Abstract
The aim of this study was to examine the antioxidant activities of Physalis peruviana L. (Solanaceae) aqueous extract (PPWE) and its protective effect against acetaminophen (APAP)-induced hepatotoxicity in rats. Using different models of antioxidant assay, namely ferric thiocyanate, 2,2-diphenyl-1-picrylhydrazyl (DPPH), and reducing power, PPWE showed a dose-dependent increase in antioxidant activities, with total antioxidant activity (IC50: 0.81 μ g/ml) close to that of vitamin C (IC50: 0.89 μ g/ml). APAP at 850 mg/kg significantly increased the levels of serum glutamic pyruvic transaminase (sGPT), glutamic oxaloacetic transaminase (sGOT) and alkaline phosphatase (sALP). However, pre-treatment with PPWE at doses 150, 300, and 600 mg/kg body weight significantly prevented the increase in these enzymes, which are the major indicators of liver hepatitis. Biochemical assays of liver homogenate showed that PPWE at 150∼ 600 mg/kg significantly enhanced superoxide dismutase (SOD), catalase (CAT), glutathione peroxidase (GPx) concentrations, and diminished the level of thiobarbituric acid reactive substances (TBARS). Furthermore, liver histological observation also showed an obvious amelioration in the liver cell necrosis, liver lesion, and fatty changes in PP-treated groups. High performance liquid chromatographic analysis showed that ellagic acid (ca. 0.2%) but not others could be the major component contributing to the antioxidant and hepatoprotective activities of PPWE. The present study concludes that PPWE possesses antioxidant activity and potent hepatoprotective effect against APAP-induced liver injury in rats.
Introduction
Physalis peruviana L. (PP) of family Solanaceae is a species indigenous to South America, now widely grown in Taiwan. It is commonly used as folk medicine for treating cancer, leukemia, hepatitis, rheumatism, and other diseases (CitationPerry, 1980; CitationWu et al., 2004a). Major bioactive compounds of Physalis spp., such as physalins (B, D and F) (CitationChiang et al., 1992; CitationMagalhães et al., 2006) and glycosides (such as myricetin-3-O-neohesperidoside) (CitationIsmail & Alam, 2001), have been shown to possess anticancer activities. Previous phytochemical studies have isolated a number of compounds from PP, such as ticloidine (CitationBeresford & Woolley, 1974), phygrine (CitationBasey et al., 1992), 28-hydroxywithanolide, and 4-β -hydroxywithanolide E (CitationBaumann & Meier, 1993; CitationDinan et al., 1997), withanolides, withaphysanolide, and viscosalactone (CitationAhmad et al., 1999). Aqueous extract of PP exhibited potent cytotoxicity on B16-F10 melanoma cells (CitationWon et al., 1988). Ethanol extract of PP showed potent cytotoxic effect against Hep G2 cells and its mechanism of action was found to relate to a mitochondria-mediated apoptotic pathway (CitationWu et al., 2004a,Citationb). This extract also showed potent xanthine oxidase inhibition and anti-lipid peroxidation activities (CitationWu et al., 2005).
Acetaminophen (APAP), a widely used analgesic and antipyretic drug, can cause hepatic necrosis in man and rats when taken in overdose (CitationBoyd & Bereczky, 1966; CitationBoyer & Rouff, 1971). Its toxic metabolite, N-acetyl-p-benzoquinoneimine (NAPQI), can deplete cellular glutathione (GSH) and covalently bind to tissue macromolecules which consequently lead to liver damage (CitationMitchell et al., 1973; CitationDahlin et al., 1984). Although the detailed mechanism of hepatocellular injury after the initial NAPQI formation, glutathione depletion and covalent binding to proteins remain unclear, recent studies have suggested that reactive oxygen and nitrogen species are major contributors to the impairment of liver function (CitationAlia et al., 2003; CitationJames et al., 2003). Administration of antioxidants prior to APAP treatment has been used as a model to test the potential preventive role of phytochemicals against acute oxidative stress. Crude drugs like B. nivea and B. nivea subsp. nippononivea (CitationLin et al., 1997), Platycodon grandiflorum (CitationLee et al., 2001), Abutilon indicum (CitationPorchezhian & Ansari, 2005) and Cistus laurifolius (CitationKupeli et al., 2006), and compounds such as n-acetylcysteine (CitationTran et al., 2001), α -tocopherol (CitationAmimoto et al., 1995), β -carotene (CitationManda & Bhatia, 2003) and flavonoids (CitationKupeli et al., 2006) have been reported to protect against APAP-induced oxidative stress in animals.
