Abstract
The species of Linaria have been used as tonic, antiscorbutic, laxative, antidiabetic, and diuretic, as well as for the treatment of wounds, hemorrhoid and vascular disorders in Turkey. The anti-inflammatory and analgesic effects of different extracts from Linaria grandiflora Desf., L. genistifolia subsp. confertiflora (Boiss.) Davis, L. aucheri Boiss. (Scrophulariaceae) and two isolates (linariin and antirrinoside) from L. aucheri were studied using carrageenan- and PGE2-induced hind paw edema, 12-O-tetradecanoyl-13-acetate (TPA)-induced ear edema, and p-benzoquinone induced writhing reflex tests in mice. Our findings showed that oral administration of L. aucheri ethyl acetate extract (LA-EtOAc) and L. aucheri 20% aqueous extract, LA-MeOH:H2O(8:2), as well as antirrinoside significantly inhibited TPA-induced ear edema in mice. The remaining extracts and linariin did not show any anti-inflammatory activity on carrageenan-, PGE2-induced hind paw edema, 12-O-tetradecanoyl-13-acetate (TPA)-induced ear edema models. Moreover, LA-MeOH:H2O (8:2) and antirrinoside displayed significant antinociceptive activity by p-benzoquninone-induced writhing reflex in mice.
Introduction
Linaria, a well known genus of the family Scrophulariaceae, is widely distributed in Turkey, and represented by 20 species in the flora of Turkey (CitationDavis, 1978). In traditional Turkish medicine, some Linaria species are used as diuretics, laxatives, and for the treatment of hemorrhoids and some wounds (CitationBaytop, 1999). In addition, several Linaria species are used as tonic, antiscorbutic, antidiabetic and to treat some vascular disorders (CitationSan Feliciano et al., 1993). Iridoids (CitationBianco et al., 1996), flavonoids (CitationOtsuka, 1992), alkaloids (CitationHua et al., 2002), and diterpenoids (CitationGordaliza et al., 1995) are the main classes of the secondary methabolites in this genus of plants. In a previous phytochemical investigation on the petroleum ether and methanol extracts from the aerial parts of Linaria aucheri Boiss., six known compounds, including linariin and antirrinoside, have been isolated (CitationErcil et al., 2004).
The present study was carried out to investigate the analgesic and anti-inflammatory activities of different extracts prepared from three Linaria species (Linaria grandiflora Desf., L. genistifolia subsp. confertiflora (Boiss.) Davis, L. aucheri) and antirrinoside and linariin previously isolated from L. aucheri by carrageenan-, PGE2-induced hind paw edema, 12-O-tetradecanoyl-13-acetate (TPA)-induced ear edema, and p-benzoquinone-induced writhing reflex tests in mice. This study is an attempt to establish a scientific basis for the traditional use of these plants in Turkish folk medicine and a possible mechanism involvement.
Materials and methods
Plant materials
Linaria grandiflora Desf., L. genistifolia subsp. confertiflora (Boiss.) Davis and L. aucheri Boiss. (Scrophulariaceae) were collected from Konya, Ankara, and çankırı, Turkey in 2000, 1999, and 1997, respectively, in flowering period and identified by Dilek Ercil, Department of Pharmacognosy, Faculty of Pharmacy, Hacettepe University, Turkey. Voucher specimens have been deposited in Herbarium of the Faculty of Pharmacy, Hacettepe University, Ankara, Turkey (HUEF 00-177, HUEF 99-094, HUEF 97-020 respectively).
Extraction and isolation
The aerial parts of three Linaria species were extracted successively with n-hexane, dichloromethane, ethyl acetate and 20% aqueous methanol and the following extracts were obtained: L. grandiflora: n-hexane extract (0.73%), dichloromethane extract (0.65%), ethylacetate extract (1.04%) and 20% aqueous methanol extract (21.40%); L. genistifolia subsp. confertiflora: n-hexane extract (0.65%), dichloromethane extract (0.51%), ethyl acetate extract (0.72%) and 20% aqueous methanol extract (19.70%); L. aucheri: n-hexane extract (1.21%), dichloromethane extract (1.39%), ethyl acetate extract (2.68%) and 20% aqueous methanol extract (33.06%).
