Abstract
This work is part of a larger screening program, which seeks to discover new antitumor plants and compounds from the Brazilian Amazon. In a prescreen of stem and leaf extracts of Tachia grandiflora. Maguire & Weaver (Gentianaceae) based on the SRB method, leaf methanol and ethanol extracts showed appreciable cytotoxicity in human breast (MCF-7) and colon (HCT-8) tumor cell lines. Liquid-liquid partitioning of the leaf ethanol extract yielded hexane, chloroform, butanol, and water-methanol fractions. Only the hexane and chloroform fractions were active, inhibiting murine melanoma (B-16) and HCT-8 cells. The chloroform fraction suffered sequential column chromatography on silica gel using different eluent systems and yielded a number of very active subfractions. In all, 25 fractions and subfractions were tested, and 10 exhibited high growth inhibition of HCT-8, and two of these presented strong inhibition of murine melanoma (B-16) cells. The most active subfractions were tested against five tumor cell lines (leukemia CEM and HL-60, as well as the three used previously) using the MTT assay, and four fractions demonstrated significant cytotoxicity based on IC50. Cell lysis was discarded as a possible mechanism for in vitro. cytotoxicity given that these fractions did not exhibit hemolytic activity. The greatest antiproliferative potential was found in the second (two samples) and third generation (two samples) chromatographic subfractions of the chloroform fraction (obtained from partitioning of the ethanol extract). These subfractions proved to be complex mixtures from which no pure substance could be isolated after further chromatographic separations.
Introduction
The use of medicinal plants in the world, and especially in South America, contributes significantly to primary health care. Many plants are used in Brazil in the form of crude extracts, infusions, or plasters to treat common infections without any scientific evidence of efficacy (Di Stasi et al., Citation1994; Araújo & Leon, Citation2001; Holetz et al., Citation2002).
The use of natural products as anticancer agents has a long history that began with folk medicine, and, throughout the years, has been incorporated into traditional medicine, although many claims for the efficacy of such treatment should be viewed with some skepticism because cancer, as a specific disease entity, is likely to be poorly defined in terms of folklore and traditional medicine. Many drugs currently used in chemotherapy were isolated from plant species or derived from natural prototypes (Wang, Citation1998; Cragg et al., Citation1999; Cragg & Newman, Citation2005). According to Cragg and Newman (Citation2000), over 50% of all drugs currently in clinical trials for anticancer activity were isolated from natural sources or are structurally related to these natural compounds.
Tachia grandiflora. Maguire & Weaver belongs to the Macrocarpaea clade of the Helieae tribe of the Gentianaceae family (Struwe et al., Citation2002). Tachia. spp. are generally found only in northern South America, and T. grandiflora. is endemic to the central and eastern Amazon forest of Amazonas and Pará States in Brazil as well as French Guyana. It is a rare, single-stemmed, perennial treelet (2–3 m) presenting hollow stems and branches, petiolate leaves and as well as sessile, solitary flowers with tubular calyxes and cream or yellow-colored corollas (Maguire & Weaver, Citation1975).
In the Brazilian Amazon, T. grandiflora. is frequently collected and improperly identified as the medicinally important and more widely distributed type species T. guianensis. Aubl., or “caferana” (Kah-Feh-RAH-Nah), as the latter is known in this region (Pio Correa, Citation1984). A recent survey showed that in ethnobotanic reports from Colombia, the Guyanas, Peru, and Brazil dating as far back as the mid-nineteenth century, T. guianensis. (syn. Myrmecia scandens. Willd.) (mainly root) decoctions are reported as effective in the treatment of fevers and malaria (Milliken, Citation1997). In addition, ethnopharmacological studies have shown that T. guianensis. root extracts present antimalarial activity in vivo. against Plasmodium berghei. (rodent malaria parasite) but are not active in vitro. against P. falciparum., the most important human malaria parasite (Brandão et al., Citation1992).
