; Ramasamy et al., Citation2005).
Cnidarians are a very diverse phylum of aquatic animals that include hydroids, jellyfish, sea anemones, and corals. Nearly ubiquitous within the phylum is the presence of nematocysts, specialized intracellular organelles, which act as lethal weapons for defense and predation (Kass-Simon & Scappaticci, Jr., Citation2002). Nematocysts are complex microscopic stinging organelles containing a rich mixture of toxins. Examples of these toxins include polypeptides with cytolytic, hemolytic, and neurotoxic effects. These active compounds can be used in pharmacology because of their potential as anesthetic, analgesic, or anticancer agents or as drugs for the treatment of infectious diseases (Faulkner Citation1998Citation2000; Haefner, Citation2003). Although the use of plants as a source of antimicrobial activity dates far back in history, this is not the case for the cnidarians. This work attempts to find these properties in six cnidarian species that are abundant in the Mexican Caribbean Sea and also to evaluate the antiprotozoal, antimicrobial, and toxic activities.
Materials and Methods
Cnidarian collections
The six cnidarian species studied were collected in the northern coast of the Mexican Caribbean Sea from May to July 2004. Three sea anemone species, Bartholomea annulata., Lebrunia danae., and Stichodactyla helianthus., were collected by scuba diving at 2 to 20 m depth. The jellyfish Cassiopea xamachana. and Carybdea marsupialis. were collected manually by snorkeling at 2 to 4 m depth, and Linuche unguiculata. was obtained by near-surface horizontal hauls of 10-min duration. In the field, the specimens were cleaned, identified, and transported to the laboratory. indicates the names of the cnidarians that were screened, the parts used for the extract preparation, and the collection localities.
Table 1.. Scientific names, the body parts used for the extract preparations, and the collection localities of cnidarian species screened from the Mexican Caribbean Sea.
Preparation of crude extracts
Samples with protease inhibitors were frozen, lyophilized, and stored at −70°C until further use. The toxins were isolated according to a modified method of Kem et al. (Citation1989). Samples were placed in vials with 1.0 mL of distilled water, and 0.5 mL of glass beads (500 µm) was added. These samples were shaken in a vortex for 1-min intervals up to three times. Later, the samples were frozen at −70°C for 30 min. This procedure was repeated three more times. The nematocyst rupture was monitored microscopically to confirm toxin release (Carrete & Seymour, Citation2004). Protein concentrations were measured by the method of Bradford (Citation1976) for each crude extract, and bovine gamma globulin was used as standard. The protein concentrations for each gram of extract were 12.5 mg mL−1 (Bartholomea annulata.), 8.5 mg mL−1 (Lebrunia danae.), 11.8 mg mL−1 (Stichodactyla helianthus.), 6.5 mg mL−1 (Cassiopea xamachana.), 2.1 mg mL−1 (Carybdea marsupialis.), and 8.0 mg mL−1 (Linuche unguiculata.).
Biological activity assays
To assess the antimicrobial and antiprotozoal effects of the six cnidarian extracts, bacteria (Gram-positive and Gram-negative) were maintained in nutritive agar (NA), Candida albicans. in potato dextrose agar (PDA), and finally, Giardia lamblia. in TYI-S · 33 modified medium (Cedillo-Rivera et al., Citation1991). The microorganisms that were used to assess the toxic activities of crude extracts of the six cnidarian species are listed in .
Table 2.. Test microorganism species used to assess the antimicrobial activity of six cnidarian extracts.
Antimicrobial assay
Antimicrobial activity was evaluated using the agar diffusion method, according to the NCCLS (Citation1993) protocol with modifications. Briefly, eight sterile cylinders of 6-mm diameter were used to make wells inside Mueller-Hinton agar plates for the bacteria and PDA for the yeast. The plates containing an inoculum size of 106 CFU mL−1 for bacteria or 2 × 105 yeast cells were used. Previously, the crude extract was prepared at 0.01 mg/mL dry weight in sterile water. One hundred microliters of each extract were added aseptically to each well. Wells containing standard concentrations of the antibiotics amikacin (0.3 mg mL−1) for bacteria and nystatin (50 U/mL) for yeast and sterile water were used as controls. The plates were incubated for 18 h at 37°C for bacteria and at 30°C for yeast. The sensitivity was recorded by measuring the clear zone of growth inhibition around the well (mm diameter). Each set was tested in triplicate.