In view of evaluating the therapeutic claims of PP in the traditional practice for treating liver disorders, the present study was conducted to examine the antioxidant activities of PP aqueous extract (PPWE), and its protective effect against APAP-induced hepatotoxicity in rats.
Materials and Methods
Chemicals
Acetaminophen (APAP), thiobarbituric acid (TBA), dimethylsulfoxide (DMSO) and Tris-HCl were purchased from Sigma Chemical Co. (St. Louis, MO, USA). Ferrous sulphate was obtained from Wako Pure Chemical Industries Ltd. (Japan). PEG 400 (polyethylene glycol) was purchased from J.T. Baker Chemicals (Deventer, Netherlands). 2,2-Diphenyl-1-picrylhydrazyl (DPPH) was obtained from MP Biomedicals Inc. (Eschwege, Germany). All other chemicals and reagents used were of analytical grade.
Plant samples and extract preparation
Plant material of P. peruviana was obtained from the Tainan District Agricultural Research and Extension Station, Taiwan. The authenticity of species was confirmed by Professor C.C. Lin from the Graduate Institute of Natural Products, Kaohsiung Medical University, Taiwan. Whole plants were dried and ground to powdered form, which was then kept in an air-tight plastic bag until use.
To prepare the test extract, one hundred grams of dried PP was decocted with 1 L of distilled water at 65°C for 3 h. The extract was filtered through filter paper (Advantec No. 1, Japan) and the residue was re-extracted under the same conditions. The filtrates obtained from two separate extractions were combined, concentrated and lyophilized. The dried PP aqueous extract (PPWE) was collected and stored at −21°C until use.
Chemical analysis
The total flavonoid and phenol contents of PPWE was determined by colorimetric and Folin-Ciocalteu methods as described by CitationZou et al. (2004) and CitationCliffe et al. (1994), respectively. High performance liquid chromatographic method coupled with a photodiode array detector was established to analyze the selected flavonoid and phenolic components according to methods described by CitationGlowniak et al. (1996) and CitationWang et al. (2003). Selected pure flavonoids (i.e. kaempterol, quercetin, and rutin) and phenolic (i.e. caffeic acid, chlorogenic acid, cinnamic acid, and ellagic acid) compounds were used as reference standards.
Antioxidant activities
The total antioxidant activity of PPWE was determined by the ferric thiocyanate method (CitationDuh et al., 1997). DPPH radical scavenging activity was determined according to the methods of CitationBlois (1958) and CitationChang et al. (2006). The reductive potential of PPWE was determined according to the method of CitationOyaizu (1986).
Animals
Male Wistar rats, at 5 weeks of age (180–200 g), were purchased from the National Laboratory of Animal Breeding and Research Center (Taipei, Taiwan). They were housed in a controlled environment with temperature maintained at 22 ± 1°C and humidity at 55 ± 5% under a 12:12 h light/dark cycle. Animals were fed a standard laboratory diet and tap water ad libitum. After 4 weeks of adaptation, animals were subjected to various treatments for hepatotoxicity studies. All experiments were carried out in accordance with guidelines of the Experimental Animal Ethic's Committee of the Council of Agriculture, Executive Yuan, Taiwan. The experimental protocol was approved by the University's Animal Ethic Committee.
