Abstract
A new O.-galloyl-C-glycosylflavone, 2″,6″-O.-digalloylvitexin (1), along with four known glycosylflavones (2–5) have been isolated from Clidemia sericea. D. Don (Melastomataceae), and four other known glycosylflavones (6–9) have been isolated from Mosquitoxylon jamaicense. Krug & Urb. (Anacardiaceae). Compound 1, 3, and 6 showed mild antimalarial activity (24 ± 1, 38 ± 2, and 44 ± 1 µM, respectively) against a chloroquine-resistant P. falciparum. strain. Additionally, tests against leishmaniasis and Trypanosoma cruzi. were made. These compounds were identified by MS, UV, IR, and 1D and 2D NMR data and by comparison with the literature data.
Introduction
Malaria is one of the tropical diseases with the greatest impact on world health, causing 300 million cases and one million deaths annually (Gelb & Hol, Citation2002). Strains of Plasmodium falciparum. that are resistant to the latest drugs, as well as chloroquine, have emerged and spread rapidly (Ridley, Citation2002aCitationb). Continuing with our search for antiprotozoal drugs, we report now the bioassay-guided isolation of five flavonoids from Clidemia sericea. D. Don (Melastomataceae): the new O.-galloyl-C-glycosylflavone 1 () and the known isovitexin 2, 2″-O.-galloylvitexin 3, rutin 4 (Lin et al., Citation2000), and vitexin 5 (Latte et al., 2000), and four known flavonoids from Mosquitoxylon jamaicense. Krug & Urb. (Anacardiaceae): phloridzin 6 (Hilt et al., Citation2003), 4-hydroxy benzenepropanal 7 (Ishikawa & Kishi, Citation2000), trilobatin 8 (Tanaka et al., Citation1983), and quercetine-3-O.-β.-D-galactoside 9 (Zhang & Mao, Citation2001) as part of the ongoing ICBG program based on Panamá (Coley et al., Citation2003).
Materials and Methods
General experimental procedures
Optical rotations were determined on an Autopol III 6971 Automatic Polarimeter (Rudolph Research Analytical, NJ, USA). Infrared (IR) spectra were measured on a Perkin-Elmer Fourier transformer infrared (FT-IR) Spectrometer Spectrum RXI (Perkin-Elmer, USA). The nuclear magnetic resonance (NMR) spectra were recorded on a Bruker Avance 300 spectrometer (300 MHz for proton and 75 MHz for carbon) (Bruker BioSpin, MA, USA). Low Resolution and High Resolution Mass Spectra (HRMS) were recorded on a Kratos MS50TC instrument using chemical Ionization (Kratos Analytical Instruments, NJ, USA). High Pressure Liquid Chromatography (HPLC) and ultraviolet (UV) spectrum were carried out on a Waters Liquid Chromatography (LC) system, with a 600 pump and 996 phodiode array detector (Waters, MA, USA).
Plant material
Young leaves of Clidemia sericea. and Mosquitoxylon jamaicense. were collected in Altos de Campana National Park and Chagres National Park, respectively, in the Republic of Panama. The material was identified by Professor Mireya Correa of the University of Panama and the Smithsonian Tropical Research Institute. Vouchers have been deposited at the Herbarium of the University of Panama, numbers PMA 53118 (C. sericea.) and PMA 53117 (M. jamaicense.).
Extraction and isolation
The leaf material of C. sericea. (300 g) and of M. jamaicense. (900 g) was homogenized and processed as previously described (Torres et al., Citation2003). The organic extracts were concentrated as described (Montenegro et al., Citation2003), obtaining 15 g (IC50: 16 µg/mL) and 44 g (IC50: 12 µg/mL), correspondingly.
All LH-20 columns were equilibrated with methanol prior to chromatography.