Antiprotozoal assay
Trophozoites were maintained in axenic culture and were used in the log phase of growth. The in vitro. assay was performed as described by Cedillo-Rivera et al. (Citation1991) and Cedillo-Rivera and Muñoz (Citation1992). Each test extract (50 mg) was dissolved in 1 mL of distilled water and introduced into microcentrifuge tubes with 1.5 mL of medium TYI-S 33 to obtain the required range of concentrations, 50–400 µg mL−1. The tubes containing the extract and medium were inoculated with 7 × 104 trophozoites of G. lamblia. (collection number IMSS: 0989:1). Each test included metronidazole (Sigma, USA) as a giardicidal drug and culture medium with trophozoites without extract as controls. The trophozoites were incubated for 48 h at 37°C and counted. The number of parasites was determined with a hemocytometer, and the percentages of trophozoite growth inhibition were calculated by comparison with the control culture. The percentage of inhibition calculated for each concentration was transformed into Probit units against log concentration. The best straight line was determined by regression analysis. The 50% inhibitory concentration (IC50) values were calculated. The experiment was performed in duplicate and repeated at least three-times.
Toxic effects in fish
The in vivo. toxic effect of cnidarian crude extracts was determined in male tilapia (Oreochromis niloticus.) (19 to 20 cm total length; 180 to 200 g weight). Tilapias were maintained in independent experimental systems with continuous water flow for 2 days for acclimation. For each cnidarian extract, three tilapias were injected intraperitoneally with four doses (25, 50, 100, and 200 mg kg−1 body weight) dissolved in distilled water, and three control fish were injected only with distilled water. Twenty-four hours after the injection was applied, the first signs of lethal effects on the fish began to be observed, and values of LD50 were calculated for each extract. Given that the major damage effects were observed in fish treated with the sea anemone Stichodactyla helianthus., a blood sample was taken from the caudal vein and the tilapias were dissected. Sections of livers and gills were removed and fixed with 10% buffered formalin. Fixed sections already fixed were embedded in paraffin wax, sectioned at 5 µm, and stained with hematoxylin and eosin. For hematologic analysis, blood smears were made, fixed with methanol, and stained with Giemsa (Undritz, Citation1979). With lymphocytes, cellular abnormalities, and degenerative cells were identified according to their morphologic characteristics, as described by Rowley and Ratcliffe (Citation1988).
Results and Discussion
Antimicrobial and antifungal assay
None of the cnidarian extracts were active against Gram-positive and Gram-negative bacteria tested at a concentration of 0.01 mg mL−1. In a previous study, crude extracts from some coral anthozoan species showed activity against Staphylococcus aureus., Mycobacterium tuberculosis., and M. avium. (Encarnación et al., Citation2000). This difference could be related to the defense mechanisms and resistance displayed by the pathogens. It is noticeable that among the six cnidarian species tested, the jellyfish Linuche unguiculata. was the only extract to exhibit an important activity against Candida albicans., being greater than nystatin, which was used as the positive control (). Among fungal pathogens, C. albicans. causes serious systemic infections, including opportunistic infection in patients infected with HIV virus (Diz-Dios et al., Citation2001; Sánchez-Vargas et al., Citation2005). The effect of the crude extract of Linuche unguiculata. demonstrating anticandidal activity could result in the discovery of novel anticandidal agents.
Table 3.. In vitro. antifungal, antiprotozoal, and toxic activity of crude extracts isolated from cnidarians from the Mexican Caribbean Sea.
Antiprotozoal assay
The antiprotozoal activities displayed by the cnidarian extract are shown in . Among the six cnidarian extracts tested, 83% showed antiprotozoal activity against axenically grow trophozoites of Giardia lamblia.. In particular, the Linuche unguiculata. extract was the most active with an IC50 of 63 µg mL−1, whereas the extract of the sea anemone Stichodactyla helianthus. showed very weak antiprotozoal activity with an IC50 of 1338 µg mL−1. Although the whole-cnidarian extracts contain a wide variety of chemical compounds that could have synergistic or antagonistic behaviors that display multiple activities, the isolation and purification of these compounds could increase the potency of their bioactive metabolites. The results obtained by Tejuca et al. (Citation1999) with the sea anemone S. helianthus. against G. lamblia. demonstrated that two different sticholysins were active with an IC50 in the nanomolar range (St I 0.5 nM and St II 1.6 nM). Further studies, including the isolation and characterization of the compounds responsible for the antiprotozoal activity, are now in progress.
Giardia lamblia. is a major cause of human diarrheal diseases in the world. Giardiasis is often asymptomatic but may also cause severe gastroenteritis. Particularly vulnerable are children and individuals affected by AIDS. Despite the existence of several medications to treat giardiasis, an ideal drug is still lacking, because those in use frequently have side effects, some are carcinogenic in animals, and none are capable of curing all infections. The massive use of antibiotics has often resulted in the development of resistance. In this way, the study of cnidarians has the potential to generate novel giardicidal metabolites.