Experimental design
The experiment was conducted according to the modified procedures as described previously (CitationLin et al., 1995). In brief, animals were randomly divided into five groups of seven rats each. The control and APAP treated groups were initially treated intragastrically with saline solution (0.9% NaCl) whereas the treatment groups were given intragastrically 150, 300, and 600 mg/kg of PPWE for 7 consecutive days. On day 8, rats were fasted 18 h and then intraperitoneally injected with 850 mg/kg APAP (dissolved in 40% propylene glycol; PEG), followed by fasting for further 24 h. On the following day, blood was taken from rats under ether anesthesia. Serum was prepared for analysis of glutamate oxalate transaminase (sGOT), glutamate pyruvate transaminase (sGPT), and alkaline phosphatase (sALP) whereas the liver was collected for biochemical and histological analyses.
Biochemical assays
sGOT and sGPT activities were analyzed according to the method described by CitationReitman and Frankel (1957) and the sALP assay was carried out according to CitationKing and King (1954). In brief, blood samples were first centrifuged at 3000 × g at 4 ° for 10 min to obtain the serum. The serum collected was then analyzed for sGOT, sGPT and sALP activities.
Liver tissues were homogenized in four volumes of ice-cold 20 mM Tris-HCl (pH 7.4) containing 0.15 M KCl using a Potter-Elvehjem homogenizer with a Teflon pestle (Kontes Vineland, NJ, USA). The homogenates were centrifuged at 3200 × g for 20 min at 4°C to produce a supernatant for the various biochemical analyses. Catalase (CAT) activity was determined according to CitationBeers and Sizer (1952), superoxide dismutase (SOD) activity was determined by the method of CitationSun and Zigman (1978), and glutathione peroxidase (GPx) activity was determined with method of CitationRotruck et al. (1973). Lipid peroxidation in the liver was measured by the thiobarbituric acid-reactive method according to CitationKimura et al. (1981). The protein concentration was measured by the method of CitationLowry et al. (1951).
Histological examinations
APAP-induced liver necrosis was evaluated using hematoxylin and eosin (H&E) staining. In brief, immediately after collecting the blood under ether anaesthesia, the rat liver was removed and fixed in 10% formalin solution, dehydrated with a series of ethanol solutions from 50 to 100%, cleared in xylene and embedded in paraffin. Liver tissues were sliced into 5 μ m sections which were then stained with haematoxylin and eosin (H-E) dye, followed by photomicroscopic assessment of liver morphological changes.
Statistical Analysis
Data were expressed as means ± standard deviations (SD). Differences between groups were evaluated using the non-parametric Kruskal-Wallis test, followed by post hoc Dunn's multiple comparison test. A value of P < 0.05 was considered significant.
Results
Chemical content
Results showed that PPWE contained 40.45 ± 0.10 mg/g of flavonoids and 19.64 ± 0.09 mg/g of phenolic compounds. Quantitative analysis of PPWE showed that ellagic acid (ca. 0.2%) but not other constituents were present in a measurable amount under the adopted chromatographic system.
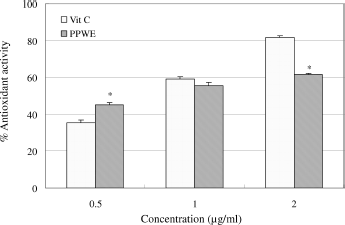
Total antioxidant activity
shows the total antioxidant activity of PPWE as determined by the ferric thiocyanate method. PPWE demonstrated an antioxidant activity in a dose-dependent manner. Its IC50 value (0.81 μ g/ml) was close to vitamin C (0.89 μg/ml). This suggests that PPWE possesses potent total antioxidant activity.
DPPH radical scavenging activity
PPWE demonstrated an ability to reduce the stable radical DPPH to the yellow-colored diphenylpicrylhydrazine, suggesting that this extract is active in DPPH radical scavenging (). PPWE also showed a dose-dependent increase in the DPPH radical scavenging activity.
Table 1. DPPH radical scavenging activity and reducing power of P. peruviana aqueous extract (PPWE).
Reducing power
As shown in , PPWE showed a dose-dependent increase in reducing power. However, its reducing power was weaker than the reference compound vitamin C. The reducing power of PPWE was about 20 fold less active than vitamin C.