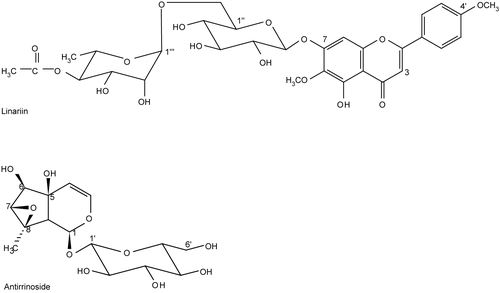
The tested pure compounds linariin and antirrinoside were isolated previously () (CitationErcil et al., 2004).
Chemicals
The following solvents and chemicals were purchased and used as received. Carraegeenan (Sigma, St.Louis, Missouri, USA), PGE2 (Fluka Chemie AG, Switzerland), 12-O-tetradecanoyl-13-acetate (TPA) (Sigma-Aldrich), p-benzoquinone (Merck, Darmstadt, Germany), indomethacin (Bayer AG, Turkey), acetylsalicylic acid (Bayer AG, Turkey), sodium carboxymethylcellulose (Sigma-Aldrich).
Pharmacological procedures
Animals
Male Swiss albino mice (20-25 g) were purchased from the animal breeding laboratories of Refik Saydam Central Institute of Health, Ankara, Turkey. The animals left for two days for acclimatization to animal room conditions were maintained on standard pellet diet and water ad libitum. The food was withdrawn on the day before the experiment, but they were allowed free access to water. A minimum of six animals was used in each group. Throughout the experiments, animals were processed according to the suggested international and national ethical guidelines for the care of laboratory animals.
Preparation of test samples for bioassay
Test samples were given orally to the test animals after suspending in a mixture of distilled H2O and 0.5% sodium carboxymethyl cellulose (CMC). The control group animals received the same experimental handling as those of the test groups except the drug treatment was replaced with appropriate volumes of dosing vehicle. Indomethacin (10 mg/kg and 0.5 mg/ear) and acetylsalicylic acid (ASA) (100 and 200 mg/kg) in 0.5% CMC were used as reference drugs.
Anti-inflammatory activity
Carrageenan-induced hind paw edema model
Carrageenan-induced hind paw edema model was used for the determination of anti-inflammatory activity (CitationYes¸ilada & Küpeli, 2007). Each mouse was injected, 60 min after the oral administration of a test sample or dosing vehicle, with freshly prepared suspension of carrageenan (0.5 mg/25 μL) in physiological saline (154 nM NaCl) into subplantar tissue of the right hind paw. As the control, 25 μL saline solution was injected into that of the left hind paw. Paw edema was then measured in every 90 min during 6 h after induction of inflammation. The difference in footpad thickness was measured by gauge calipers (Ozaki, Tokyo, Japan). Mean values of treated groups were compared with those of a control group and analyzed by using statistical methods. Indomethacin (10 mg/kg) was used as the reference drug.
PGE2-induced hind paw edema model
PGE2-induced hind paw edema model used for the determination of anti-inflammatory activity was evaluated as given by CitationKasahara et al. (1985). Each mouse was injected, 60 min after the oral administration of a test sample or dosing vehicle, with freshly prepared suspension of PGE2 (5 μg/5 μL) in Tyrode’s solution into subplantar tissue of the right hind paw. As the control, 5 μL Tyrode’s solution was injected into that of the left hind paw. Paw edema was measured every 15 min during a period of 75 min after the induction of inflammation. The difference in footpad thickness was measured by gauge calipers. Mean values of treated groups were compared with mean values of a control group and statistically analyzed. Indomethacin (10 mg/kg) was used as reference drug.
TPA-induced mouse ear edema
Each mouse received 2.5 μg of TPA (12-O-tetradecanoylphorbol 13-acetate) dissolved in 20 μL of EtOH 70% (CitationDe Young et al., 1989). This was applied by an automatic pipette in 20 μL volumes to both anterior and posterior surfaces of the right ear. The left ear (control) received the same volume of solvent (EtOH 70%), simultaneously with TPA. Indomethacin (0.5 mg/ear) was used as reference drug. For the evaluation of the activity, two different ways were followed up as given below.
The thickness of each ear was measured 4 h after induction of inflammation using gauge calipers. The edema was expressed as the difference between the right and left ears due to TPA application and consequently inhibition percentage was expressed as a reduction thickness with respect to the control group.
After 4 h of the administration the animals were killed under deep ether anesthesia. Discs of 6 mm diameter were removed from each ear and weighed in balance. The swelling was estimated as the difference in weight between the punches from right and left ears and expressed as an increase in ear thickness.