Clidemia sericea
The methanol crude extract of C. sericea. (15 g) was subjected to liquid-liquid partition with n.-hexane and methanol. The methanol fraction was evaporated and subjected to a second solvent partition using EtOAc and H2O. The EtOAc portion (2.7 g, IC50: 5 µg/mL) was subjected to vacuum-liquid chromatography (VLC) on silica gel (7GF, VWR Scientific) using hexane-EtOAc-MeOH mixtures of increasing polarity. The fractions were combined according to TLC composition into fractions 1–7 and dried under vacuum. 950 mg of fraction 5 (IC50: 8 µg/mL), between 650–700 mL, was chromatographed on Sephadex LH-20 (2.5 × 70 cm) (25–100 µm Sigma) and eluted with EtOH:H2O 80:20 (800 mL), EtOH:H2O 90:10 (300 mL), and 100% EtOH (500 mL). The fractions were combined according to TLC composition into Frs. 5a–5i. Fraction 5d (42 mg) was recrystallized from MeOH, yielding compound 2 (30 mg). Purification of fraction 5e used isocratic reverse phase HPLC (XTerra® 10 µm, 10 × 250 mm) with MeOH:H2O (50:50) as eluent, flow 2 mL/min, yielding compounds 3 (20 mg, tR: 17 min) and 4 (7 mg, tR: 32 min). Fraction 5 h was recrystallized from EtOAc and purified using RP-HPLC (XTerra 10 µm, 10 × 250 mm) with MeOH:H2O (40:60), flow 2 mL/min, yielding the galloylglycosylflavone 1 (6.4 mg, tR: 17.8 min). Fraction 6 (267 mg), between 700–800 mL, was redisolved in MeOH and chromatographed on Sephadex LH-20 (2.5 × 70 cm, 25–100 µm, Sigma) and eluted with EtOH:H2O 80:20 (800 mL), EtOH:H2O 90:10 (300 mL), and 100% EtOH (500 mL). The fractions were combined according to TLC composition into Frs. 6a–6j. Fraction 6d (45 mg) was recrystallized from MeOH, yielding 5 (5 mg).
Mosquitoxylon jamaicense
The methanol crude extract of M. jamaicense. (44 g) was subjected to liquid-liquid partition with n.-hexane and MeOH. The methanol fraction was evaporated and subjected to a second solvent partition using EtOAc and H2O. The EtOAc part (6.6 g, IC50: 7 µg/mL) was subjected to vaccum-liquid chromatography (VLC) on silica gel (7GF, VWR Scientific) using hexane-AcOEt-MeOH mixtures of increasing polarity.
The fractions were combined according to TLC composition into Frs. 1–6. Fraction 5 (2.5 g, IC50: 17 µg/mL) between 1260–1350 mL was chromatographed on Sephadex LH-20 (2.5 × 70 cm, 25–100 µ, Sigma) and eluted with EtOH:H2O 80:20 (800 mL), EtOH:H2O 90:10 (300 mL), 100% EtOH (500 mL), and MeOH 100% (500 mL). The fractions were combined according to TLC composition into Frs. 5a–5k. Fraction 5d (132 mg) was filtered on a solid-phase extraction cartridge of RP-18 (Merck) and recrystallized from MeOH. This fraction was purified using RP-HPLC (XTerra 10 µm, 10 × 250 mm) with MeOH:H2O (40:60), flow 2 mL/min, yielding 6 (16 mg, tR: 19 min). Purification of fraction 5e using preparative RP-HPLC (Novapack RP-18 6 µm, 25 × 200 mm) with gradient MeOH:H2O (10:90–70:30 in 40 min and 70:30–100 MeOH in 50 min) flow 4 mL/min, yielding 8 (38 mg, tR: 20 min) and 7 (6 mg, tR: 40 min). Fraction 5i was recrystallized from MeOH, yielding 9 (12 mg).
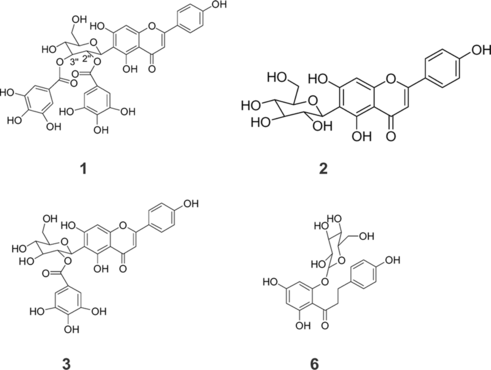
Galloylglycosylflavone 1
Yellow amorphous powder; [α]D22: −14.25 (MeOH, c 0.25); IRv.max cm−1: 3352, 2924, 1624, 1598, 1516, 1452, 1262, 1206, 1174, 1076, 1046, 1024, 994, 828; UV λ.max (MeOH) nm 225.0, 269.8, 281.1, 339.9, 343.4; 1H NMR (MeOD; 300 MHz) δ. 7.76 (2H, d, J. = 8.3 Hz, H-2′ and H-6′), 7.11 (2H, s, H-2″″, and H-6″″), 6.88 (2H, d, J. = 8.3 Hz, H-3′ and H-5′), 6.82 (2H, s, H-2″′ and H-6″′), 6.52 (1H, s, H-3), 6.42 (1H, s, H-8), 5.50 (1H, t, J. = 7.8 Hz, H-2″), 5.33 (H, m, H-3″), 5.32 (H, d, J. = 7.8 Hz, H-1″), 3.67 (1H, m, H-5″), 3.89 (1H, m, H-6″), 3.83 (3H, m, H-4″, H-6″); 13C NMR (MeOD; 75 MHz) δ. 184.2 (C-4), 168.5 (C=O 3″ ), 167.5 (C=O 2″), 166.5 (C-2), 164.9 (C-7), 163.0 (C-5 and C-4′), 159.2 (C-9), 146.6 (C-3″″ and C-5″″), 146.5 (C-3″′ and C-5″′), 140.2 (C-4″′ and C-4″″), 129.8 (C-2′ and C-6′), 123.4 (C-1′), 121.8 (C-1″″), 121.3 (C-1″′), 117.3 (C-3′ and C-5′), 110.8 (C-2″″ and C-6″″), 110.7 (C-2″′ and C-6″′), 107.8 (C-10), 105.3 (C-6), 104.2 (C-3), 95.4 (C-8), 83.2 (C-5″), 79.4 (C-3″), 73.4 (C-2″), 72.0 (C-4″), 63.0 (C-6′); HRFABMS (NBA, positive mode) [M]+736.12760 (calculated C35H28O18, 736.12756).