Recently, as part of a general program oriented toward investigation of the bioactivity of Amazonian plants, extracts of different parts of T. grandiflora. were found to be lethal to Aedes aegypti. larvae, the hemorrhagic dengue fever vector (Pohlit et al., Citation2004). However, no significant activity was observed in the brine shrimp assay (Quignard et al., Citation2003) for T. grandiflora. extracts. To date, there have been no pharmacological reports that demonstrate the potential antitumor activity of Tachia. spp.
Cytotoxic screening models provide important preliminary data to select plant extracts with potential antineoplasic properties. The present study is part of our ongoing investigations directed toward the discovery of potential antitumor agents in fractions of the leaves of Tachia grandiflora.. In what follows, we report our results on the fractionation of the ethanol leaf extract of T. grandiflora. and the cytotoxic activity of its fractions and chromatographic subfractions.
Materials and Methods
Collection and identification
Plant materials were collected in INPA's Adolpho Ducke Forest Reserve in Manaus, Amazonas, Brazil, and at INPA's Experimental Station for Tropical Silviculture (50 km north of Manaus) in October 2000. Voucher specimens (numbers 205.948 and 20.8104) were deposited at the INPA Herbarium where they were identified as Tachia grandiflora. Maguire & Weaver by Herbarium employees. This botanic identification was later corroborated by Dr. Lena Struwe, on a visit from Rutgers University (New Jersey, USA). Plant parts were allowed to dry separately in the shade and were next cut into small pieces.
Extraction techniques
In the preparation of extracts for prescreen, dry, ground stems or leaves were separately extracted with methanol in a Soxhlet apparatus (Ceará, Brazil) (3 × 6 h), and the combined extracts were totally evaporated using rotary evaporation followed by freeze-drying. Water extracts were prepared by infusion (100°C, 15 min) followed by hot filtration and total evaporation as above. For fractionation, an extract was prepared in the following way. Hot ethanol was poured onto dry, ground leaves (0.21 kg), and the mixture was allowed to cool to room temperature over 1 week. The resulting solution was filtered off, and the above treatment was repeated using fresh, hot ethanol. The ethanol solutions were totally evaporated and freeze-dried. The yield of dry extract was 50.2 g, 24% based on extracted plant material.
Chemical screening for major classes of secondary metabolites
The leaf ethanol extract was screened using standard bench-top tests for the presence of alkaloids, anthocyanidines, anthocyanines, phenols, tannins, flavonoids, flavanones, steroids, and xanthones (Matos, Citation1997). These tests were positive for quaternary alkaloids, tannins, flavonones, steroids, and xanthones.
Liquid–liquid partitioning of the leaf ethanol extract
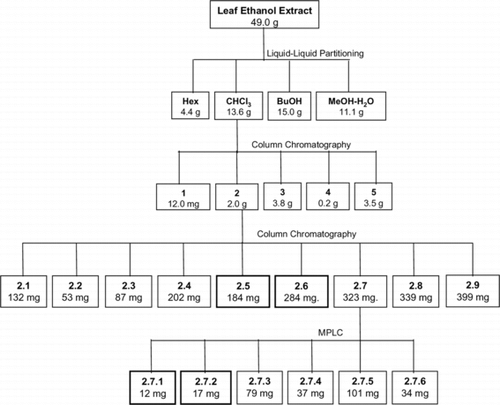
Dry extract (49.0 g) was dissolved in 9:1 methanol-water (1.2 L) and partitioned with hexanes (3 × 500 mL). Next, the concentration of water in the methanol-water phase was adjusted to 30%, and further partitioning was performed, first with chloroform (3 × 500 mL) and then with butanol (4 × 500 mL). After concentration on a rotary evaporator under mild conditions and freeze-drying, dry hexane (4.35 g), chloroform (13.6 g), butanol (15.0 g), and methanol-water (11.1 g) fractions were obtained ().
