Abstract
The pentacyclic triterpenes 3-O-α-l-rhamnopyranosyl-(1 → 4)-β -d-glucopyranosyl-(1 → 3)-[β -d-glucopyranosyl-(1 → 2)]-β -d- fucopyranosyl-23,28-dihydroxy-oleane-11, 13(18)-diene (1) and 3-O-α-l-rhamnopyranosyl-(1 → 4)-β -d-glucopyranosyl-(1 → 3)-[β -d-glucopyranosyl-(1 → 2)]-β -d-fucopyranosyl-16,23,28-trihydroxy-oleane-11,13(18)-diene (2) isolated from leaves of Buddleia scordioides Kunth (Loganiaceae) were studied for the effect on wound healing, using excision and incision wound modes. Significant increase in granulation tissue, tensile strength, hydroxyproline, total protein, and collagen contents was observed. There was significant reduction in the wound area measurement of the test group compared with that of controls. The efficacious prohealing effect may be due to increased collagen deposition as well as to better alignment and maturation.
Introduction
Buddleia scordioides Kunth (Loganiaceae) is a common herb that grows wild and abundantly in the fields of Mexico. A water extract of the leaves has long been used by indigenous Guerrero peoples for the treatment of wounds. The treatment of ulcers with this plant in the state of Aguascalientes is reported by CitationArgueta et al. (1994). Decoction is also used for treatment of hepatitis, to wash wounds, and a powder of the same plant parts is applied topically as a wound healer (CitationHoughton & Mensah, 1999). Buddleia is reported to be applied topically as a poultice or lotion for healing wounds (CitationHoughton, 1984; CitationHoughton & Manby, 1985). A literature survey indicated that the flavonoid rutin was isolated from Buddleia scordioides (CitationArgueta et al., 1994). Other compounds isolated include syringin, saikogenin A, verbacoside, linarine (CitationHoughton & Mensah, 1999), and triterpenoid saponins (CitationAvila & Romo, 2002). The current study was undertaken to evaluate this healing.
Materials and Methods
General experimental methods
IR spectra were recorded on a PerkinElmer 1710 spectrophotometer (USA). 1H NMR, 13C NMR, 1H-1H-COSY, and HMBC spectra were performed on a Varian 300 Hz spectrometer (USA) with TMS as internal standard.
Plant material
B. scordioides stems were collected in January 2004 in Guerrero State, Mexico. The material was identified by Edith Lopez Villafranca of the Department of Botany of ENEP-Iztacala UNAM, and a voucher specimen of the plant (no. 7976) is deposited in the herbarium of this department for reference.
Extraction and isolation of compounds
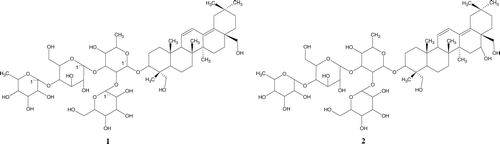
Aerial parts of B. scordiodes were dried at room temperature and ground into a fine powder (200 mesh) of which 5 kg was extracted by heating to reflux temperature (hexane, 30 L) threetimes during 4 h and combined extracts concentrated to dryness at 50°C under reduced pressure. Plant was extracted successively with CHCl3 and methanol. The most active MeOH concentration gave a brown viscous liquid (380 g) that were chromatographed over silica EtOAc-hexane (1:1), and 9 fractions were collected. According to pharmacologic testing, fractions F6A and F7B showed a wound-healing effect, and were combind and chromatographed on silica gel eluting with Et-OAc-CHCl3(7:1) to yield fractions. Further column chromatography was performed on silica of active 5B and F6B eluting with CH3Cl3-MeOH-hexane (10:1.0:2) to yield six fractions. The most polar fraction was chromatographed over Sephadex LH-20 to yield four fractions. Yields were 300 mg of compound 1 and 250 mg of 2 ().
