Abstract
This study investigated the effects of the Securidaca longepedunculata. Fres. (Polygalaceae) lyophilized root aqueous extract on calcium signaling in rat cultured skeletal muscle cells. In Africa, this plant is used to treat snakebites, to calm inflammation, and to cure antibacterial infections. This wide spectrum of pharmacological activities requires multiple studies to identify the different mechanisms of action. The results of the study showed that the lyophilized root aqueous extract (1 µg/ml) increased the resting free calcium level (viewed as the fluorescence ratio). This effect was blocked by ryanodine and nifedipine but not by 2-aminoethoxydiphenylborate (2-APB). It was therefore concluded that the main effect of the SL extract operates through the dihydropyridine (DHP) receptors.
Introduction
According to the World Health Organization, a large segment of the human population still depends on traditional medicine. In Africa, many medical plants are listed in the pharmacopoeia to treat a variety of diseases (Adjanohoun, Citation1990). Among them is Securidaca longepedunculata. Fres. (Polygalaceae), which is used to treat snakebites (Diakite et al., Citation1977; Chippaux et al, Citation1997) and for its anti-inflammatory, antimalarial, and antibacterial effects (Adjanohoun et al., Citation1989; Ancolio et al., Citation2002). This wide spectrum of pharmacological activities requires multiple studies to identify the different mechanisms of action.
Securida longepedunculata. (SL) is common to West African countries (Abbiw, Citation1990). Previous results showed that the SL bark contains β.-D-(3,4 disinapoyl) fructofuranosyl-α.-D-(6-sinapoyl) glucopyranoside and β.-D-(3-sinapoyl) fructofuranosyl-α.-D-(6-sinapoyl) glucopyranoside (De Tommasi et al., 1993) and that the SL root bark contains methyl 2-hydroxybenzoate and methyl 2-hydrxy-6-methoxybenzoate (Jayasekara et al., Citation2002). The root extract has been shown to have many effects on frog muscle fiber, such as a curare-like effect at the neuromuscular junction, an increase in action potential duration, and some competition with the Naja nigricollis. Reinhardt (Elapidae) venom (Kone, Citation1980). The root extract of SL increases the sodium current and contraction of cultured rat skeletal muscle cells (Mouzou et al., Citation1999). In previous work, we have shown an important augmentation of cultured rat skeletal muscle cell contraction without a significant effect on L-type calcium current (Mouzou et al., Citation1999). This initial finding suggests an exploration of the possible interference of the lyophilized root extract with calcium mobilizing systems in rat skeletal muscle cells.
We have undertaken the present work to investigate the possible interference of SL lyophilized root extract with intracellular free calcium homeostasis. During the course of this study, three compounds were used to address the depolarization/release processes involved in the SL root extract effects. The first of these compounds was nifedipine, a molecule that has been demonstrated to be a potent inhibitor of charge movements attributed to the voltage-sensing molecules involved in excitation-contraction coupling (ECC) (Rios & Brum, Citation1987; Cognard et al., Citation1990). Nifedipine was used to investigate a possible implication of the T-tubule membrane. Ryanodine, the second compound, which remains the best-known inhibitor of RS calcium channels (Fill et al., Citation1990), was used to investigate the possible alteration of RyRs by root extract. The third compound used was 2-aminoethoxydiphenylborate (2-APB), which is known to inhibit InsP3R (Diver et al., Citation2001; Powell et al., Citation2001). It has been demonstrated that a release of calcium from intracellular stores via the inositol 1,4,5-triphosphate receptor (InsP3R) could exist (Vergara et al., Citation1985; Volpe et al., Citation1985, Melzer et al., Citation1995).
Materials and Methods
Vegetal material
The root specimens of Securidaca longepedunculata. were collected from Anié (located 120 km north of Lomé in Togo, West Africa) in December 2001 and authenticated by Dr. Kouami Kokou and Professor Koffi Akpagana, Department of Botany, University of Lomé, Togo. A sample specimen has been deposited in the herbarium of the Department of Botany. The root bark was dried in the shade and crushed into powder before being mixed in deionized water (40 g/500 ml) for 4 h at 37°C, and then boiled for 10 min. The resulting extract was filtered through Whatman paper and lyophilized.