Antinociceptive activity
The p-benzoquinone-induced abdominal constriction test (CitationOkun et al., 1963) was performed on mice for the determination of antinociceptive activity. According to the method evaluated, 60 min after the oral administration of a test sample, the mice were intraperitonally injected with 0.1 mL/10 g body weight of 2.5% (w/v) p-benzoquinone (PBQ) solution in distilled water. Control animals received an appropriate volume of dosing vehicle. The mice were then kept individually for observation and the total number of the abdominal contractions (writhing movements) was counted for the following 15 min, starting 5 min after the PBQ injection. The data represent the average of the total number of writhes observed. Antinociceptive activity was then expressed as the percentage change from writhing controls. Acetylsalicylic acid (ASA) at 100 and 200 mg/kg doses was used as the reference drug in this test.
Acute toxicity
Animals employed in the carrageenan-induced paw edema experiment were observed during 48 h and morbidity or mortality was recorded, if present, for each group at the end of observation period.
Gastric-ulcerogenic effect
After the employment of antinociceptive activity experiment, mice were killed under deep ether anesthesia and the stomachs of each mouse were removed. Then the abdomen of each mouse was opened through the greater curvature and examined under a dissecting microscope for lesions or bleedings.
Statistical analysis of data
Data obtained from animal experiments were expressed as the mean standard error (± SEM). Statistical differences between the treated and the control groups were evaluated by ANOVA and Students-Newman-Keuls post-hoc tests. p < 0.05 was considered to be significant (* p < 0.05; ** p < 0.01; *** p < 0.001).
Results and discussion
The anti-inflammatory properties of Linaria species were studied using several standard pharmacological methods. The results generally revealed that the n-hexane, dichloromethane, ethyl acetate and 20% aqueous methanol extracts of Linaria grandiflora, L. genistifolia subsp. confertiflora and L. aucheri elicited anti-inflammatory activities of different intensities, as reflected by inhibition of mice paw edema caused by both carrageenan and PGE2, and ear edema by TPA.
The carrageenan test is highly sensitive to non-steroidal anti-inflammatory drugs, and it has been accepted as a useful phlogistic tool for the investigation of new anti-inflammatory drugs (CitationJust et al., 1998). The results obtained with the extracts, and the isolated compounds together with indomethacin on the carrageenan-induced paw edema test are shown in . The degree of swelling of the carrageenan-injected paws was maximal 3 h after injection and the mean increase in volume at that time was about 100% in the control group. Statistical analysis showed that the edema inhibition of extracts and the isolated compounds at the dose of 100 mg/kg were not significantly different from those of the control group.
Table 1. Effect of the extracts and active principles on carrageenan-induced hind paw edema in mice.
A similar activity pattern was also observed in PGE2-induced hind paw edema model. As shown in , none of the extracts and the isolates exhibited an inhibition at 100 mg/kg dose level.
Table 2. Effect of the extracts and active principles against PGE2-induced paw edema in mice.
The results obtained from TPA-induced mice ear edema test were demonstrated in . It was observed that the L. aucheri ethyl acetate extract (LA-EtOAc) and L. aucheri 20% aqueous extract, LA-MeOH:H2O (8:2), as well as antirrinoside showed a significant inhibition of the TPA-induced ear edema in a dose-dependent manner as compared to control. It has been established that the phorbol ester (TPA) exert its inflammatory effect through the protein kinase C activation with subsequent cytosolic phospholipase A2 stimulation, AA mobilization, and biosynthesis of prostaglandins and leukotrienes (CitationJust et al., 1998). Phospholipase A2, cyclooxygenase and lipoxygenase inhibitors as well as corticoids are found to be effective in this model (CitationCarlson et al., 1985; CitationDe Young et al., 1989), and the extracts of the studied Linaria species may have interfered with these mediators to inhibit TPA-induced inflammation.
Table 3. Effect of the extracts and active principles against TPA-induced ear edema in mice as measurement of swelling thickness and weight measurement of edema.
Effects of the studied extracts and the isolated compounds on p-benzoquinone writhing reflex test have been shown in . Statistical analysis showed that none of the test groups was significantly different from those of the control group.
Table 4. Effect of the extracts and active principles against p-benzoquinone-induced writhings in mice.