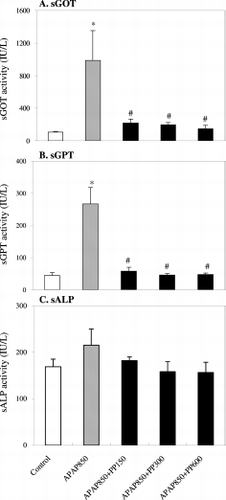
Effect of P. peruviana extract on sGOT, sGPT and sALP concentrations in APAP- induced rats
The results on the hepatoprotective effect of PPWE against APAP-induced acute liver damage in rats are shown in . At 24 h after the administration of APAP (850 mg/kg), the sGOT and sGPT levels were significantly increased. However, pretreatment with PPWE significantly reduced the levels of these enzymes which were major indicators of liver hepatitis. Although a similar trend in response was noted on the level of sALP for the PP-treated group, there was no significant difference between the different treatments.
Figure 2. Effects of P. peruviana aqueous extract (PPWE) on the levels of (A) serum glutamic oxaloacetic transaminase (sGOT), (B) serum glutamic pyruvic transaminase (sGPT) and (C) serum alkaline phosphatase (sALP) in APAP-induced rats. Data are presented as means ± SD of seven independent analyses. *P < 0.05 vs control group; #P < 0.05 vs APAP group as analyzed by Dunn's test.
Effect of P. peruviana extract on the levels of antioxidant enzymes in APAP-induced rats
shows the results of CAT, SOD and GPx concentrations in rat liver after challenging with APAP. Compared to the untreated group, a trend of increase in GPx level was noted after rats were treated with PPWE. APAP administration significantly decreased the SOD concentration from 2.64 ± 0.19 U/mg protein (control group) to 1.84 ± 0.30 U/mg protein in liver. It is interesting to note that at 600 mg/kg PPWE, the SOD levels of the control group and APAP+PP-treated groups were not significantly different. In the PP-treated groups, CAT and GPx concentrations tended to increase significantly with values ranging from 31.2∼ 43.4 U/mg protein and 5.83∼ 7.19 U/mg protein, respectively. At 300 and 600 mg/kg, the levels of CAT and GPx were significantly higher than the control group.
Table 2. Biochemical assessment of APAP-induced liver injury.
Effect of P. peruviana extract on the liver TBARS concentration in APAP-induced rats
Compared to the untreated control group, the APAP treatment significantly increased the concentration of TBARS in the liver (). However, when rats were administered with PPWE, the liver TBARS concentration was found to maintain at the level of the control group (1.34 ± 0.43 nmol/g) and was significantly different from the APAP treated group (2.04 ± 0.36 nmol/g). With increasing concentration of PPWE, a trend of reduction in TBARS concentration was noted. Compared to the control group, the effect of APAP administration on liver TBARS concentration of the PP-treated groups was not significant.
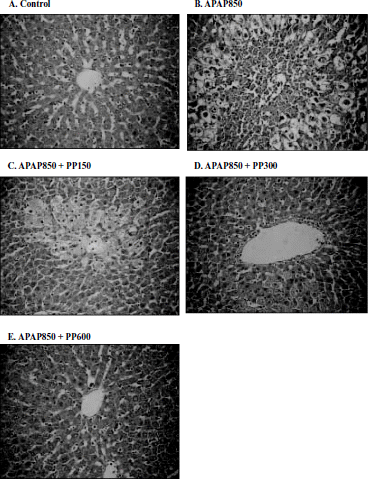
Histopathological conditions of liver
shows a representative photomicrograph of the protective effect of PPWE against APAP-induced liver injury in rats. Rats treated with normal saline/PEG showed no necrosis, inflammation, or vascular degeneration. In rats administered APAP alone, patch-like necrotic and hemorrhagic necrosis in the central and middle zones were prominent with many pyknotic cells around the lesions (). However, pretreatment with PPWE in a dose-dependent fashion, ameliorated the hepatic lesions produced by APAP ().
Figure 3. Histopathological observation of liver sections in APAP alone and APAP + PP treated rats. Liver sections were stained with haematoxylin and eosin (HE stain 176 x). A: Control; B: APAP (850 μg/ml) alone; C: APAP(850 μg/ml) + PP(150 μ g/ml); D: APAP(850 μg/ml) + PP(300 μ g/ml); E: APAP(850 μ g/ml) + PP(600 μ g/ml).