Culture of mammalian skeletal muscle cells
Primary cultures of mammalian skeletal muscle cells were initiated from satellite cells obtained by trypsinisation of muscle pieces of 1-to 3-day-old Wistar rats' hindlimbs as previously detailed (Rivet et al., Citation1989; Cognard et al., Citation1993a). Cells were maintained for two days in a growth medium at 37°C in a water-saturated atmosphere of 95% air and 5% CO2. This initial growth medium consisted of HamF12 (Gibco, Cergy Pontoise, France), supplemented with 10% heat-inactivated horse serum (Gibco), 10% fetal calf serum (Boehringer Mannheim, Meylan, France), and 1% antibiotics (penicillin-G, 100 U/ml, Sigma and streptomycin, 50 µg/ml, Sigma). The growth medium was then changed for a fusion-promoting medium composed of Dulbeco's Modified Eagle Medium (DMEM, Gibco) supplemented with 5% heat-inactivated horse serum and 1% antibiotics. This culture medium prompted the fusion of mononucleated myoblasts into multinucleated myotubes. The day on which the culture medium was changed was referred to as “zero age.” Experiments were carried out on 3-to 4-day-old myotubes.
Experimental solutions
The experiments were performed in control solution containing (mM): NaCl 130, KCl, 5.4, CaCl2 2.5, MgCl2 0.8, HEPES 10, pH 7.4. The lyophilized extract was added directly to the control solution at 1 µg/ml to make the test solution (Mouzou et al., Citation1999). Rapid changes of solution in the surroundings of tested cells were achieved by means of a homemade (original) microperfusion system.
Intracellular free calcium measurements
Intracellular free calcium was measured with the fluorescence dye Indo-1. Cells were loaded with 3 µM of the lipophilic form of Indo-1 (AM form, dissolved in dimethylsulfoxide) diluted in control solution. The cells were incubated for 45 min in the dark at room temperature, washed twice with the control solution, and incubated for 15 min at 37°C to obtain a complete de-esterification of the probe. Fluorescence was recorded at room temperature using an OSP100 microscopic photometry system (Olympus).
Excitation of Indo-1 was set in the UV range (band-pass filter centred at 360 nm) by means of a xenon lamp. Fluorescence emission was collected through a dichroic filter (455 nm) by means of two photomultiplier tubes with two band-pass filters centered at 405 nm for the emission of the calcium bound form and at 485 nm for the calcium free form of the probe.
Data analysis
Data analysis was performed with the GraphPad Prism 3.0 (GraphPad Software Inc., San Diego, CA, USA) and Origin 5.0 (Microcal Software Inc., Northampton, MA, USA) software. Data are expressed as mean±SEM, and paired or impaired Student's t.-test was used to test for statistical significance between the means. P < 0.05 was considered as significant.
Results
In the presence of lyophilized SL root extract (100 µg/ml), the fluorescence ratio (405/485) significantly increased from 1.4±0.03 to 2.9±0.04, corresponding to an increase of 53%±2 (P < 0.05). This effect was partially reversible (A, n = 12).
Figure 1 Representative recordings showing the effects of SL lyophilized extract (100 µg/ml) on the free calcium level (viewed as the fluorescence ratio) in F + 3 and F + 4 myotubes from cultured rat skeletal muscle cells. In the presence of SL lyophilized extract, the fluorescence ratio increased in control conditions (A). When the cells were incubated with nifedipine (5 µM), the SL extract-induced effect was drastically reduced (B).
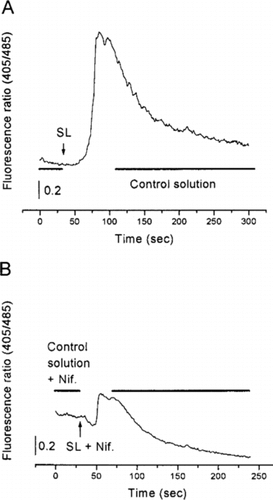
To search for a possible activation effect on the voltage-sensing molecules involved in excitation-contraction coupling, cells were incubated with nifedipine (5 µM). In this case, the application of the lyophilized extract only increased the fluorescence ratio by 10%±1 (B, n = 12), a weak increase as compared with the one under control conditions.