Chromatographic fractionation of the chloroform fraction
Chloroform fraction (10.0 g) was column chromatographed (Merck, silica gel 60, 0.063–0.200 mm; diameter 7 cm, height 10 cm) using first hexanes (0.5 L), then chloroform (2.5 L), and finally a gradient of acetone (5–100%) in chloroform (total 6 L) as eluents. A total of 38 subfractions (125–250 mL) were collected. After TLC analysis and combination, sub-fractions 1–5, having masses of 0.10, 2.00, 3.80, 0.20, and 3.50 g (with respect to order) were obtained.
Chromatographic fractionation of subfraction 2.. Subfraction 2 (2.0 g) was column chromatographed (Merck, silica gel 60, 0.063–0.200 mm, diameter 4.5 cm, height 30 cm) using a gradient of acetone (0–40%) in chloroform (total 5.4 L), and, after TLC analysis and combination, subfractions 2.1–2.9, presenting masses in the range 53–399 mg, were obtained. MPLC fractionation of subfraction 2.7.. Subfraction 2.7 (323 mg) was subjected to medium pressure liquid chromatographic separation (silica gel, diameter 21, 5 mm, height 250 mm, 16 mL min−1) using hexanes (1.0 L), then a gradient of hexanes-chloroform-isopropanol (20:4:1, 15 min), and, finally, chloroform-isopropanol (4:1, 60 min). After TLC analysis and combination, subfractions 2.7.1–2.7.6 were obtained ().
Cytotoxicity studies
In the prescreen, cytotoxicity of fractions from the leaves of Tachia grandiflora. was assessed using the sulforhodamine B method (SRB assay), as described by Skehan et al. (Citation1990), at a single sample concentration (125 µg/mL) in three tumor cell lines, B-16 (murine skin cancer), HCT-8 (human colon), and MCF-7 (human breast) in 96-well microplates at densities of 0.6 × 105, 0.4 × 105, and 0.7 × 105 cells per 100 µL per well, respectively. After 48 h, cells were stained with SRB followed by solubilization of cell-bound SRB with Trizma base, and colorimetric assessment of the plates was then performed using a multiplate reader (Spectra Count, Packard, Ontario, Canada). Absorbances were read at a wavelength of 540 nm. The percentage of cell growth (%G) was calculated by comparing the absorbance of test samples with that of the control (+ 100%), zero-time (0%) and cytotoxic standard etoposide (− 100%, which means total growth inhibition or cytostatic level effect). The fractions were classified as possessing no activity (NA, % G ≥ − 25%), low activity (LA, − 25 > %G ≥ − 50%), moderate activity (MA, − 50 > % G ≥ − 75%), and high activity (HA, %G < − 75%) for each cell line tested. Only the fractions that showed high activity were studied further in a second cytotoxicity test.
The MTT assay (Mosmann, Citation1983) was used for evaluation of the cytotoxic potential of these fractions against five tumor cell lines: CEM and HL60 (human leukemia) at densities of 3 × 105 cells per 100 µL per well for both lineages as well as the the lineages used previously. Tumor cell growth was quantified by the ability of living cells to reduce the yellow dye 3-(4,5-dimethyl-2-thiazolyl)-2,5-diphenyl-2H-tetrazolium bromide (MTT) to a purple formazan product. The fractions (0.039 to 25 µg/mL) dissolved in DMSO (1%) were added to each well and incubated for 3 days (72 h). Control groups received the same amount of DMSO. Doxorubicin (0.3 µg/mL) was used as positive control. Thereafter, the plates were centrifuged and then the medium was replaced by fresh medium (200 µL) containing 0.5 mg/mL MTT. Three hours later, the MTT formazan product was dissolved in DMSO, and absorbance was measured using a multiplate reader (Spectra Count, Packard, Ontario, Canada). Drug effect was quantified as the percentage of control absorbance of reduced dye at 550 nm. For the MTT assay, the IC50 values and their 95% confidence intervals (CI 95%) were obtained by nonlinear regression using the GRAPHPAD program (Intuitive Software for Science, San Diego, CA). For both cytotoxicity tests, all cell lines were provided by National Cancer Institute, Bethesda, MD, USA.