Discussion
The present study demonstrated that PPWE possesses potent total antioxidant activity and provided hepatoprotection against APAP-induced oxidative damage in rats. This observation can be supported by the significant decrease in the levels of sGPT, sGOT, and sALP. Besides decreasing the level of lipid peroxides in the liver, pre-treatment of PPWE also led to an increase in CAT, SOD, and GPx levels in this organ in APAP-treated rats. Histopathological observation further revealed that PPWE could reduce the incidence of liver lesions. These results support that PPWE possesses hepatoprotective effects against acute APAP-induced oxidative stress in rats through its antioxidant activities and induced enhancement production of antioxidant enzymes.
Under the present model systems, the tested extracts, in some terms, were specific in their antioxidant activity. For example, PPWE was as good as vitamin C in total antioxidant activity but was weaker in DPPH radical scavenging and reducing power. This discrepancy in antioxidant activities could be attributed to the different antioxidants present in the PP extracts. To identify if these antioxidant activities were derived from specific flavonoids (i.e. kaempterol, quercetin, and rutin) and phenolic (i.e. caffeic acid, chlorogenic acid, cinnamic acid, and ellagic acid) compounds, PPWE was subjected to quantitative analysis using a high performance liquid chromatographic system coupled with a photodiode array detector. Surprisingly, only ellagic acid (ca. 0.2%) but not others was measurable detected under our adopted chromatographic system (CitationGlowniak et al., 1996; CitationWang et al., 2003). However, it appears that PPWE contains antioxidant compounds, other than the selected flavonoids and phenols, which wait to be isolated and characterized. Ellagic acid has been reported to possess activity against tetrachloride-induced hepatotoxicity in rats (CitationIto et al., 1990; CitationSingh et al., 1999). Similarly, it could have contributed to the hepatoprotection of PPWE against APAP-induced toxicity.
In detailed phytochemical studies, CitationPun (2005) found that the same PP plant material contains nineteen compounds, namely 4β -hydroxywithanolide E, peruvianolide A, withanolide E, withanolide S, withanolide C, withaperuvin, whysalolactone, peruvianolide B, peruvianolide C, peruvianolide D, peruvianolide E, withaphysanolide, peruvianolide F, physalactone, peruvianolide G, peruvianolide H, withaperuvin D, peruvianoxide, and loliolide; of which 4β -hydroxywithanolide E, peruvianolide A, withanolide C, and withaphysanolide showed cytotoxicity toward lung (A 549), liver (Hep G2 and Hep 3B), and breast (MDA-MB-231 and MCF 7) cancer cells. It is possible that some of these compounds might contribute to the antioxidant and hepatoprotective activities of PPWE. Physalin, one of the major bioactive compounds of Physalis spp., was reported to cause kidney and liver toxicity (CitationMagalhães et al., 2006). Interestingly, this compound was not found in the PP material used in this study.
Liver injuries induced by APAP are commonly used for the screening of hepatoprotective drugs (CitationDavis et al., 1974). The rise in serum levels of GOT, GPT, and ALP has been attributed to the damaged structural integrity of the liver because these are normally located in the cytoplasm and are released into the circulatory system after cellular damage (CitationVermeulen et al., 1992). Our results provide strong evidence that PPWE significantly inhibits the acute liver toxicity induced by high doses of APAP in rats, as shown by a reduction of serum liver enzyme activities and hepatic lipid peroxidation, as well as the preservation of the liver histopathology. These protective effects were dose-dependent, and the effects of all doses were significant. The decrease in the serum transaminase enzymes (GOT and GPT) level in APAP-induced hepatic damage by PPWE could explain the prevention of leakage of the intracellular enzymes by its membrane stabilizing activity (CitationThabrew et al., 1987).