For the acute toxicity evaluation of the extracts and isolated compounds, morbidity and mortality were monitored for 48 h after the administration of the extracts in mice and no negative symptom that might be attributed to morbidity or death was recorded. Neither the extracts nor the isolated compounds induced any apparent gastric lesion in the administered doses.
The genus Linaria contains several species used in folk medicine as tonic, antiscorbutic, laxative, antidiabetic and diuretic, as well as for the treatment of wounds, hemorrhoids and vascular disorders (CitationBaytop, 1999; CitationSan Feliciano et al., 1993). However, there are no phytochemical or biological reports on Linaria grandiflora, L. genistifolia subsp. confertiflora, and L. aucheri except one previous phytochemical study (CitationErcil et al., 2004).
Iridoids, particularly antirrinoside and antirride together with flavonoid glycosides, particularly pectolinarin and linariin, are considered as good chemotaxonomic markers for the Scrophulariaceae family.
Antirrinoside is one of the major iridoid glycosides of Linaria aucheri. It is a derivative of catalpol. Previous studies showed that catalpol has no effect against carrageenan-induced edema, however, antirrinoside was found to possess a significant activity in similar experimental protocols. A double bond between C-7 and C-8 is considered as one of the most positive characters for the activity of iridoids, and its oxidation to an epoxy function leads to a remarkable decrease in the anti-inflammatory activity, as occurs in catalpol. Moreover, the presence of an additional hydroxy group at C-6 of the iridoid skeleton further reduces topical anti-inflammatory activity, whereas tertiary hydroxy group at C-5 causes the opposite effect. However, an iridoid compound that possesses both 5 and 6 hydroxy groups produces an edema reduction. In addition, hydroxylation at C-8 seriously decreases the topical activity (CitationRecio et al., 1994). However, in contrast with the previous studies, antirrinoside was found to be active against carrageenan-induced edema in the present study. This study was the first report to demostrate that antirrinoside possesses a significant induced ear edema. Therefore, it can be concluded that despite the epoxy function, the presence of hydroxy groups at C-5 and C-6, as well as a methyl function at C-8, might be necessary for significant activity.
The presence of 4-oxo functional group at C-ring together with a double bond between C-2 and C-3 and the number and the position of hydroxyl and/or methoxyl groups seem to be necessary for the highest anti-inflammatory effect of the flavonoids. In particular, the A-ring 5,7-dihydroxyl and B-ring 4′-or 3′,4′-dihydroxyl groups are important for their inhibitory activities. Substitution of methyl groups into the flavone molecule increase this activity. 3′,4′-Dimethoxy groups of B-ring are also important for the activity (CitationCarvalho et al., 1999; CitationKim et al., 2004; CitationSchneider & Bucar, 2005; CitationTakano -Ishikawa et al., 2006). A polymethoxyflavone, 5-O-demethylnobiletin (6,7,8,3′,4′-pentametoxy flavone) was found to be active (CitationBas et al., 2006). The glycosides of various flavonoids exhibit lower inhibitory activities than their corresponding aglycones, showing that the glycosylation diminishes the inhibitory activity (CitationSchneider & Bucar, 2005; CitationWalgren et al., 1998). These findings help to partially explain the pharmacological efficacy of flavonoids as anti-inflammatory compounds. However, linariin being a 4′-methoxy flavone did not show any effect. Therefore, it might be concluded that the glycosylation in the flavone skeleton was important and the difference in the activity might be due to the pharmacokinetic behavior of each compound in the body.
On the TLC analysis of the fractions, linariin and antirrinoside were found to be the main components of the ethyl acetate and especially the aqueous methanol extracts of L. aucheri. This finding may help to explain the significant inhibition of the TPA-induced ear edema of these extracts. However, only small antirrinoside spots were detected in the TLC analysis of the ethyl acetate and aqueous methanol extracts of the remaining Linaria species, which could explain the weak activity of the extracts of these plants.
Further studies are necessary to elucidate the mechanism behind the traditional effects of Linaria grandiflora, L. genistifolia subsp. confertiflora and L. aucheri. The results of pharmacological tests performed in the present study suggest that all the tested extracts of Linaria species present analgesic and anti-inflammatory effects, with no toxicity.
Acknowledgment
Declaration of interest: The authors report no conflicts of interest.
References
- Bas E, Recio MC, Giner RM, Manez S, Cerda-Nicolas M, Rios JL (2006): Anti-inflammatory activity of 5-O-demethylnobiletin, a polymethoxyflavone isolated from Sideritis tragoriganum. Planta Med 72: 136–142.