Acute toxicity in fish
The LD50 values of cnidarian toxins for male tilapias in the respective time intervals are given in . The 24 h LD50 are basic values for the acute toxicity test. For the tilapias, the 24 h LD50 values were 44.0 mg kg−1,104.0 mg kg−1, and 104.0 mg kg−1 for Carybdea marsupialis., Bartholomea annulata., and Stichodactyla helianthus. toxins, respectively. Even though the C. marsupialis. toxin was very lethal, a neural paralytic syndrome was typical for fish poisoned with the Stichodactyla helianthus. toxin. Strong restlessness began when fish came into contact with the poison. Fish excitation was reflected by an increased reaction to exogenous stimuli and by cramp-like movements of the fins and mouth. Loss of coordination began, as well as a loss of orientation in water. The fish turned on their side and swam in half-circles. The reaction to excitation was manifested by sudden movements and fin tremor. Body surface darkening was noticeable in this phase of poisoning, mainly on the dorsal part. Weakening of jerks or areflexia, paralysis, arrhythmia, and blockage of respiration began in the terminal phase of poisoning. The fish died during the first 24 h after injection. Over the course of the 24 h toxicity test of cnidarian toxins on adult tilapias, no fish died in the control aquarium. The oxygen saturation of water did not drop below 65% in any of the toxin concentrations tested nor in the control group. Based on the observed 24 h LD50 value (44.0 mg kg−1), Carybdea marsupialis. can be classified in the group of toxins harmful for fish. Within the same order of magnitude of effect are the Bartholomea annulata. and Stichodactyla helianthus. toxins, with 104.0 mg kg−1 that were the 24 h LD50.
On the other hand, the pathologic and anatomical signs found in fish treated with Stichodactyla helianthus. toxin were not specific. The body surface became opaque due to a slightly increased amount of mucus and with expressive pigmentation mainly on the dorsal part. No changes were noticed in the eyes. Gills had straight edges, normal color, and slightly increased amount of mucus. Within the body cavity there was an evidence of an effective hyperemia of the liver.
Hematologic profile
The results of the leukocyte profile of both the control and experimental tilapias under study are given in and in . The main hematologic response of tilapias exposed to the Stichodactyla helianthus. toxin (104.0 mg kg−1) was a significant decrease in the count of lymphocytes, monocytes, and trombocytes compared with control fish. In contrast, there was an increase in both the relative and absolute count of developmental forms of neutrophils and degenerative cells in fish injected with the toxin. A singular characteristic of this anemone is that the nematocyst contains cytolysins with a hemolytic effect (Michaels, Citation1979; Varanda & Finkelstein, Citation1980; Maček, Citation1992; Belmonte et al., Citation1993).
Table 4.. Differential leukocyte counts from Nile tilapia (Oreochromis niloticus.) exposed to the toxin of the sea anemone Stichodactyla helianthus..
Figure 1 Normal and degenerative cells in tilapias (Oreochromis niloticus.) injected with (a) distilled water, and (b, c) Stichodactyla. toxin.
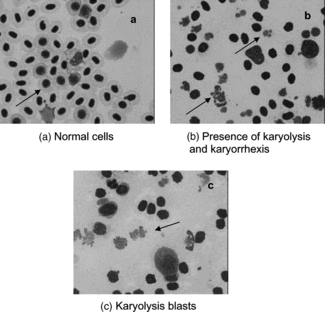
A significant decrease in the lymphocyte count and hemoglobin content were observed in the tilapia Oreochromis mossambicus. after acute exposure to different toxic substances (Sampath et al., Citation1993). Ghosh and Banerjee (Citation1993) report lymphopenia and both neutrophil and eosinophil granulocytosis in Heteropneustes fossilis. using an algal toxin. A decreased nonspecific immunity in fish can be expected after acute exposure to cnidarian toxins due to decreased leukocyte count, lymphopenia, and granulocytosis. Numerous authors have described the same behavior (lymphopenia and granulocytosis) after exposure to many pollutants (Wlasow, Citation1985; Murad & Houston, Citation1988; Thakur & Sahai, Citation1993, Alkahem, Citation1994; Svobodova et al., Citation1996). These changes in differential leukocyte count also provide evidence for a decreased level of nonspecific immunity in fish after acute exposure to cnidarian toxins.