Crucial components of the antioxidant defense system in the body are cellular antioxidant enzymes (CAT, SOD, GPx), which are involved in the reduction of reactive oxygen species (ROS) and peroxides produced in the living organism as well as in the detoxification of certain compounds of exogenous origin, thus playing a primary role in the maintenance of a balanced redox status. An induction of antioxidant enzymes has been suggested to reflect an enhancement in cellular protection, ensuring that potential oxidants are metabolized and eliminated more rapidly. Conversely, decreased enzymatic activity would result in an increased steady-state level of oxidants, contributing to cell injury. In this study, the protective effect of PPWE against APAP-induced liver injury in rat might have been manifested by maintaining the hepatic SOD level, and enhancing the concentrations of CAT and GPx. It is also possible that the active compounds present in PPWE may have biological significance in the elimination of reactive free radicals (CitationWu et al., 2005).
The TBARS concentration was found to increase significantly following APAP-induced hepatotoxicity as compared to the untreated control group, suggesting an obvious lipid peroxidation occurring in the liver. PPWE at concentrations 150∼ 600 mg/kg effectively inhibited the lipid peroxidation as demonstrated by maintaining the TBARS concentration at the level of the control group. This suggests that PPWE may play an important role in cytoprotection as well as protection against peroxidation-induced membrane damage.
In histopathological examination, APAP caused an extensive centrilobular necrosis and fatty degenerative changes. In contrast, PP-pretreated groups showed a significant protective effect against APAP-induced liver injury. The inflammation of hepatocytes and ballooning degeneration were less severe with the prior administration of PPWE. Interestingly, livers from PPWE treatments at concentrations 300 and 600 mg/kg appeared to have a similar appearance as that of the control. The histological changes associated with the hepatoprotective activity of PPWE basically support the results of the serum enzymes estimation. These results also suggest that the inhibition of serum transaminase elevation and hepatic damage may play an important role in the protective effect of PPWE on APAP-induced hepatocellular destruction.
In conclusion, the present study concludes that PPWE possesses an antioxidant activity in a concentration-dependent manner. Its mode in affording the hepatoprotective activity against APAP may be due to cell membrane stabilization, hepatic cell regeneration and enhancement of antioxidative enzymes such as CAT, SOD and GPx production. In spite of the unknown mechanism(s) of action, the obvious hepatoprotective effects of PPWE on APAP-induced liver injuries may have potential clinical applications. PP could contain compounds possessing hepatoprotective activity that warrant further investigation.
References
- S Ahmad, A Malik, R Yasmin, N Ullah, W Gul, P M Khan, H R Nawaz, and N Afza. (1999). Withanolides from Physalis peruviana. Phytochemistry 50:647–651.
- M Alia, C Horcajo, L Bravo, and L Goya. (2003). Effect of grape antioxidant dietary fiber on the total antioxidant capacity and the activity of liver antioxidant enzymes in rats. Nutr Res 23:1251–1267.
- T Amimoto, T Matsura, S Y Koyama, T Nakanishi, K Yamada, and G Kajiyama. (1995). Acetominophen-induced hepatic injury in mice: the role of lipid peroxidation and effects of pretreatment with coenzyme Q10 and α -tocopherol. Free Rad Biol Med 19:169–176.
- K Basey, B A McGaw, and J G Wolley. (1992). Phygrine, an alkaloid from Physalis species. Phytochemistry 31:4173–4176.
- T W Baumann, and C M Meier. (1993). Chemical defence by withanolides during fruit development in Physalis peruviana. Phytochemistry 33:317–321.
- R F BeersJr, and I W Sizer. (1952). A spectrophotometric method for measuring the breakdown of hydrogen peroxide by catalase. J Biol Chem 195:133–140.
- P J Beresford, and J G Woolley. (1974). Biosynthesis of ticloidine in Physalis peruviana. Phytochemistry 13:2143–2144.
- M S Blois. (1958). Antioxidant determinations by the use of stable free radical. Nature 26:1199–1200.
- E D Boyd, and G M Bereczky. (1966). Liver necrosis from paracetamol. Br J Pharmacol 26:606–614.
- T D Boyer, and S L Rouff. (1971). Acetaminophen-induced hepatic necrosis and renal failure. J Am Med Assoc 218:440–441.