Compound 1. An amorphous solid, its fast atom bombardment mass spectroscopy (FABMS) showed an [M + Na + H]+ ion at m/z 1094 indicating a molecular weight of 1070 compatible with a molecular formula of C54H88O21. IRν max (KBr) cm− 1: 3400 (OH), 2930 (CH); 1H NMR (CD3OD), aglycoe moiety: δ 1.98 (1H,m, H-2), 2.31(1H, d, J = 10.0 Hz, H-2), 3.11 (m, H-3), 2.06 (br,s, H-9), 5.34 (dd, J = 10.4, 1.1 Hz, H-11), 5.87 (d, J = 10.4 Hz, H-12), 3.8 (d, J = 6 Hz, H-23), 1.05 (s, H-24), 0.93 (s, H-25), 0.83 (s, H-26), 1.15 (s, H-27), 4.1 (dd, J = 10.9, 10.8 Hz, H-28), 0.70 (s, H-29), 0.87 (s, H-30); (br,s, H-4′), 5.0 (m, H-5′), 1.74 (d, J = 6.4 Hz, H-6′); 13C NMR δ 37.4 (C-1), 26.7 (C-2), 89.8 (C-3), 43.9 (C-4), 48.1 (C-5), 17.2 (C-6), 31.2 (C-7), 38.3 (C-8), 54.2 (C-9), 35.7 (C-10), 132.6 (C-11), 136.4 (C-12), 126.5 (C-13), 44.1 (C-14), 32.2 (C-15), 40.8 (C-16), 41.9 (C-17), 127.1 (C-18), 48.7 (C-19), 29.3 (C-20), 32.7 (C-21), 31.8 (C-22), 65.1 (C-23), 13.6 (C-24), 14.3 (C-25), 16.4 (C-26), 24.6 (C-27), 69.1 (C-28), 33.5 (C-29), 23.9 (C-30); Sugar moiety: Fucose δ 5.54 (d, J = 8.0 Hz), 4.79 (br,s, H-2′), 4.5 (d, J = 9 Hz, H-3′), 4.61 (br, s, H-4′), 5.0 (m, H-5′), 1.74 (d, J = 6.4 Hz, H-6′); δ 104 (C-1′), 85.3 (C-2′), 82.7 (C-3′), 72.1 (C-4′), 70.7 (C-5′), 18.3 (C-6′); glucose δ 6.01 (d, J = 7.8 Hz, H-1”), 4.1 (dd, J = 7.9, 8.4 Hz, H-2”), 4.3 (dd, J = 8.4, 8 Hz, H-3”), 4.1 (dd, J = 8, 8.8 Hz, H-4”), 3.93 (m, H-5”), 4.1 (m, H-6”); δ 103 (C-1”), 76.2 (C-2”), 78.7 (C-3”), 72.5 (C-4”), 77.6 (C-5”), 63.1 (C-6”); glucose δ 5.8 (d, J = 8 Hz, H-1”′), 3.8 (dd, J = 8, 8.5 Hz, H-2”′), 4.1 (dd, J = 8.5, 8.7 Hz, H-3”′), 4.2 (m, H-4”′), 4.1 (m, H-5”′), 4.4 (m, H-6”′); δ 101.2 (C- 1”′), 74.4 (C- 2”′), 76.4 (C- 3”′), 80.1 (C- 4”), 75.3 (C- 5”), 61.6 (C- 6”); rhamnose δ 5.76 (d, J = 8.0 Hz, H-1””), 4.8 (br, J = 3.1 Hz, H-2””), 5.8 (dd, J = 9.2, 3.4 Hz, H-3””), 4.41 (dd, J = 9.2, 9.4 Hz, H-4””), 4.8 (dq, J = 9.5, 6.1 Hz, H-5””), 1.7 (d, J = 6.2 Hz, H-6””); 102 (C-1””), 72.4 (C-2””), 72.9 (C-3””), 83.9 (C-4””), 70.3 (C-5””), 18 (C-6””); HMBC NMR spectra: H-23/H-24/C-1′, H-27/C-8, H-27/C-15, H-19/C-19, H-29/C-20, H-29/C-21, H-3/C-23, H-3/C-24, H-3/H-2′/H-3′/C-1′, H-1”/H-3′/C-2′, H-2′/H-2′/H-1”′/C-3′, H-2′/H-3′/C-4′,H-4′/H-5′/C-6, H-3′/H-2”′/H-3”′/C-1”′, H-1”′/H-3”′/C-2”′, H-2”′/C-3”′, H-2”′/H-5”′/C-4”′, H-6”′/H-5”′/C-5”′, H-4”′/H-5”′/C-6”′, H-1”/′C-2′, H-1”′/C-3′, H-1””/C-4”′, H-3”/C-6”.