Histopathology analysis
Histopathology observations for both control and Stichodactyla. toxin–treated tilapias with representative images of the tissues are displayed in . The most significant differences between control and animals exposed to Stichodactyla helianthus. toxin were observed in the gills. There was an increased number of mucous cells in primary lamellae, hypertrophy of the gill, damage to epithelial cells, and edema ().
Figure 2 Normal and abnormal gills in tilapia (Oreochromis niloticus.) injected with (a) distilled water, and (b, c) Stichodactyla. toxin.
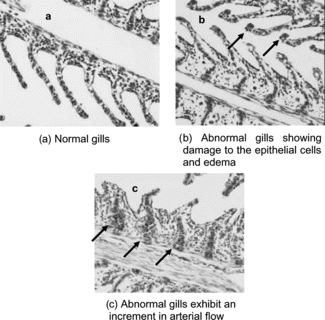
Here, we report that the toxin from Stichodactyla helianthus. leads to hypertrophy of the gills and alterations in lamellae. These results are similar to several studies in yellow perch, mirror carp, and trout exposed to different toxins. Specifically, in those studies there was depletion of lymphocytes count, and necrosis of epithelial cells in gills was observed.
In summary, the results presented in this work show that cnidarian extracts had a high potential to cause biological effects in the organisms. Some of them (Linuche unguiculata. and Cassiopea xamachana.) were very toxic against the protozoan Giardia lamblia.. This is important because these toxins can be used to develop new pharmaceutical products for the treatment of giardiasis.
On the other hand, even though the toxins of the other marine organisms (Carybdea marsupialis., Stichodactyla helianthus., and Bartholomea annulata.) did not show significant activity against Giardia., they were capable of causing rapid death in a vertebrate model (fish). In this way, the information generated about these toxins may play an important role in the search for new antivenoms. Future work will be focused to isolate, purify, and identify the cnidarian toxins and to determine whether the toxins contain useful compounds suitable for other pharmaceutical purposes.
Acknowledgments
The authors wish to acknowledge MS Fernando Negrete-Soto and Amauri Mendoza-López from the ICMyL-UNAM for invaluable help in specimen collection, QBB César Puerto and Carlos Luna from CINVESTAV-IPN for their excellent technical assistance in culture of Nile tilapia, and QFB Clara Salazar Salazar and Hospital General Regional, “Dr. Jesús Kumate Rodríguez,” Cancún, Mexico, for their excellent collaboration and technical assistance in the microbiological lab. Dr. Anastazia Banaszak kindly checked the English language of the text. This work was supported by CONACyT (Scholarship No. 142401) to the first author.
References
- Alkahem HF (1994): The toxicity of nickel and the effects of sublethal levels on haematological parameters and behavior of the fish, Orechromis niloticus.. J Univ Kuwait Sci 21: 243–252.
- Belarbi EH, Contreras-Gómez A, Chisti Y, García-Camacho F, Molina-Grima E (2003): Producing drugs from marine sponges. Biotech Adv 21: 585–598.
- Belmonte G, Pederzolli C, Maček P, Menestrina G (1993): Pore formation by the anemone cytolysin equinatoxina II in the red blood cells and model lipid membranes. J Membr Biol 131: 11–22.
- Bosmans F, Aneiros A, Tytgat J (2002): The anemone Bunodosoma granulifera. contains surprisingly efficacious and potent insect-selective toxins. FEBS Lett 532: 131–134.
- Bradford MM (1976): A rapid and sensitive method for the quantitation of microgram quantities of proteins utilizing the principle of protein-dye binding. Anal Biochem 72: 248–254.
- Carrete T, Seymour J (2004): A rapid and repeatable method for venom extraction from Cubozoan nematocysts. Toxicon 44: 135–139.
- Cedillo-Rivera R, Enciso-Moreno JA, Martínez-Palomo A, Orega-Pierres G (1991): Isolation and axenization of Giardia lamblia. from symptomatic and asymptomatic patients in México. Arch Invest Med (Mexico). 22: 79–85.
- Cedillo-Rivera R, Darby JM, Enciso-Moreno JA, Ortega-Pierres G, Ey PL (2003): Genetic homogeneity of axenic isolates of Giardia intestinalis. derived from acute and chronically infected individuals in Mexico. Parasitol Res 90: 119–123.
- Cedillo-Rivera R, Muñoz O (1992): In-vitro susceptibility of Giardia lamblia to adbendazole, mebendazole and other chemotherapectic agens. J Med Microbiol 37: 221–224.
- Diz-Dios P, Ocampo A, Otero I, Iglesias I, Martínez C (2001): Changes in oropharyngeal colonization and infection by Candida albicans. in human immunodeficiency virus-infected patients. J Infect Dis 183: 355–356.