Assays
All assays were based on inhibition of growth of the parasites by added compounds or extracts, as described previously (Molinar-Toribio et al., Citation2006 and 2004; Corbett et al., Citation2004; Torres et al., Citation2003 and Citation2004; Williams et al., Citation2003).
Cytotoxicity assay
Vero cells adhering to 96-well plates were used to evaluate the toxicity of the compounds purified on the basis of the reduction of 3-(4, 5-dimethylthiazol-2-yl)-2, 5-diphenyltetrazolium bromide (MTT, Sigma) (Torres et al., Citation2004). After treatment with the test compound and 4 h of incubation at 37°, cell viability was evaluated in an ELISA reader at 570 nm.
Results and Discussion
Clidemia sericea
The ethyl acetate partition fraction of the crude extract of C. sericea. showed significant antiplasmodial activity (IC50: 5 µg/mL), and was selected for bioguided fractionation, yielding the novel galloylglycosylflavone (1) and four known compounds (2–5).
Compound 1 was obtained as a yellow powder and showed a pseudomolecular ion from its HRFABMS at m/z. 737.12760 (C35H28O18, calcd 736.12756), which was consistent with 35 carbons observed in the 13C NMR spectra, sorted by DEPT experiments into 1-CH2 oxygenate, 15-CH (5 oxygenate), and 19 quaternary. The UV spectra of compound 1 exhibited three absorption bands at 222, 272, and 337 nm, consistent with a flavone derivative (Latte et al., 2000). The IR spectrum showed bands consistent with the presence of one or more hydroxyl groups (3352 cm−1), an ester carbonyl (1624 cm−1), a conjugated carbonyl (1598 cm−1), and a phenyl group (1516, 1452 cm−1). The 1H NMR spectrum showed signals due to two galloyl groups δ. 6.82 (2H, s) and 7.11 (2H, s) and 13C NMR signals at δ., 110.7, 121.3, 140.2, 146.5, 167.5 (COO), due to one galloyl group, and signals at δ. 110.8, 121.8, 140.2, 146.6, 168.5 (COO), due to a second galloyl group.
The 1H NMR spectrum revealed the presence of two protons at δ. 6.42 (H-8) and δ. 6.52 (H-3) belonging to the flavone skeleton, assigned from HMQC and HMBC correlations. A para.-substituted phenol was characterized by aromatic A2B2-spin system of the B-ring at δ. 7.76 and δ. 6.88 (each 2H, d, J. = 8.3 Hz). The anomeric proton of the β.-D-glucopyranosyl moiety (δ. 5.32, d, J. = 7.8 Hz) had correlations with C-2″ and C-3″ and a long-range correlation with C-6″. A methylene proton at δ. 5.50 (t, J. = 7.89 Hz) correlated with C-3″ and had a long range correlation with the carbonyl (167.5) of a galloyl group, indicating that one of the galloyl group is attached to C-2″ as in 3. A second methines proton at δ. 5.33 (m) correlated with C-2″ and another carbonyl group (168.5) of a second galloyl group, indicating that this second galloyl group was attached to C-3.″
Mosquitoxylon jamaicense
The EtOAc liquid-liquid partition fraction of the crude MeOH-EtOAc extract of M. jamaicense. with an activity IC50: 12 µg/mL against P. falciparum. was selected to bioassay-guided fractionation; yielding four known compounds (6–9).
From the nine compounds reported here, three (i.e., 1, 3, and 6) showed moderate activity against W2, a Plasmodium falciparum. strain (chloroquine-resistant), while a further six isolated compounds were with activities that exceeded 50 µg/mL (). Compounds 1 and 3 are more active than 2, and this suggests that the galloyl moiety is necessary for the activity as previously reported by our group (Corbett et al., Citation2004). Likewise, none of the isolated compounds demonstrated activity against Leishmania. and T. cruzi. parasites at concentrations of 40 and 50 µg/mL, respectively, or significant cytotoxic activity when tested against Vero cells (100 µg/mL).