- W C Chang, E L Lee, and L T Ng. (2006). Factors influencing the 2,2-diphenyl-1-picrylhydrazyl (DPPH) radical scavenging assay in the natural product analysis. Tajen J 28:17–36.
- H C Chiang, S M Jaw, and P M Chen. (1992). Inhibitory effects of physalin B and physalin F on various human leukemıa cells in vitro.. Anticancer Res 12:1155–1162.
- S Cliffe, M S Fawer, G Maier, K Takata, and G Ritter. (1994). Enzyme assays for the phenolic content of natural juices. J Agric Food Chem 42:1824–1828.
- D C Dahlin, G T Miwa, A Y Lu, and S D Nelson. (1984). N-acetyl-p-benzoquinone imine: A cytochrome P450-mediated oxidation product of acetaminophen. Proc Natl Acad Sci USA 81:1327–1331.
- D C Davis, W Z Potter, D J Jollow, and J R Mitchell. (1974). Species differences in hepatic glutathione depletion, covalent binding and hepatic necrosis after acetaminophen. Life Sci 14:2099–2109.
- L N Dinan, S D Sarker, and V Sik. (1997). 28-Hydroxywithanolide E from Physalis peruviana. Phytochemistry 44:509–512.
- P D Duh, W J Yen, P C Du, and G C Yen. (1997). Antioxidant activity of mung bean hulls. J Am Oil Chem Soc 74:1059–1063.
- K Glowniak, G Zgorka, and M Kozyra. (1996). Solid-phase extraction and reversed-phase high performance liquid chromatography of free phenolic acids in some Echinacea species. J Chromatogr 730:25–29.
- N Ismail, and M Alam. (2001). A novel cytotoxic flavonoid glycoside from Physalis angulata. Fitoterapia 72:676–679.
- M Ito, H Shimura, N Watanabe, M Tamai, K Hanada, A Takahashi, Y Tanaka, K Arai, P L Zhang, R Chang, W M Chen, J S Yang, Y L Su, and Y L Wang. (1990). Hepatoprotective compounds from Canarium album Euphorbia nematocypha. Chem Pharm Bull 38:2201–2203.
- L P James, P R Mayeux, and J A Hinson. (2003). Acetominophen-induced hepatotoxicity. Am Soc Pharmacol Exp Ther 31:1499–1506.
- Y M Kimura, M Kubo, T Tani, S Arichi, and H Okuda. (1981). Studies on Scutellariae radix IV: Effects on lipid peroxidation in rat liver. Chem Pharm Bull 29:2610–2617.
- P R N King, and E J King. (1954). Estimation of plasma phosphatase by determination of hydrolysed phenol with amino-antipyrine. J Clin Pathol 7:322–326.
- E Kupeli, D D Orhan, and E Yesilada. (2006). Effect of Cistus laurifolius L. leaf extracts and flavonoids on acetaminophen-induced hepatotoxicity in mice. J Ethnopharmacol 103:455–460.
- K J Lee, H J You, S J Park, Y S Kim, Y C Chung, T C Jeong, and H G Jeong. (2001). Hepatoprotective effects of Platycodon grandiflorum on acetaminophen-induced liver damage in mice. Cancer Lett 174:73–81.
- C C Lin, C C Tsai, and M H Yen. (1995). The evaluation of hepatoprotective effects of Taiwan folk medicine ‘Teng-Khia-U’. J Ethnopharmacol 45:133–123.
- C C Lin, M H Yen, T S Lo, and C F Lin. (1997). The anti-inflammatory and liver protective effects on B. nivea B. nivea subsp. nippononivea in rats. Phytomedicine 4:301–308.
- O H Lowry, N J Rosebrough, A L Farr, and R J Randall. (1951). Protein measurement with the Folin phenol reagent. J Biol Chem 193:265–275.
- H I Magalhães, M L Veras, M R Torres, A P Alves, O D Pessoa, E R Silveira, L V Costa-Lotufo, M O de Moraes, and C Pessoa. (2006). In vitro in vivo antitumour activity of physalins B and D from Physalis angulata. J Pharm Pharmacol 58:235–241.