Compound 2. An amorphous solid, its FARMS showed an [M + Na + H]+ ion at m/z 1112 indicating a molecular weight of 1088, compatible with a molecular formula of C54H88O22. 1H NMR and 13C NMR spectra of 2 were similar to those of 1, except for the signals at δ 4.8 (1H, br, s, H-16) and 77.1 originated from C-16 with a hydroxyl group.
Biological experimental procedures
Wistar rats (170 to 200 g) of both sexes were used for all experiments. Animals were housed in a cage under conditions of standard light (light on from 7 a.m. to 7 p.m.), temperature (22 ± 1°C), and room humidity (60 ± 10%) conditions for 1 week before the experimental sessions. Rats were given a commercial feed prepared by Purina (Mexico) and were allowed tap water ad libitum. The procedures involving animals and their care was done accordingly, as suggested by the Institutional Animal Ethics Committee.
Diabetic animals
Wistar rats were made diabetic by a single injection of streptozotocin (STZ) prepared in citrate buffer (0.1 M, pH 4.5) (50 mg/kg, i.p.) after overnight fasting (CitationJunod et al., 1969). Blood glucose levels were estimated at time of wound creation as well as at wound excision to check whether the plant has any antidiabetic activity per se.
Wound-healing activity
Excision (CitationVillegas et al., 1997) and incision (CitationDarias et al., 1996) wound models were used to evaluate wound-healing activity.
The epithelialization time was noted as the number of days required after wounding for the scar to fall off leaving no raw wound behind. The wound contraction rate was monitored by measurement of the wound area by tracing the wound margin on a graph paper every alternate day. Reduction in the wound area was expressed as the percentage of the original wound size (CitationMukherjee et al., 2000). The tensile strength of wound tissues was measured by the method of CitationVogel (1971).
Biochemical analyses
A sample of granulation tissues weighing around 300 mg was homogenized in a glass homogenizer, and 10 mL of 6 N hydrochloric acid was added to the homogenates in glass test tubes. The test tubes were then opened and contents were decanted to graduated glass cylinders, the test tubes were washed thoroughly with water, and the washings were combined with the hydrolytes (CitationWoessner, 1961). The hexosamine content of granulation tissues were then estimated by the method of CitationElson and Morgan (1933).
For estimates of total protein and DNA content, wet granulation tissues were first extracted with TCA by the method of CitationSchneider (1957). Briefly, the tissue was first homogenized in 5% TCA and centrifuged. The pellet was washed with 100% TCA, resuspended in 5% TCA, and kept for 15 min in a water bath maintained at 90°C. Contents were centrifuged, and the supernatant was used for determination of DNA content by the method of CitationBurton (1956). Precipitates were suspended in 0.1 M Tris-HCl, pH 7.4, and protein content estimated by the method of CitationLowry et al. (1951).
Histopathologic studies
The sections of the skin were stained with hematoxylin-eosin and were examined microscopically for keratinization, epithelialization, fibrosis, and angiogenesis (CitationMcManus & Mowry, 1965). The sections from the regenerated tissues (10 days) were observed under light microscope for keratinization, epithelialization, inflammation, fibrosis, and neovascularization (CitationYeo et al., 2000).
Statistical analysis
All wound area measurements were expressed as percentage of the initial wound size. p values of 0.05 or less were taken as significant.
Results
Compounds 1 and 2 were obtained as white amorphous powders. Their FABMS showed an [M + Na + H]+ ion at m/z 1096 and 1112 indicating a molecular weight of 1072 and 1088, compatible with a molecular formula of C54H88O21 and C54H88O22, respectively. In addition d-glucose, l-rhamnose, and d-fucose were identified by TLC and paper chromatography with an authentic sample. lH NMR and l3C NMR spectra of 1 and 2 indicate that most of the signals of the aglycon were in agreement with oleanane literature (CitationDing et al., 1992). Glycosylation shifts were observed at C-3, the aglycon, indicating that the saccharide units were attached at this position signal at a downfield shift by 10 ppm when compared with analogous data for oleanane. Both compounds indicate four sugar residues in a HMBC NMR experiment, which revealed correlations between anomeric carbons in the δ 104–101 range and anomeric resonating between δ 4.0 and 6.1. Thus, anomeric 13C NMR signals at δ 104 and 102 gave cross-peaks with anomeric protons at δ 5.54 (d, J = 8.0 Hz), 6.01 (d, J = 7.8 Hz, H-1”), 5.8 (d, J = 8 Hz, H-1”′), and 5.76 (d, J = 8.0 Hz, H-1””), respectively (CitationDing, 1992). Some of the more important connections were as follows (detailed for 1 but similar connectivities were observed for 2): the proton at C-3 of the triterpene portion of 1 showed a long-range correlation with the carbon resonating at δ 104, which must be the C-1′ of d-fucose (CitationTori et al., 1976). Sugar moieties were assigned mainly from the HMBC NMR spectra obtained. Accumulated evidence described above indicate that the structure of 1 was 3-O-α-l-rhamnopyranosyl-(1 → 4)-β -d-glucopyranosyl-(1 → 3)-[β -d-glucopyranosyl-(1 → 2)]-β -d-fucopyranosyl-23,28-dihydroxyoleane-11,13(18)-diene and 3-O-α-l-rhamnopyranosyl-(1 → 4)-β -d-glucopyranosyl-(1 → 3)-[β -d-glucopyranosyl-(1 → 2)]-β -d-fucopyranosyl-16,23,28-trihydroxyoleane-11,13(18)-diene previously isolated from B. scordiodes (CitationAvila & Romo, 2002).
