Abstract
The antibacterial activity of the ethanol, aqueous, and organic extracts from the root of Rheum ribes. Linn (Polygonaceae) was examined. Four anthraquinone aglycone components, chrysopahnol, physcion, aloe emodin, and emodin, were isolated from the biologically active extract and identified by spectroscopic analysis. Emodin is recorded for the first time in this species. The MIC values of the biologically active extracts, aloe emodin, and emodin, were 500, 125, 250, and 63 µg/mL against Staphylococcus aureus., respectively. The extracts and compounds did not inhibit Pseudomonas aeruginosa. and Escherichia coli. at the highest concentration tested, 4000 and 250 µg/mL, respectively.
Introduction
Rheum. Linn (Polygonaceae) (English name: rhubarb) is a large genus of perennial, stout herbs that are distributed in the temperate and subtropical regions of the world, chiefly in Asian countries. Several species of this plant are used in medicine, some for culinary purposes, and a few others are grown as ornamental plants. There are three main types of rhubarb, the Chinese rhubarb, the Indian or Himalayan rhubarb, and the Rhapontic rhubarb (Agarwal et al., Citation2001). Rheum ribes. Linn grows between 1000 and 4000 m on dunite rocks, among stones and slopes, and is distributed in Iraq, Turkey, Iran, Pakistan, Afghanistan, and Russia (Davis, Citation1967). In Turkey, Rheum ribes. young shoots and petioles are eaten as a vegetable and used to promote digestion and improve appetite (Tuzlaci et al., Citation1992; Tabata et al., Citation1994). Roots of Rheum ribes. are used to treat diabetes, hemorrhoids, ulcer, diarrhea, and are reported to have anthelmintic and expectorant activity (Tabata et al., Citation1994; Fatma & Cigdem, Citation2003). The naturally occurring Rheum ribes. young shoots and petioles are very popular vegetables in Kurdistan during spring, and the roots are used in folk medicine to treat hypertension, diabetes, and some infectious dermatologic diseases (Razavi, Citation2003).
Therefore, the current in vitro. biological study was undertaken to validate the effects of different Rheum ribes. root extracts for their antimicrobial and anifungal activity against four strains of bacteria and one strain of fungus, and then the phytochemical profiles were evaluated to correlate possible biological activity of this plant.
Materials and Methods
Medicinal plant materials
Rheum ribes. roots (Kurdish name, “rewas”; Arabic name, “Rawand”) were collected from the mountains of Haje Omaran, Kurdistan Region, Iraq, during May 2006. The plant was identified by the Pharmacognosy Department, College of Pharmacy, University of Baghdad. A voucher sample is stored at the Department of Medicinal Chemistry, the Danish University of Pharmaceutical Sciences (voucher no. Alaadin1).
Preparation of the medicinal plant extracts
Freshly collected Rheum ribes. roots were cleaned, cut into small pieces, air-dried under shade for 5–7 days, and powdered with a mechanical grinder. Dried powdered plant materials (100 g) were refluxed with 80% ethanol, yielding 13.3 g total extract (TE). Liquid partitioning of TE between CHCl3:H2O yielded 0.6 g of chloroform extract (CE1) and 10.7 g aqueous fraction (AE). The AE was hydrolyzed by refluxing with 60% FeCl3 and 5 N HCl, yielding 0.2 g of CHCl3 fraction (CE2) after partitioning.
Microorganisms
The following microorganisms were used for detecting antimicrobial compounds: Staphylococcus aureus. ATCC 6538, Pseudomonas aeruginosa. ATCC 9027, Escherichia coli. ATCC 11229, Bacillus subtilis. ATCC 6633 and the fungus Candida albicans..
Culture media
For bacterial strains, Mueller-Hinton medium with 0.6% Bacto agar (6 g/L) and 0.02% phenol red (0.2 g/L) was used, and for fungal strain Sabouraud broth medium with 0.6% Bacto agar (6 g/L) and 0.02% phenol red (0.2 g/L) was used for overlay. These media were autoclaved at 121°C for 20 min and then maintained in the liquid state at 45°C in a water bath prior to overlay. Cultures were grown overnight in Mueller-Hinton medium (for bacteria) or Sabouraud medium (for Candida.) and diluted to 106 cells/mL by serial dilution, just before bioautography. The final concentration of each strain in the agar-containing medium was 106 cells/mL, which gave uniform growth of strains in overlay media (Saxena et al., Citation1995).
Thin-layer chromatography
TLC evaluation of TE, AE, CE1, and CE2 was carried out on silica gel GF254, 0.2 mm thickness aluminum plates, with petroleum ether:ethyl acetate:formic acid (75:25:1) as a mobile phase (HMSO, Citation1993). Derivatization was with 10% KOH.
Bioautography
For bioautography, developed TLC plates were dried overnight and overlayed with nutrient agar inoculated with Staphylococcus aureus. ATCC 6538, Pseudomonas aeruginosa. ATCC 9027, Escherichia coli. ATCC 11229, Bacillus subtilis. ATCC 6633, or the fungus Candida albicans. (Saxena et al., Citation1995). After overnight incubation under humid conditions, the plates were sprayed with MTT [3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyl tetrazolium bromide], and yellow spots on a purple background indicated antimicrobial activity. Streptomycin and amphotericin B were used as standards.