- K Manda, and A L Bhatia. (2003). Role of β -carotene against acetominophen-induced hepatotoxicity in mice. Nutr Res 23:1097–1103.
- J R Mitchell, D J Jollow, W Z Potter, D C Davis, J R Gillette, and B B Brodi. (1973). Acetaminophen-induced hepatic necrosis. I. Role of covalent binding in vivo. J Pharmacol Exp Ther 187:185–194.
- M Oyaizu. (1986). Studies on product of browning reaction prepared from glucose amine. Japn J Nutr 44:307–315.
- L M Perry. (1980): Medicinal Plants of East and Southeast Asia - Attributed Properties and Uses. The MIT Press, Massachusetts, USA, p. 393.
- E Porchezhian, and S H Ansari. (2005). Hepatoprotective activity of Abutilon indicum on experimental liver damage in rats. Phytomedicine 12:62–64.
- M I Pun. (2005): Studies on the chemical constituents and cytotoxicity of Physalis peruvianaL. MSc Thesis, Graduate Institute of Natural Products, College of Pharmacy, Kaohsiung Medical University, Kaohsiung, Taiwan. 134 pp.
- S Reitman, and S Frankel. (1957). A chlorotrimetric method for the determination of sGOT and sGPT. Am J Clin Pathol 28:56–63.
- J T Rotruck, A L Pope, H E Ganther, A B Swanson, D G Hafeman, and W G Hoekstra. (1973). Selenium: biochemical role as a component of glutathione peroxidase. Science 179:588–590.
- K Singh, A K Khanna, and R Chander. (1999). Hepatoprotective activity of ellagic acid against carbon tetrachloride induced hepatotoxicity in rats. Indian J Exp Biol 37:1025–1026.
- M Sun, and S Zigman. (1978). An improved spectrophotometric assay for superoxide dismutase based on epinephrine autoxidation. Anal Biochem 90:81–89.
- M Thabrew, P Joice, and W Rajatissa. (1987). A comparative study of the efficacy of Pavetta indica and Osbeckia octandra in the treatment of liver dysfunction. Planta Med 53:239–241.
- A Tran, J M Treluyer, E Rey, J Barbet, G Ferracci, P d'Athis, J Vincent, and G Pons. (2001). Protective effect of stiripentol on acetaminophen-induced hepatotoxicity in rat. Toxicol Appl Pharmacol 170:145–152.
- N P E Vermeulen, J GM Bessems, and R van de Straat. (1992). Molecular aspects of paracetomol-induced hepatotoxicity and its mechanisms-based prevention. Drug Metab Rev 24:367–407.
- F M Wang, T W Yao, and S Zeng. (2003). Determination of quercetin and kaempferol in human urine after orally administrated tablet of Ginkgo biloba extract by HPLC. J Pharm Biomed Analy 33:317–321.
- S J Won, H C Chiang, W S Kan, and M T Lin. (1988). Augmentation of mouse splenic NK cytotoxic activity by extracts of Physalis angulata L. J Biomed Lab Sci 1:83–97.
- S J Wu, L T Ng, Y M Huang, D L Lin, S S Wang, S N Huang, and C C Lin. (2005). Antioxidant activities of Physalis peruviana. Biol Pharm Bull 28:963–966.
- S J Wu, L T Ng, C H Chen, D L Lin, S S Wang, and C C Lin. (2004a). Antihepatoma activity of Physalis peruviana P. Peruviana extract and their effects on apoptosis in human Hep G2 cells. Life Sci 74:2061–2073.
- S J Wu, L T Ng, D L Lin, S N Huang, S S Wang, and C C Lin. (2004b). Physalis peruviana extract induced apoptosis in human Hep G2 cell through CD95/CD95L system and the mitochondrial signaling transduction pathway. Cancer Lett 215:199–208.
- Y P Zou, Y H Lu, and D Z Wei. (2004). Antioxidant activity of a flavonoid-rich extract of Hypericum perforatum L. in vitro. J Agric Food Chem 52:5032–5039.