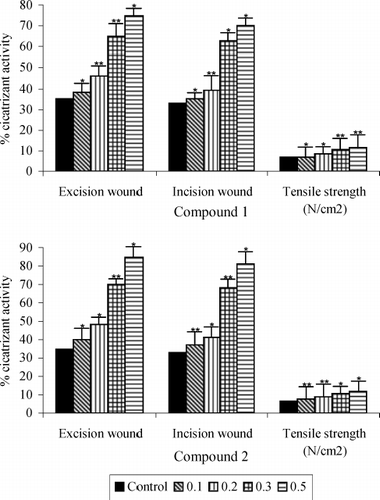
shows the rate of wound contraction expressed in terms of percentage of wound area that had healed. It may be seen that in group V the rats treated with 0.5% of the saponins 1 and 2, it decreased with 75% and 85% in the incision wound test and 70% and 81% in the wound test when compared with the controls (35% and 33% healing, respectively), on the 7th day.
Figure 1 Wound healing properties of compounds 1 and 2 and tensile strength of the wound in diabetic rats after 7 days. Values are mean + S.E. (n = 10 animals). *P < 0.05 and ** P < 0.01 as compared to vehicle (water) control. All wound area measurements were expressed as a percentage of the initial wound size.
shows collagen content (hydroxyproline) in granulation tissues of topically treated, diabetic rats. A common pattern of change was observed in all the groups. Collagen content of granulation tissues reached maximum levels 7 days after wound creation. Moreover, the steep increase in collagen content observed after day 4 was followed by almost equally steep decreases after day 7 in the treated groups. Levels continued to decrease after day 12, but at a much slower rate.
Figure 2 Collagen content of saponins 1 and 2 treated and untreated wound granulation tissue from diabetic rats. Data are given as mean + S.E. for eight animals in each group. Statistically significant results are indicated as *P < 0.001 and **P < 0.01.
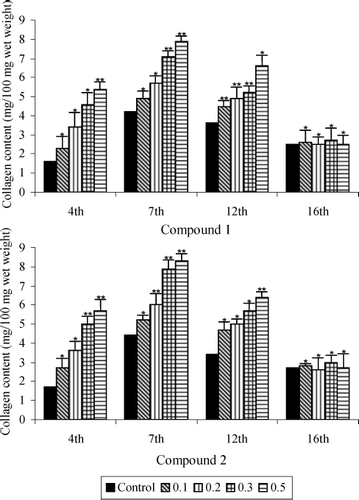
Wounds topically treated with saponins 1 and 2 (0.5%) showed (on day 7) an 87.6% and 88.6%, respectively, increase in the collagen content of their granulation tissues compared with the untreated control wounds. Results indicated enhanced synthesis of collagen, an important constituent of the extracellular matrix essential for healing.
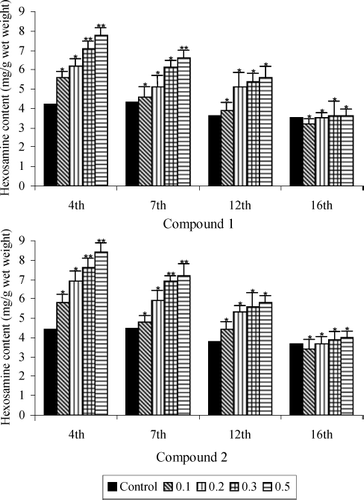
shows the hexosamine content in granulation tissues of the five groups. It was observed that groups IV and V (doses 0.5% of saponins 1 and 2) reached maximum levels 4 days after wound creation. Moreover, the steep increase in hexosamine content observed after day 4 was followed by almost equally steep decreases after day 7 in the treated groups. In all treated rats the hexosamine levels were found to decrease gradually and reach near normal levels by day 16.