Table 1.. AntimalarialFootnotea. activity and cytotoxicitiesFootnoteb. of flavonoid glycosides.
Acknowledgments
The authors extend special thanks to Drs. Phyllis D. Coley and Thomas A. Kursar from the University of Utah, who were instrumental in establishing the present bioprospecting program in Panama and for the technical assistance from R. Aizprua, N. Flores, and B. Arauz. Funding comes from the International Cooperative Biodiversity Groups Program (grant number 1U01 TW006634-01) from the NIH, National Science Foundation, and U.S. Department of Agriculture.
References
- Coley PD, Heller MV, Aizprua R, Araúz B, Flores N, Correa M, Gupta M, Solis PN, Ortega-Barría E, Romero LI, Gómez B, Ramos M, Cubilla-Ríos L, Capson T, Kursar T (2003): Using ecological criteria to design plant collection strategies for discovery. Front Ecol Environ 1: 421–428.
- Corbett Y, Herrera L, González J, Cubilla L, Capson T, Coley P, Kursar T, Romero L, Ortega-Barría E (2004): A novel DNA-based microfluorimetric method to evaluate anti-malarial drug activity. Am J Trop Med Hyg 70: 119–124.
- Gelb M, Hol W (2002): Drugs to combat tropical protozoan parasites. Science 297: 343–344.
- Hilt P, Schieber A, Yildirim C, Arnold IK, Conrad J, Beifuss U, Carle R (2003): Detection of phloridzin in strawberries (Fragaria. × ananassa. Duch.) by HPLC-MS/MS and NMR spectroscopy. J Agric Food Chem 51: 2896–2899.
- Ishikawa Y, Kishi K (2000): Molecular orbital approach to odor molecules: Normal fatty acids and cyclamenaldehydes. Int J Quantum Chem 79: 101–108.
- Latte KP, Ferreira D, Venkatraman MS, Kolodziej H (2002): O.-Galloyl-C-glycosylflavones from Pelargonium reniforme.. Phytochemistry 59: 419–424.
- Lin YL, Kao YH, Shiao MS, Chen CC, Ou JC (2000): Flavonoid glycosides from Terminalla catappa. L. J Chin Chem Soc 47: 253–256.
- Molinar-Toribio E, González J, Ortega-Barría E, Capson TL, Coley PD, Kursar TA, McPhail K, Cubilla-Ríos L (2006): Antiprotozoal activity against Plasmodium falciparum. and Trypanosoma cruzi. of xanthones isolated from Chrysochlamys tenuis.. Pharm Biol 44: 550–553.
- Montenegro H, Gutiérrez M, Romero LI, Ortega-Barría E, Capson T, Cubilla-Ríos L (2003): Aporphine alkaloids from Guatteria spp. with leishmanicidal activity. Planta Med 69: 677–679.
- Ridley R (2002a): Medical need scientific opportunity and the drive for antimalarial drugs. Nature 415: 686–693.
- Ridley R (2002b): Introduction. Antimalarial drug resistance: Ramifications, explanations and challenges. Microb Infect 4: 155–156.
- Tanaka T, Tanaka O, Kohda H, Chou WH, Chen FH (1983): Isolation of trilobatin, a sweet dihydrochalcone-glucoside from leaves of Vitis piasezkii. Maxim. and V. saccharifera makino.. Agric Biol Chem 47: 2403–2404.
- Torres D, Ureña L, Ortega-Barría E, Capson T, Cubilla-Ríos L (2003): Five new cassane diterpenes from Myrospermum frutescens. with activity against Tripanosoma cruzi.. J Nat Prod 66: 928–932.
- Torres D, Ureña L, Ortega-Barría E, Coley PD, Kursar TA, Capson T, McPhail K, Cubilla-Ríos L (2004): Novel cassane and cleistanthane diterpenes from Myrospermum frutescens.: Absolute stereochemistry of the cassane diterpene series. J Nat Prod 67: 1711–1715.
- Williams C, Espinosa OA, Montenegro H, Cubilla L, Capson L, Ortega-Barría E, Romero LI (2003): Hydrosoluble formazan XTT: Its application to natural drug discovery for Leishmania.. J Microbial Methods 55: 813–816.
- Zhang T, Mao TA (2001): Constants for chemical exchange between water and hydroxyl protons in natural compounds evaluated with NMR two-dimensional exchange spectroscopy and geometric average method. Appl Magn Reson 20: 189–202.