Abstract
Vernonia amygdalina. Del. (Asteraceae) is a plant widely used both for nutritional and medicinal purposes throughout the tropical Africa. The health-promoting ability of this plant species might be related to the antioxidative effect of its constituents. In this study,the antioxidant activity of this plant was evaluated by comparing the reducing capacity and the DPPH radical scavenging of two previously isolated sesquiterpene lactones (vernolide and vernodalol) with the ethanol extract from which the two compounds were isolated. Results indicated that vernolide had a higher reducing power than vernodalol and the ethanol extract. At 0.25 mg/mL, vernolide had an absorbance value of 0.15 while vernolide and the ethanol extract had absorbencies of 0.042 and 0.144, respectively. Catechin (a standard antioxidant compound), however, exhibited a higher reducing power than all the three samples. In the DPPH radical scavenging, both the sesquiterpene lactones and the ethanol extract exhibited appreciable activity. At 0.25 mg/mL, the activity order was ethanol extract > vernodalol > vernolide. At all concentrations, the ethanol extract had higher radical scavenging activity than the sesquiterpene lactones, suggesting a possible synergistic effect of these and any other antioxidant constituents.
Introduction
The generation of reactive oxygen species and free radicals in humans has recently been suggested to be a contributor to a wide range of pathologic disturbances such as myocardial and cerebral ischemia, atherosclerosis, renal failure, inflammation, cancer, diabetes, and arthritis (Haraguchi et al., Citation1997; Shindo et al., Citation2004). The generation of active oxygen species is normally catalyzed by transition metals such as Fe2+ and Cu+ and subsequently leads to lipid peroxidation, which can result in cell death (and neoplasia) (Smith, Citation1985). The role of antioxidants may therefore include decreasing reactive oxygen species concentrations, interception of singlet oxygen, prevention of the initiation of lipid peroxidation by scavenging primary radicals, binding metal ion catalysts, decomposing primary products to nonradical compounds, and chain breaking to prevent continued hydrogen abstraction from lipid substrates (Saha et al., Citation2004).
Natural products such as sesquiterpenoids and flavonoids have been reported to be potential antioxidants (Bork et al., Citation1997; Haraguchi et al., Citation1997; Jodynis-Liebert et al., 2000). These products are naturally obtained from food and use of medicinal plants. Vernonia amygdalina. Del. (Asteraceae) is one of the prominent plant species used for both medicinal and culinary purposes in Africa (Bullogh & Leary, Citation1982; Aka & Ekekwe, 1995; Igile et al., Citation1995; John et al., 1995; Hamill et al., Citation2000; Otshudi et al., Citation2000; Kambizi & Afolayan, Citation2001; Ajebesone & Aina, 2004).
As an attempt to further evaluate the ethnomedical potential of V. amygdalina., two sesquiterpene lactones (vernolide and vernodalol) together with the crude ethanol extract of the leaves of this plant were screened for comparison of their radical scavenging activity and reducing power. This was done with the aim of further validating the medicinal properties of V. amygdalina..
Materials and Methods
General analysis
The 1H, 13C, and DEPT 135 NMR spectra (in methanol-d.4) as well as 2D (COSY HSQC, HMBC) spectra were obtained on a Bruker Avance DPX 300 (300 MHz); mass spectra were recorded on Finnigan MAT LCQ DECA instrument; melting points were recorded on Stuart Scientific (SMP1) apparatus; chromatographic separation experiments were performed on silica gel 60 (particle size 0.063–0.200 mm, Merck); preparative TLC was carried out using silica gel 60 PF 254+366 precoated glass plates (Merck, South Africa); visualization of compounds was done under UV lamp (254 and 266 nm) and also using vanillin– sulfuric acid spray.
Chemicals
Catechin, potassium ferricyanide [K3Fe(CN)6], trichloroacetic acid (Cl3CCO2H), ferric chloride (FeCl3), and 1,1-diphenyl-2-picryl-hydrazyl (DPPH) were purchased from Sigma Aldrich (South Africa). All solvents were supplied by Merck Laboratory supplies (South Africa).
Plant material, extraction and isolation of compounds
The leaves of V. amygdalina. were collected in May 2004 from a cultivated garden in East London, South Africa. The plant was authenticated by Prof. Afolayan, and a voucher specimen (Erasto12) was deposited in the herbarium of the University of Fort Hare. The air-dried leaves (500 g) were pulverized and shaken in 99% ethanol at room temperature for 24 h, and the extract was concentrated under reduced pressure to afford 67 g of a crude extract. It was then treated with activated charcoal to remove chlorophyll leaving 42.6 g of extract, which was then subjected to vacuum liquid chromatography using an elution gradient as follows: n.-hexane (100%); n.-hexane/CHCl3 (7:3); CHCl3 (100%); CHCl3/EtOAc (8:2); EtOAc (100%); and finally EtOAc/MeOH (7:3). A total of 20 fractions of 150 mL each were collected.