Similar experiments were performed using ryanodine (10 µM), which is known to inhibit the sarcoplasmic reticulum calcium release channels. Under these conditions, SL increased the fluorescence ratio by about 8%±1 (see example, A, n = 10).
Figure 2 Representative recordings showing the effects of SL lyophilized extract (100 µg/ml) on the free calcium level (viewed as the fluorescence ratio) in F + 3 and F + 4 myotubes from cultured rat skeletal muscle cells. In the presence of SL lyophilized extract, the fluorescence ratio increased when cells were incubated with ryanodine (10 µM) (A) or 2-APB (50 µM) (B).
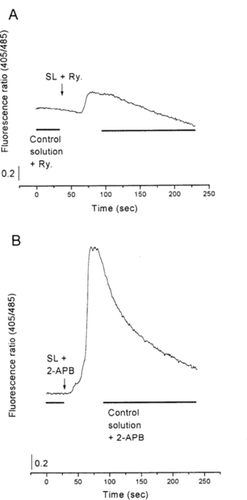
Finally, similar experiments were performed using 2-aminoethoxydiphenylborate (2-APB), known to inhibit sarcoplasmic reticulum IP3R. In this case, the increase in the fluorescence ratio (55%±2; B, n = 10) was similar to the one under control conditions.
Discussion
The experiments described in the present work were performed to investigate the possible interference of lyophilized SL extract with calcium mobilizing systems in skeletal muscle cells. SL applied alone increased the intracellular free calcium level. The partial reversibility agrees with the results of Kone (Citation1980) and Mouzou (Citation1999). Data and reasoning can be summarized as follows:
IP3 receptors were not involved in the SL effect, as the induced calcium increase remained even in the presence of 2-aminoethoxydiphenylborate (2-APB), an inhibitor of calcium-releasing IP3 receptors (Diver et al., Citation2001; Powell et al., Citation2001).
By contrast, the calcium level-increasing effect induced by application of the SL extract could be related to the ryanodine receptor, since in the presence of ryanodine the amplitude of the calcium transient induced by the SL extract was drastically reduced. In this way, two hypotheses could be constructed: 1) the SL extract was able to directly induce calcium release in a way similar to the action of caffeine (Cognard et al., Citation1993b; Herrmann-Frank et al., 1999); or 2) the voltage-sensing molecules of the voltage-dependent calcium channel (DHP receptors) are involved in excitation-contraction coupling (Rios & Brum, Citation1987; Cognard et al., Citation1990).
This latter hypothesis was tested by blocking the voltage-sensing molecules of the DHP receptors with nifedipine. The drastic inhibiting effect showed clearly that the SL extract effect operates through the activation of the DHP receptors. The remaining effect in the presence of ryanodine or nifedipine could be interpreted as an incomplete inhibition of the targeted mechanisms or, alternatively, as the action of secondary compounds, since the SL extract probably contains several active molecules.
The experimental results of the present study can explain previous findings showing increases in frog skeletal muscle fiber contraction (Kone et al., 1979; Kone, Citation1980) and in cultured rat skeletal muscle cell contraction (Mouzou et al., Citation1999).
Conclusion
The present result demonstrates that one (or several) active component(s) of the Securidaca longepedunculata. lyophilized extract has a significant effect on voltage-sensing molecules. Therefore, extraction and isolation of the different components are required to explain the details of this main effect as well as the wide spectrum of pharmacological activities of this plant.
Acknowledgments
The authors thank Dr. Jean-Mari Coustard (Département de Chimie, Université de Poitiers) for the lyophilized extract and Sarah WOOD (Peace Corps in Togo) for help in the revision of the English version of the manuscript. This work was supported by CNRS/Université de Poitiers UMR 6558 (France).
References
- Abbiw W (1990): Useful Plants of Ghana. Intermediate Technology. London: Royal Botanic Garden Kew, pp. 218–220.
- Adjanohoun A (1989): Contribution aux Ė tudes Ethnobotaniques et Floristiques en République Populaire du Bénin.. Paris: Agence de Coopération Culturelle et Technique, p. 853.
- Adjanohoun A (1990): Etat d'évolution de l'ethnopharmacopée africaine. Bul. Med Trad Pharmacol 1: 159–163.