Figure 3 Hexosamine content of saponins 1 and 2 treated and untreated wound granulation tissue from diabetic rats. Data are given as mean + S.E. for eight animals in each group. Statistically significant results are indicated as *P < 0.001 and **P < 0.01.
Protein and DNA contents of the granulation tissues of all the five groups are presented in and , respectively. There was a rapid increase in both protein and DNA contents, their values reaching maximum levels 7 days after wound creation. Their levels decreased after day 7, but the rate of decrease was much slower. On all days, both the protein and DNA levels were significantly higher in the groups treated with the saponins isolated from B. scordioides compared with the untreated control wounds.
Figure 4 Total protein content of saponins 1 and 2 treated and untreated wound granulation tissue from diabetic rats. Data are given as mean + S.E. for eight animals in each group. Statistically significant results are indicated as *P < 0.001 and **P < 0.01.
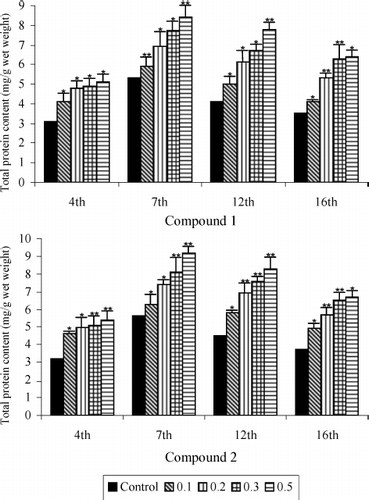
Figure 5 DNA content of saponins 1 and 2 treated and untreated wound granulation tissue from diabetic rats. Data are given as mean + S.E. for eight animals in each group. Statistically significant results are indicated as *P < 0.001 and **P < 0.01.
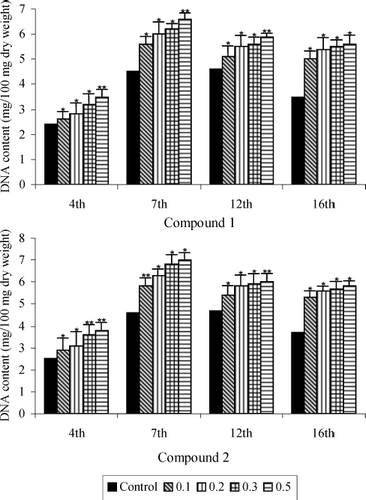
DNA level was elevated by 46.6% in the wounds receiving 0.5% topical application of 1, compared with the control. Protein also exhibited a highly significant increasing pattern resulting in a 58.4% and 64.2% rise in the wounds of the group treated with saponins 1 and 2, respectively, at control dose.
shows period of epithelialization of control and treated wounds. Wounds treated with compounds heal significantly faster than do untreated controls.
Table 1 Period of ephithelialization of saponins 1 and 2 treated wounds on diabetic rats.
Discussion
Compounds 1 and 2 isolated from B. scordioides were evaluated for wound-healing activity in Wistar rats. The wound area measurement by graph paper showed the wound size of the test group was reduced early compared with the control group. In addition to the reduction in wound size, the test group also showed a faster rate of healing. Wound contraction and tensile strength measurements were used to evaluate the effect of triterpenes on wound healing. The measurement of tensile strength was in agreement. The estimated increase in hydroxyproline content of the granulation tissue of the excision wounds indicated rapid collagen turnover thus, leading to rapid healing of wounds (CitationFreifelder, 1987; CitationPrasad & Dorle, 2006). The study also revealed a significant increase in the granulation tissue. The granulation tissue was subjected to histopathologic examination to determine the pattern lay-down for collagen. Protein and DNA contents of granulation tissue indicate the levels of protein synthesis and mitogenic profile of compounds and that this action significantly contributes to wound healing (CitationDarias et al., 1996). It has been evident that triterpenes significantly improve the quality of wound healing and scar formation. Our result validate their use in traditional medicine for treating injuries. The results obtained prompt us to carry out a more profound study of this plant to obtain better knowledge of its therapeutic possibilities.