From the fractions eluted with CHCl3/EtOAc (8:2), white needle-like crystals formed, and were purified by recrystallizing in CHCl3/EtOAc (9:1) to give 78 mg of a compound identified as vernolide. After spotting the remaining fractions on analytical Thin Layer Chromatography (TLC) and developing with CHCl3/acetone/MeOH (8:1:1), the EtOAc (100%) and EtOAc/MeOH (7:3) fractions were combined and chromatographed on a silica gel column. The elution was done using CHCl3 (100%) followed by CHCl3/EtOAc (8:2), CHCl3/EtOAc/MeOH (7:2.5:0.5), with increasing polarity to yield 55 fractions. Guided by analytical TLC, fractions 30–41 were combined and subjected to preparative TLC and developed with CHCl3/acetone/MeOH (8:1:1) to give 243 mg of a creamy feathery crystalline compound identified as vernodalol. The identification of these compounds was achieved using NMR and MS spectroscopic techniques.
Reducing capacity
Adopting the method of Oyaizu (Citation1986), 0.025, 0.05, 0.1, and 0.25 mg/mL of pure compounds and the ethanol extract were mixed with 2.5 mL of 0.2 M phosphate buffer (pH 6.6) and 2.5 mL of 1% potassium ferricyanide [K3Fe(CN)6]. The mixture was then incubated at 50°C for 20 min. Aliquots (2.5 mL) of 10% trichloroacetic acid were added to the mixture, which was then centrifuged for 10 min at 1000 × g.. The upper layer of the solution (2.5 mL) was mixed with 2.5 mL of distilled water and 0.5 mL of 0.1% FeCl3, and the absorbance was measured at 700 nm in a Beckman spectrophotometer (Sigma, South Africa). Catechin was used as a standard antioxidant compound. The analysis of each assay solution was replicated three-times.
DPPH radical scavenging activity
The method described by Liyana-Pathirana and Shahidi (2005) was used to assess the DPPH radical scavenging activity of the two sesquiterpene lactones and the ethanol extract. The DPPH solution (0.1 mL of 0.12 mM) in methanol was separately mixed with 0.025, 0.05, 0.1, and 0.25 mg/mL of the samples, respectively, and vortexed thoroughly. The absorbance of the mixtures at ambient temperature was recorded for 60 min at 10-min intervals. Catechin was used as a reference antioxidant compound. The absorbance of the remaining DPPH Radicals was read at 517 nm using a Beckman spectrophotometer (model DU 7400). The analysis of each assay solution was replicated three-times. The scavenging of DPPH radicals was calculated according to the following equation:
where Abscontrol is the absorbance of DPPH radical + methanol, and Abssample is the absorbance of DPPH radical + sample extract/standard.
The results were expressed as mean values±SD (n. = 3). Statistical significance was determined using the MUSTAT program, and the differences at P < 0.05 were considered to be significant.
Results and Discussion
Structural identification of sesquiterpene lactones
Vernolide was obtained as white needle crystals with melting point 176–177°C and gave an Electron Impact-Mass Spectrum (EI-MS) molecular ion peak at m./z. 385 [M + Na]+ consistent with molecular formula C19H22O7 when m./z. 23 for sodium ion is subtracted. The 1H and 13C NMR spectral data of vernolide were in good agreement with published data (Rabe et al., Citation2002) (). This compound has previously been reported from the aerial parts of Vernonia amygdalina. Del., Vernonia colorata. willd, and Vernonia hindei. S. Moore (Jisaka et al., Citation1993; Zdero et al., 1991; Rabe et al., Citation2002).
Table 1.. NMR data for vernolide and vernodalol (300 MHz, CD3OD) isolated from the leaves of Vernonia amygdalina.
Vernodalol was isolated as creamy feathery crystals with melting point 132–136°C and gave an EI-MS molecular ion peak at m./z. 415 [M + Na]+ consistent with molecular formula C20H24O8 when m./z. 23 for sodium ion is subtracted. The 1H NMR spectral data of vernodalol were in agreement with the earlier report of this compound by Asaka et al. (1977) (). Jisaka et al. (Citation1993) reported vernodalol to be an artifact of vernodalin, presumed to be the product of methanol and extract solvolysis reaction during the extraction process. However, a recent report by Erasto et al. (Citation2006) confirmed the identity of this compound.