- Encarnación DR, Franzblau SG, Tapia CA, Cedillo-Rivera R (2000): Screening of marine organisms for antimicrobial and antiprotozoal activity. Pharm Biol 38: 379–384.
- Faulkner DJ (1998). Marine natural products. Nat Prod Rep 15: 113–158.
- Faulkner DJ (2000): Marine pharmacology. Antonie van Leeuwenhock 77: 135–145.
- Ghosh K, Banerjee V (1993): Alteration in blood parameters in the fish Heteropneustes fossilis. exposed to dimethoate. Environ Ecol 11: 979–981.
- Haefner B (2003): Drugs from the deep: Marine natural products as drug candidates. Drug Discov Today 8 (12): 536–544.
- Harvey AL (2002): Toxins ‘R’ Us: More pharmacological tools from nature's superstore. Trends Pharmacol Sci 23(5): 201–203.
- Kass-Simon G Scappaticci AA Jr (2002): The behavioral and developmental physiology of nematocysts. Can J Zool 80: 1772–1794.
- Kem WR, Parten B, Pennington MW, Dunn BM, Priece D (1989): Isolation and characterization, and amino acid sequence of a polypeptide neurotoxin occurring in the sea anemone Stichodactyla helianthus.. Biochemistry 28: 3483–3489.
- Maček P (1992): Polypeptide cytolytic toxins from sea anemones (Actinarias.). FEMS Microbiol Immun 105: 121–130.
- Michaels D (1979): Membrane damage by a toxin from the sea anemone Stoichactis helianthus.. Biochim Biophys Acta 555: 67–78.
- Murad A, Houston AH (1988): Leucocytes and leucopoietic capacity in goldfish, Carassius auratus. exposed to sublethal levels of cadmium. Aquat Toxicol 13: 141–154.
- NCCLS (1993): Performance standards for antimicrobial disk susceptibility tests. Approved standard. NCCLS document M2-A5. Wayne, Pa 28.
- Radwan FFY, Gershwin LA, Burnett JW (2000): Toxinological studies on the nematocyst venom of Chrysaora achlyos.. Toxicon 38: 1581–1591.
- Ramasamy S, Isbirter GK, Seymour JE, Hodgson WC (2005): Pharmacologically distinct cardiovascular effects of box jellyfish (Chironex fleckeri.) venom and tentacle-only extract in rats. Toxicol Lett 155: 219–226.
- Rowley AF, Ratcliffe NA (1988): Vertebrate Blood Cells. Cambridge: Cambridge University Press, pp. 127.
- Sánchez-Vargas LO, Ortíz-López NG, Villar M, Moraguez MD, Aguirre JM, Cashat-Cruz M, López-Ribot JL, Gaitán-Cepeda LA, Quindóis G (2005). Oral Candida. isolates colonizing or infecting human immunodeficiency virus-infected and healthy persons in Mexico. J Clin Microbiol 43: 4159–4162.
- Sampath K, Velammal S, Kennedy IJ, James R (1993): Haematological changes and their recovery in Oreochromis mossambicus. as a function of exposure period and sublethal levels of ekalux. Acta Hydrobiol 35: 73–83.
- Svobodova Z, Machova J, Kolaova J, Vykusova B, Piaaka V (1996): The effect of selected negative factors on haematological parameters of common carp, Cyprinus carpio. L. and Tench, Tinca tinca. L. Proc. Sci. Papers to the 75th Anniversary of Foundation of the RIFCH Vodnany, pp. 95–105.
- Thakur N, Sahai S (1993): Differential leukocyte counts of some fishes during malathion intoxication. Environ Ecol 11: 875–878.
- Tejuca M, Anderluh G, Maček P, Marce R, Torres D, Sarracent J, Alvarez C, Lanio ME, Serra M, Menestrina G (1999): Antiparasite activity of sea-anemone cytolysins on Giardia duodenalis. and specific targeting with anti-Giardia antibodies. Int J Parasitol 29: 489–498.
- Undritz E (1979). Atlas of Haematology, 2nd ed. Basel, Sandoz Ltd., pp. 234.
- Varanda W, Finkelstein A (1980): Ion and nonelectrolyte permeability properties of channels formed in planar lipid bilayer membranes by cytolytic toxin from the sea anemone Stoichactis helianthus.. J Membr Biol 55: 203–211.
- Wlasow T (1985): The leukocyte system in rainbow trout, Salmo gairdneri. Rich. affected by prolonged subacute phenol intoxication. Acta Ichthyol Piscator 15: 83–94.