- Ancolio C, Azas N, Mahiou V, Ollivier E, Di Giorgio C, Keita A, Timon-David P, Balansard G (2002): Antimalarial activity of extracts and alkaloids isolated from six plants used in traditional medicine in Mali and Sao Tome. Phytother Res Nov 16: 646–649.
- Chippaux JP, Rakotonirina VS, Rakotonirina A, Dzikouk G (1997): Drug or plant substances which antagonize venoms or potentiate antivenins. Bull Soc Pathol Exot 90: 282–285.
- Cognard C, Constantin B, Rivet-Bastide M, Imbert N, Besse C, Raymond G (1993a): Appearance and evolution of calcium currents and contraction during the early post-fusional stages of rat skeletal muscle cells developing in primary culture. Development 117: 1153–1161.
- Cognard C, Constantin B, Rivet-Bastide M, Raymond G (1993b): Intracellular calcium transients induced by different kinds of stimulation during myogenesis of rat skeletal muscle cells studies by laser cytofluorimetry with Indo-1. Cell Calcium 14: 333–348.
- Cognard C, Rivet M, Raymond G (1990): The blockade of excitation-contraction coupling by nifedipine in patch-clamped rat skeletal muscle cells in culture. Pflügers Arch 416: 98–105.
- De Tommasi N, Placente S, De Simone F, Pizza C (1993): New sucrose derivatives from the bark of Securidaca longepedunculata.. J Nat Prod 56: 134–137.
- Diakite D (1977): Premier inventaire de la faune ophidienne du Mali. Etude épidémilogique, clinique et thérapeutique des accidents d'envenimation. Bamako, Thèse de Médécine, p. 155.
- Diver JM, Sage SO, Rosado JA (2001): The inositol trisphosphate receptor antagonist 2-aminoethoxydiphenylborate (2-APB) blocks calcium entry channels in human platelets: Cautions for its use in studying calcium influx. Cell Calcium 30: 323–329.
- Fill MD, Coronado R, Mickelson R, Vilven J, Jacobson BA, Louis CF (1990): Abnormal ryanodine receptor channels in malignant hyperthermia. Biophy J 57: 471–475.
- Jayasekara TK, Stevenson PC, Belmain SR, Farman DI, Hall DR (2002): Identification of methyl salicylate as the principal volatile component in the methanol extract of root bark of Securidaca longepedunculata.. J Mass Spectrom 37: 577–580.
- Kone P (1980): Etude toxicologique électrophysiologique et pharmacologique du venin de Naja nigrocollis. (Elapidè de Côte d'Ivoire) et d'une substance antivenimeuse de la pharmacopée traditionnelle africaine (extrait de Securidaca longepedunculata., Polygalacea). Thèse d'état Abidjan (Côte d'Ivoire), p. 172.
- Melzer V, Hermann-Frank A, Lüttgau HCh (1995): The role of calcium ions in excitation-contraction coupling of skeletal muscle fibres. Biochem Biophys Acta 1241: 59–116.
- Mouzou AP, Bulteau L, Raymond G (1999): The effects of Securidaca longepedunculata. root extract on ionic currents and contraction of cultured rat skeletal muscle cells. J Ethnopharmacol 65: 157–164.
- Powell JA, Carrasco MA, Adams DS, Drouet B, Rios J, Müller M, Estrada M, Jaimovich E. (2001): IP3 receptor function and localization in myotubes: An unexplored calcium signalling pathway in skeletal muscle. J Cell Sc 114: 3673–3683.
- Rios E, Brum G (1987): Involvement of dihydropiridine receptors in excitation contraction coupling in skeletal muscle. Nature 325: 717–720.
- Rivet M, Cognard C, Raymond G (1989): The slow inward calcium current is responsible for a part of patch-clamped rat myoballs. Pflügers Arch 413: 316–318.
- Vergara J, Tsien RY, Delay M (1985): Inositol 1,4,5triphosphate: A possible chemical link in excitation-contraction coupling in muscle. Proc Natl Acad Sci USA 82: 6352–6356.
- Volpe P, Salviati G, Divirgilio F, Pozzan T (1985): Inositol 1,4,5-triphosphate induces calcium release from sarcoplasmic reticulum of skeletal muscle. Nature 316: 347–349.