Reducing power
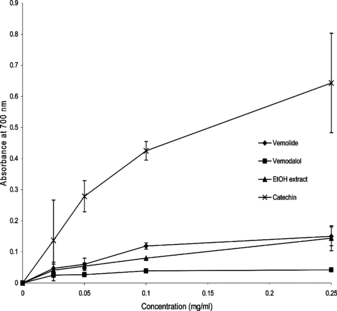
The antioxidant activity of the two isolated sesquiterpene lactones (vernolide and vernodalol) and the ethanol extract was manifested through their reducing power as shown in . In this assay, the Fe3+ → Fe2+ transformation was established as reducing capacity. Catechin exhibited a superior reducing power at all concentrations compared with the sesquiterpenoids and the ethanol extract. The reducing capacity of the samples, increased with increasing concentration of the sample, which implied that the samples are electron donors. At 0.05 mg/mL, the absorbencies of vernolide, vernodalol, and the ethanol extract (at 700 nm) were 0.06, 0.027, and 0.054, whereas at 0.25 mg/mL the absorbencies were 0.15, 0.042 and 0.144, respectively. These values reflect the following reducing capability: vernolide > ethanol extract > vernodalol ().
Figure 1 Reducing capacity of different amounts of vernolide, vernodalol, and ethanol extract from the leaves of V. amygdalina. compared with catechin (a standard antioxidant compound) using photospectroscopic detection of the Fe3+ → Fe2+ transformation. Each value is expressed as mean ±SD (n. = 3).
DPPH radical scavenging activity
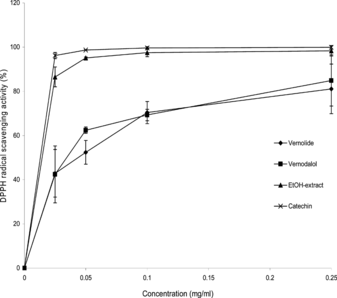
All the samples showed an appreciable DPPH radical scavenging effect at all concentrations. However, the ethanol extract was more active compared with vernolide and vernodalol (). The radical scavenging activity of all the samples exhibited a concentration- and time-dependent reaction trend. Increasing the concentration of the assay sample increased the radical scavenging effect. The time-dependency factor was particularly observed during the ethanol extract and free radicals reaction. During the first 30 min of reaction, the activity of the ethanol extract was significantly lower compared with catechin, whereas after 60 min the activities of both the ethanol extract and catechin were of the same order ().
Figure 2 The DPPH radical scavenging activity of vernolide, vernodalol, and ethanol extract from the leaves of V. amygdalina. compared with catechin after 60 min of reaction. Each value is expressed as mean±SD (n. = 3).
The percentages of free radical inhibition for the two compounds showed vernodalol to be slightly more active than vernolide. It had an IC50 value of 0.03 mg/mL compared with 0.04 mg/mL of vernolide (). The disparity may be due to some structural differences between the two compounds that are based on the number of functional groups and substituents. Vernodalol has three α, β-unsaturated carbonyl carbons and two hydroxyl groups, whereas vernolide has two α, β-unsaturated carbonyl carbons and one OH group. An interesting structural difference is the presence of an ethenyl (–CH=CH2) group at C-10 of vernodalol (). This group is free to rotate through the C1–C10 bond so as to attain stable configuration and thus make it more accessible for a free radical addition reaction. These comparative structural advantages could be responsible for a higher radical scavenging activity for vernodalol compared with that of vernolide.
Table 2.. The IC50 value of two sesquiterpene lactones (vernolide and vernodalol) and the ethanol extract from the leaves of Vernonia amygdalina..
Conclusions
The ethanol extract and the two sesquiterpene lactones (vernolide and vernodalol) isolated from V. amygdalina. exhibited appreciable antioxidant activities as manifested through their reducing capacity and free radical (DPPH) scavenging properties. The ethanol extract, however, had the highest free radical scavenging activity, therefore suggesting a possible synergistic effect of these compounds and other constituents. It has previously been observed that the antioxidant activity of natural products may be correlated with their reducing capacity (Yen & Duh, Citation1995; Duh & Yen, Citation1997). In this study, however, the reducing power of both the ethanol extract and the sesquiterpene lactones exhibited appreciable electron-donating capacity. This fact further strengthens the antioxidative potential of Vernonia amygdalina.. The results of this study further indicate that not only flavonoids are responsible for any antioxidant effects of this plant species as reported by Igile et al. (Citation1994) but that sesquiterpene lactones might also contribute.
Acknowledgment
The authors thank the National Research Foundation for financial support.
References
- Ajebesome PE, Aina JO (2004): Potential African substitutes for hops in tropical beer brewing. J Food Tech Africa 9: 13–16.
- Akah PA, Ekekwe RK (1995): Ethnopharmacology of some Asteraceae family used in Nigerian traditional medicine. Fitoterapia 66: 351–355.