Hemolytic assay
The hemolytic test was performed in 96-well plates following the method described by Berlinck et al. (Citation1996). Each well received 50 µL of 0.85% NaCl solution containing 10 mM CaCl2. The first well was the negative control that contained only the vehicle (1% DMSO), and, in the second well, 50 µL of test substance that was diluted in half was added. The fractions were tested at concentrations ranging from 18.75–300 µg/mL. The last well received 50 µL of 0.2% triton X-100 (in 0.85% saline) to obtain 100% hemolysis. Then each well received 50 µL of a 2% suspension of mouse erythrocytes in 0.85% saline containing 10 mM CaCl2. After incubation at room temperature for 1 h and centrifugation, the supernatant was removed, and the liberated hemoglobin was measured spectroscopically as absorbance at 540 nM.
Results and Discussion
The leaf ethanol extract, its simple fractions obtained by liquid-liquid partitioning, and chromatographic subfractions of the chloroform fraction, 25 samples in all, were screened for cytotoxic action using the SRB assay, and the results are presented in . Eleven samples, including the leaf ethanol extract, showed no inhibition of the tumor cells, while four fractions and chromatographic subfractions showed low and/or moderate activity in one or more cell lines, and 10 chromatographic subfractions showed high growth inhibition in HCT-8 cells, with two of these also very active against melanoma cells.
Table 1.. Results of the in vitro. screening of fractions derived from Tachia grandiflora. leaf ethanol extract for cytotoxic activity in 3 cell lines.†
Of the 10 chromatographic fractions that presented high activity toward human colon and murine skin (melanoma) cell lines (), only subfractions 2.7.1, 2.7.2, 2.6, and 2.5 showed interesting potential cytotoxic (IC50 < 10 µg/mL) in at least one cell line (). In order to verify whether active substances acted through membrane disruption, these four subfractions were tested for the ability to induce lysis in mouse erythrocytes. The result showed that none of these four samples caused membrane damage (EC50 > 300 µg/mL).
Table 2.. Inhibitory effect on cultured cell growth of fractions from the leaves of Tachia grandiflora. and doxorubicin (positive controls) on five different tumor cell lines. Data are presented as IC50 (µg/mL) values, and their 95% confidence interval (IC95) obtained by nonlinear regression.
However, according to the criteria of the American National Cancer Institute, the IC50 limit to consider a crude extract promising for further purification is lower than 30 µg/mL (Cragg & Suffness, Citation1988).
The present data suggest that the chloroform fraction obtained from partitioning of the ethanol extract of Tachia grandiflora. could be considered a potential source of anticancer compounds. Further studies are necessary for chemical characterization of the active principles and more extensive biological evaluation.
Acknowledgment
The authors acknowledge the financial support and fellowships provided by the Brazilian government's National Council for Scientific and Technological Development (CNPq/PRONEX and CNPq/PNOPG grant nos. 520354/99-0 and 550260/01-3), the Bioamazonia-Bazah-Feh-Pad (BASA-FEPAD) contract, and Fee-Neh-Pee (FINEP). The authors thank Silvana França dos Santos and Luciana França Silva for technical assistance.
References
- Araújo CAC, Leon LL (2001): Biological activities of Curcuma longa. L. Mem Inst Oswaldo Cruz. 96: 723–728.
- Berlinck RGS, Ogawa CA, Almeida AMP, Andrade MAS, Malpezzi ELA, Costa LV, Hajdu EM, Freitas JC (1996): Chemical and pharmacological characterization of halitoxin from Amphimedon viridis. (PORIFERA) from the southeastern Brazilian coast. Comp Biochem Physiol 115C: 155–163.
- Brandão MGL, Grandi TSM, Rocha EMM, Sawyer DR, Krettli AU (1992): Survey of medicinal plants used as antimalarials in the Amazon. J Ethnopharmacol 36: 175–182.