Isolation of active constituents
Quantitative separation of free anthraquinone aglycones from the biologically active fractions was carried out by preparative TLC, using silica gel GF254, 0.5 mm thickness, with concentrating zone using petroleum ether: ethyl acetate: formic acid (50:50:1) as a mobile phase. 1H NMR data were recorded (CD3OD) on a 300 MHz Varian Mercury Plus instrument.
Minimum inhibitory concentration (MIC)
MIC determinations were carried out according to Eloff (Citation1998) in microtiter plates. Concentrations of 31 to 4000 µg/mL were tested for extracts and 2 to 250 µg/ml for pure compounds against S. aureus., P. aeruginosa., and E. coli.; streptomycin was used as a control.
Results and Discussion
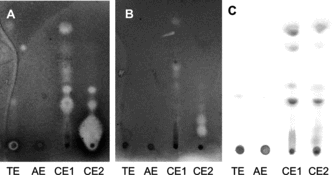
The CE1 and CE2 fractions showed a number of clear zones of inhibition against S. aureus. (A), and E. coli. (B) in the bioautographic assay. Only very weak activity of CE1 and CE2 were observed against P. aeruginosa. and no activity against B. subtilis. and C. albicans.. TE and AE did not have any activity. A reference plate sprayed with KOH for detection of anthraquinones indicated that several of the active spots were anthraquinones (C). These compounds were isolated by preparative TLC and identified by 1H NMR by comparison with data in the literature as chrysophanol (Rf, 0.72), physcion (Rf, 0.7), aloe emodin (Rf, 0.24) (Liu et al., Citation2004), and emodin (Rf, 0.37) (Meselhy, Citation2003; Liu et al., Citation2004; Shun et al., 2006).
Figure 1 (A) Bioautographic assay with S. aureus. and (B) E. coli.. (C) TLC plate sprayed with 10% KOH viewed in VIS.
MIC values were determined for the CE1, CE2, and the four isolated constituents. The MICs against S. aureus. were 500 µg/mL for CE1, 125 µg/mL for CE2, 250 µg/mL for aloe emodin, 63 µg/mL for emodin, and 3.13 µg/mL for streptomycin. Emodin has recently been shown to have activity against three Bacillus. spp. and P. aeruginosa. (Subhalakshmi et al., Citation2005). The MIC of emodin against S. aureus. was recorded as 39 µg/mL (Chukwujekwu et al., Citation2006). The extracts and compounds did not inhibit P. aeruginosa. and E. coli. at the highest concentrations tested, nor did chrysophanol and physcion inhibit S. aureus. at the concentration of 250 µg/mL.
Acknowledgments
The authors thank the Danish University of Pharmaceutical Sciences and Hawler Medical University for financial support and the College of Pharmacy, University of Baghdad, for identification of the plant material.
References
- Agarwal SK, Singh SS, Lakshmi V, Verma S, Kumar S (2001): Chemistry and pharmacology of rhubarb (Rheum. species)—A review. J Sci Ind Res 60: 1–9.
- Chukwujekwu JC, Coombes PH, Mulholland DA, Staden JV (2006): Emodin, an antibacterial anthraquinone from the roots of Cassia occidentalis.. S Afr J Bot 72: 295–297.
- Davis PH (1967): Flora of Turkey and East Aegean Islands. Cullen J 2: 268–269.
- Eloff JN (1998): A sensitive and quick microplate method to determine the minimal inhibitory concentration of plant extracts for bacteria. Planta Med 64: 711–713.
- Fatma T, Kizilay CA (2003): Anthraquinones and flavonoids from Rheum ribes.. J Fac Pharm Ankara 32: 31–32.
- HMSO (1993): British Pharmacopoeia, Vol 1. London, Her Majesty Stationery Office (HOMO), pp. 577–578.
- Liu R, Li A, Sun A (2004): Preparative isolation and purification of hydroxyanthraquinones and cinnamic acid from the Chinese medicinal herb Rheum officinale. Baill. by high-speed counter-current chromatography. J Chromatogr A 1052: 217–221.
- Meselhy R (2003): Constituents from Moghat, the roots of Glossostemon bruguieri. (Desf.). Molecules 8: 614–621.
- Razavi SM (2003): Medicinal Plants. Tehran, Talash Publication, pp. 165–166.
- Saxena G, Farmer S, Towers GHN, Hancock REW (1995): Use of specific dyes in the detection of antimicrobial compounds from crude plant extracts using a thin layer chromatography agar overlay technique. Phytochem Anal 6: 125–129.
- Subhalakshmi B, Ghosh A, Hazra B (2005): Evaluation of the antibacterial activity of Ventilago madraspatana. Gaertn, Rubia cordifolia. Linn. and Lantana camara. Linn. Isolation of emodin and physcion as active antibacterial agents. Phytother Res 19: 888–894.
- Tabata M, Sezik E, Honda G, Yesilada E, Fuki H, Goto K, Ikeshiro Y (1994): Traditional medicine in Turkey III. Folk medicine in East Anatolia, Van and Bitlis Provinces. Int J Pharmacog 32: 3–12.
- Tuzlaci E, Mericli AH (1992): Some studies on Isgin (Rheum ribes.) and its distribution in Turkey. In: Baser KCH, ed. Proceedings of the IX Symposium on Plant Drugs. Eskisehir: Publication of Anadolu University, No. 641, pp. 336–341.
- Yao S, Li Y, Kong L (2006): Preparative isolation and purification of chemical constituents from the root of Polygonum multiflorum. by high-speed counter-current chromatography. J Chromatogr A 1115: 64–71.