- Asaka Y, Kubota T, Kulkami AB (1977): Studies on a bitter principle from Vernonia anthelmintica. Phytochemistry 16: 1838–1839.
- Bork PM, Schimitz ML, Kuhnt M, Escher C, Heinrich M (1997): Sesquiterpene lactone containing Mexican Indian medicinal plants and pure sesquiterpene lactones as potent inhibitors of transcription factor NF-κB. FEBS Lett 402: 85–90.
- Bullogh CHW, Leary WP (1982): Herbal medicines used by traditional birth attendants in Malawi. Trop Geogr Med 34: 81–85.
- Duh PD, Yen GC (1997): Antioxidant activity of three herbal water extracts. Food Chem 60: 639–645.
- Erasto P, Grierson DS, Afolayan AJ (2006): Bioactive sesquiterpene lactones from the leaves of Vernonia amygdalina.. J Ethnopharmacol 106: 117–120.
- Hamill FA, Apio S, Murbiru NK, Mosango M, Bukenya-Ziraba R, Maganyi OW, Soejarto DD (2000): Traditional herbal drugs of southern Uganda. J Ethnopharmacol 70: 281–300.
- Haraguchi H, Ishikawa H, Sanchez Y, Ogura T, Kubo Y, Kubo I (1997): Antioxidative constituents of Heterotheca inuloides.. Bioorg Med Chem 5: 865–871.
- Igile GO, Oleszek W, Jurzysta M, Burda S, Fanfunso M, Fasanmade AA (1994): Flavonoids from Vernonia amygdalina. and their antioxidant activities. J Agric Food Chem 42: 2445–2448.
- Igile GO, Oleszek W, Burda S, Jurzysta M (1995): Nutritional assessment of Vernonia amygdalina. leaves in growing mice. J Agric Food Chem 43: 2162–2166.
- Jisaka M, Ohigashi H, Takegawa K, Huffman MA, Koshimizu K (1993): Antitumoral and antimicrobial activities of bitter sesquiterpene lactones of Vernonia amygdalina., a possible medicinal plant used by wild chimpanzee. Biosci Biotech Biochem 57: 833–834.
- Jodynis-Liebert J, Murias M, Bloszyk E (2000): Effect of sesquiterpene lactones on antioxidant enzymes and some drug-metabolizing enzymes in rat liver and kidney. Planta Med 66: 199–205.
- Johns T, Faubert GM, Kokwaro JO, Mahunnah RLA, Kimanani EK (1995): Anti-giardial activity of gastrointestinal remedies of the Luo of East Africa. J Ethnopharmacol 46: 17–23.
- Kambizi L, Afolayan AJ (2001): An ethnobotanical study of plants used for the treatment of sexually transmitted diseases (njovhera) in Guruve District, Zimbabwe. J Ethnopharmacol 77: 5–9.
- Liyana-Pathirana CM, Shahidi F (2005): Antioxidant activity of commercial soft and hard wheat (Triticum aestivum.L.) as affected by gastric pH conditions. J Agric Food Chem 53: 2433–2440.
- Otshudi AL, Vercruysse A, Foriers A (2000): Contribution of the ethnobotanical, phytochemical and pharmacological studies of traditionally used medicinal plants in the treatment of dysentery and diarrhea in Lomela area, Democratic Republic of Congo. J Ethnopharmacol 71: 411–423.
- Oyaizu M (1986): Studies on product of browning reaction prepared from glucose amine. Jpn J Nutri 44: 307–315.
- Rabe T, Mullholland D, van Staden J (2002): Isolation and identification of antibacterial compounds from Vernonia colorata. leaves. J Ethnopharmacol 80: 91–94.
- Saha K, Lajis NH, Israf DA, Hamzah AS, Khozirah S, Khamis S, Syahida A (2004): Evaluation of antioxidant and nitric oxide inhibitory activities of selected Malaysian medicinal plants. J Ethnopharmacol 92: 263–267.
- Shindo K, Kimura M, Iga M (2004): Potent antioxidative activity of cacalol, a sesquiterpene contained in Cacalia delphiniifolia. Sleb et Zucc. Biosci Biotech Biochem 68: 1393–1394.
- Smith MT (1985): In: Quintaniha A, ed., Reactive Oxygen Species in Chemistry, Biology, and Medicine. New York, Plenum Press, pp. 157–166.
- Yen GC, Duh PD (1995): Scavenging effect of methanolic extracts of peanut hulls on free radical and active oxygen species. J Agric Food Chem 42: 629–632.
- Zdero C, Bohlmann F, Mungai GM (1991): Glaucolides and hirsutinolide from African Vernonia. species. Phytochemistry 30: 2653–2654