References
- AV Argueta, LMA Cano, and ME Rodarte. Atlas de las Plantas de la Medicina tradicional Mexicana. Vol. II. Instituto Nacional Indigenista México, , (1994)750–752.
- AJG Avila, and VA Romo. (2002). Triterpenoid saponins and other glycosides from Buddleja scordioides. Biochem System Ecology 30:1003–1005.
- K Burton. (1956). A study of the condition and mechanism of the diphenyleamine reaction for the colorimetric estimation of deoxyribonucleic acid. Biochem J 62:315–321.
- RAF Clark. Wound Repair: Overview and General Considerations. Plenum Press, New York, (1996)3–6.
- V Darias, D Martin, S Abdala, and JM Vivas. (1996). Preliminary study of the cicatrizant, haemostatic and chemotherapic activities of Ceropegia fusca Bolle. Phytother Res 10:S1–S2.
- N Ding, S Yahara, and T Nohara. (1992). Structure of mimengosides A and B, new triterpenoid glycosides from Buddlejae flos produced in China. Chem Pharm Bull 40:780–782.
- LA Elson, and WTJ Morgan. (1933). A colorimetric method for the determination of glucosamine and chondrosamine. Biochem J 27:1824–1828.
- D Freifelder. Collagen—a multiprotein assemblyMolecular Biology. Narosa, Calcutta, (1987)56–58.
- PJ Houghton. (1984). Ethnopharmacology of some Buddleja species. J Ethnopharmacol 11:293–308.
- PJ Houghton, and J Manby. (1985). Medicinal plants of the Mapuche. J Ethnopharmacol 13:89–103.
- PJ Houghton, and AY Mensah. Biologically-active compounds from Buddleja speciesPhytochemicals; Human Health Protection, Nutrition and Plant Defense. Vol. 33. Kluwer Academic/Plenum, New York, (1999)343–368.
- LAE Junod, W Stauffacher, and AE Renold. (1969). Diabetogenic action of streptozotocin relationship of dose to metabolic response. J Clin Invest 48:2129–2139.
- YH Lien, MM Tseng, and R Stem. (1992). Glucose and glucose analogs modulate collagen metabolism. Exp Mol Pathol 57:215–221.
- OH Lowry, NJ Rosebrough, AL Farr, and RJ Randall. (1951). Protein measurement with Folin phenol reagent. J Biochem 193:265–275.
- LFA McManus, and RW Mowry. Staining Methods, Histologic and Histochemical. Harper, New York, (1965)325–328.
- PK Mukherjee, R Verpoorte, and B Suresh. (2000). Evaluation of in vivo wound healing activity of Hypericum patulum (family-Hypericaceae) leaf extract on different wound models in rats. J Ethnopharmacol 70:315–321.
- V Prasad, and AK Dorle. (2006). Evaluation of ghee based formulation for wound healing activity. J Ethnopharmacol 107:38–47.
- R Raghow. (1994). The role of extracellular matrix in post inflammatory wound healing and fibrosis. Fed Am Soc Exp Biol J 8:823–831.
- WC Schneider. (1957). Nucleic acids and derivatives. Methods Enzymol 3:680–684.
- K Tori, S Seo, Y Yoshimura, M Nakamura, Y Tomita, and H Ishii. (1976). Carbon-13 NMR spectra of saikosaponins A, C, D and F isolated from Bupléurum falcatum L. Tetrahedron Lett 46:4167–4170.
- LF Villegas, ID Fernandez, H Maldonado, R Torres, A Zavaleta, AJ Vaisberg, and GB Hammond. (1997). Evaluation of the wound-healing activity of selected traditional medicinal plants from Peru. J Ethnopharmacol 55:193–200.
- HG Vogel. (1971). Antagonistic effect of aminoacetonitrile and prednisolone on mechanical properties of rat skin. Biochim Biophys Acta 252:580–583.
- JF Woessner. (1961). The determination of hydroxyproline in tissue and protein samples containing small portion of this imino acid. Arch Bioch and Biophys 193:440–447.
- JH Yeo, KW Lee, HC Kim, Y Lyun, and SY Kim. (2000). The effect of PVA/chitosan/fibran blended spongy sheets on wound healing in rats. Biol Pharm Bull 23:1220–1223.