- Cragg GM, Boyd MR, Khanna R, Kneller R, Mays TD, Mazan KD, Newman DJ, Sausville EA (1999): International collaboration in drug discovery and development: the NCI experience. Pure Appl Chem 71: 1619–1633.
- Cragg GM, Newman DJ (2000): Antineoplasic agents from natural sources: Achievements and future directions. Exp Opin Invest Drugs 9: 1–15.
- Cragg GM, Newman DJ (2005): Plants as a source of anti-cancer agents. J Ethnophramacol 100: 72–79.
- Cragg GM, Suffness M (1988): Metabolism of plant-derived anticancer agents. Pharmacol Ther 37: 425–461.
- Di Stasi LC, Hiruma CA, Guimarães EM, Santos CM (1994): Medicinal plants popularly in Brazilian Amazon. Fitoterapia 65: 529–540.
- Holetz FB, Pessini GL, Sanches NR, Cortez DAG, Nakamura CV, Dias Filho BP (2002): Screening of some plants used in Brazilian folk medicine for treatment of infectious diseases. Mem Inst Oswaldo Cruz 97: 1027–1031.
- Maguire B, Weaver RE (1975): The neotropical genus Tachia. (Gentianaceae). J Arnold Arboretum 56: 103–125.
- Matos FJA (1997): Introdução a Fitoquímica Experimental, 2nd ed. Fortaleza, Ceará State, Brazil, Edições Universidade Federal do Ceará.
- Milliken W (1997): Plants for Malaria Plant for Fever: Medicinal Species in Latin America—A Bibliographic Survey. Kew, United Kingdom, Royal Botanic Gardens.
- Mosmann T (1983): Rapid colorimetric assay for cellular growth and survival: Application to proliferation and cytotoxicity assays. J Immunol Meth 16: 55–63.
- Pio Correa M (1984): Dicionário de Plantas Úteis do Brasil e das Exóticas Cultivadas, Vol. 1. Brasília, Federal District, Brazil, Ministério da Agricultura, Instituto Brasileiro de Desenvolvimento Florestal.
- Pohlit AM, Quignard ELJ, Nunomura SM, Tadei WP, Hidalgo AF, Pinto ACS, Santos EVM, Morais SKR, Saraiva RCG, MING LC, Alecrim AM, Ferraz AB, Pedroso ACS, Diniz EV, Finney EK, Gomes EO, Dias HB, Souza KS, Oliveira LCP, Don LC, Queiroz MMA, Henrique MC, Santos M, Lacerda Jr, OS, Pinto PS, Silva SG, Graça YR (2004): Screening of plants found in Amazonas State, Brazil for activity against Aedes aegypti. larvae. Acta Amazonica 34: 97–105.
- Quignard ELJ, Pohlit AM, Nunomura SM, Pinto ACS, Santos EVM, Morais SKR, Alecrim AM, Pedroso ACS, Cyrino BRB, Melo CS, Finney E K, Gomes EO, Souza KS, Oliveira LCP, Don LC, Silva LFR, Queiroz MMA, Henrique MC, Santos M, Pinto PS, Silva SG (2003): Screening of plants found in Amazonas State for lethality towards brine shrimp, Artemia franciscana.. Acta Amazonica 33: 93–104.
- Skehan P, Storeng R, Scudiero D, Monks A, McMahon J, Visitica D, Warren JT, Bokesch H, Kenney S, Boyd MR (1990): New colorimetric cytotoxicity assay for anticancer-drug screening. J Natl Cancer Inst 13: 1107–1112.
- Struwe L, Kadereit JW, Klackenberg J, Nilsson S, Thiv M, von Hagen KB, Albert VA (2002): Systematics, character evolution, and biogeography of Gentianaceae, including a new tribal and subtribal classification. In: Struwe L Albert VA, eds., Gentianaceae: Systematics and Natural History. Cambridge, United Kingdom, Cambridge University Press pp. 21–309.
- Wang HK (1998): Plant-derived anticancer agents currently in clinical use or clinical trials. Exp Opin Invest Drugs 1: 92–102.