- Baytop T (1999): Therapy with Medicinal Plants (Past and Present), 2nd edn. Istanbul, Nobel Tip Kitabevleri, p. 373.
- Bianco A, Guiso M, Mazzei RA, Piccioni F, Nicoletti M, Serafini M, Ballero M (1996): 69-O-Acetylantirrhinoside, a new iridoid glucoside from Linaria flava subsp. sardoa. Fitoterapia 67: 364–366.
- Carlson RP, O’Neill-Davis L, Chang J, Lewis AJ (1985): Modulation of mouse ear edema by cyclooxygenase and lipoxygenase inhibitors and other pharmacological agents. Agents Actions 17: 197–204.
- Carvalho JCT, Ferreira LP, Santos LS, Correa MJC, Campos LMO, Bastos JK, Sarti SJ (1999): Anti-inflammatory activity of flavone and some of its derivates from Virola michelli Heckel. J Ethnopharmacol 64: 173–177.
- Davis PH (1978): Flora of Turkey and the East Aegean Island, Vol. 6. Edinburgh, University Press, p. 654–672.
- De Young LM, Kheifets JB, Ballaron SJ, Young JM (1989): Edema and cell infiltration in the phorbol ester treated mouse ear are temporally separate and can be differently modulated by pharmacologic agents. Agents Activity 26: 335–341.
- Ercil D, Sakar MK, Del Olmo E, San Feliciano A (2004): Chemical constituents of Linaria aucheri. Turk J Chem 28: 133–139.
- Gordaliza M, Del Corral JMM, Mahiques MM, Castro A, San Feliciano A (1995): Neo-clerodane diterpenoids from roots of Linaria saxatilis var. saxatilis. Phytochemistry 40: 1307–1309.
- Hua H, Cheng M, Li X, Pei Y (2002): A new pyrroloquinazoline alkaloid from Linaria vulgaris. Chem Pharm Bull 50: 1393–1394.
- Just MJ, Recio MC, Giner RM, Cuellar MJ, Manez S, Bilia AR, Rois JL (1998): Anti-inflammatory activity of unusual lupane saponins from Bupleurum fruticescens. Planta Med 64: 404–407.
- Kasahara Y, Hikino H, Tsurufiji S, Watanabe M, Ohuchi K (1985): Anti-inflammatory actions of ephedrines in acute inflammations. Planta Med 51: 325–331.
- Kim HP, Son KH, Chang HW, Kang SS (2004): Anti-inflammatory plant flavonoids and cellular action mechanisms. J Pharm Sci 96: 229–245.
- Okun R, Liddon SC, Lasagnal L (1963): The effect of aggregation, electric shock and adrenergic blocking drugs on inhibition of the “writhing syndrome”. J Pharmacol Exp Ther 139: 107–109.
- Otsuka HJ (1992): Isolation of isolinariin A and B, new flavonoid glycosides from Linaria japonica. J Nat Prod 55: 1252–1255.
- Recio MC, Giner RM, Manez S, Rios JL (1994): Structural considerations on the iridoits as anti-inflammatory agents. Planta Med 60: 232–234.
- San Feliciano A, Gordaliza M, Del Corral JMM, De La Puente ML (1993): Neo-clerodane diterpenoids from roots of Linaria saxatilis var. glutinosa. Phytochemistry 33: 631–633.
- Schneider I, Bucar F (2005): Lipoxygenase inhibitors from natural plant sources. Part 1: Medical plants with inhibitory activity on arachidonate 5-lipoxygenase and 5-lipoxygenase/cyclooxygenase. Phytother Res 19: 81–102.
- Takano-Ishikawa Y, Goto M, Yamaki K (2006): Structure-activity relations of inhibitory effects of various flavonoids on lipopolysaccharide-induced prostaglandin E2 production in rat peritoneal macrophages: Comparison between subclasses of flavonoids. Phytochemistry 13: 310–317.
- Walgren RA, Walle UK, Walle T (1998): Transport of quercetin and its glucosides across human intestinal epithelial caco-2 cells. Biochem Pharmacol 55: 1721–1727.
- Yes¸ilada E, Küpeli E (2007): Clematis vitalba L. aerial part exhibits potent anti-inflammatory, antinociceptive and antipyretic effects. J Ethnopharmacol 110: 504–515.