Abstract
The objective of the present study was to investigate the in vitro antibacterial activity of the extracts and their fractions obtained from either the flowers or the leaves of Helichrysum plicatum DC. subsp. plicatum (Asteraceae), by studying inhibition of the growth of a dangerous food pathogen, Escherichia coli O157:H7. The antibacterial effects of the ethanol and water extracts of the flowers and leaves were examined first. Subsequently sub-extracts of the flower ethanol extract (FEE), which was found to be the most effective extract, were examined. As a result, the antibacterial effect of FEE was found to be stronger than that of the chloroform and ethyl acetate sub-extracts. This may due to synergistic activity of several components of the ethanol extract of the flowers.
Introduction
Strains of Escherichia coli reside in the intestine of man as an integral part of the normal bacterial flora. These common strains may perform some essential functions for the host. However, a few strains are pathogenic, and can cause distinct diarrheal syndromes. Among these are enteropathogenic, enteroinvasive, enterotoxigenic, and enterohemorrhagic E. coli (EHEC) (CitationKim et al., 2004), with the latter being known as one of the most dangerous pathogens. Human and animal EHEC strains which cause serious infections belong to a large number of O:H serotypes, but most outbreaks have been attributed to the E. coli O157:H7 strain (CitationMora et al., 2005). E. coli O157:H7 was first recognized as a pathogen in 1982 and is considered the main cause of hemorrhagic colitis, hemolytic uremic syndrome, and thrombocytopenic purpura (CitationKim et al., 2004; CitationLi & Logue, 2005; CitationMora et al., 2005; CitationMoreira et al., 2005). This serovar possesses a number of undesirable characteristics that combine to make it one of the most serious food-associated outbreak causative factors in recent years (CitationSag˘dıç et al., 2002). Despite new control measures, outbreaks caused by this microorganism continue to occur from apple cider, ground beef, poultry, milk, hamburgers, or ham and cheese sandwiches, and are accompanied by an increase in the number of cases of infections in many countries (CitationSag˘dıç et al., 2002; CitationKim et al., 2004). As a result, antimicrobial drugs have been used for the control of bacterial infections. Nevertheless, it is evident that some pathogens have become resistant to many of these drugs during the last decade (CitationBarbour et al., 2004).
Medicinal plants are natural resources yielding valuable herbal products which are often used in the treatment of various ailments. The genus Helichrysum L. (Asteraceae) consists of a few hundred species widespread throughout the world (CitationBianchini et al., 2001). Members of the genus have been used in folk medicine mainly for their antimicrobial, anti-inflammatory, digestive, choleretic, and diuretic properties (CitationSezik et al., 1991, Citation2001; CitationFujita et al., 1995). In addition, several species have minor uses as a tea or a spice when added to rice, vegetables, and savory dishes to give a mild curry flavor. A number of in vitro and in vivo studies have been conducted to evaluate the biological effects of several Helichrysum species; namely, antioxidant (CitationTepe et al., 2005), antiviral (CitationMeyer et al., 1997), antimalarial (Citationvan Vuuren et al., 2006), and anti-inflammatory (CitationSchinella et al., 2002) activities.
Medicinal properties of Helichrysum species are mainly attributed to the presence of flavonoids. However, several different classes of constituents have also been reported; namely, triterpenoids, diterpenoids, steroids, hydroxyisopentenyl acetophenone, and phloroglucinol (CitationTomas-Barberan et al., 1990). Helichrysum plicatum DC. subsp. plicatum is known to possess a rich flavonoid content, comprising 4.83% flavonoids such as helichrysins A and B, apigenin, naringenin, isoastragalin, and isosalopurposide (CitationErdogrul et al., 2001).
Antimicrobial compounds from plants may inhibit bacterial growth by different mechanisms from those of antimicrobials currently used (CitationEloff, 1988). Several Helichrysum species have been reported to possess significant antimicrobial activity, such as H. caespititium (DC.) Harv. (CitationMathekga et al. 2000), H. gymnocomum DC. (CitationDrewes & van Vuuren, 2008), H. italicum G.Don (CitationNostro et al., 2001), H. literoum Guss. (Guida et al., 1999), H. plicatum DC. subsp. plicatum (CitationErdogrul et al., 2001), H. stoechas L. Moench. (CitationRios et al., 1991), and H. tenax M.D. Hend. (CitationDrewes et al., 2006). These species were found to be effective against Staphylococcus aureus (CitationMathekga et al., 2000; CitationNostro et al., 2001; CitationDrewes & van Vuuren, 2008), multidrug-resistant S. aureus (MRSA) (CitationDrewes & van Vuuren, 2008), Bacillus cereus, Bacillus pumilus, Bacillus subtilis, Staphylococcus epidermidis (CitationDrewes et al., 2006), Micrococcus kristinae (CitationMathekga et al., 2000), and E. coli (CitationErdogrul et al., 2001).
Despite numerous investigations on the antimicrobial activity profiles of different Helichrysum species, the effect on E. coli O157:H7 has not yet been evaluated in detail. The present study investigated the antibacterial activity of H. plicatum subsp. plicatum (HPP) against E. coli O157:H7.
Materials
Bacteria
E. coli strain O157:H7, kindly provided by Professor M.P. Doyle, The University of Georgia, USA, was used throughout the study.
Plant material
HPP was collected from the locality of Mount Palandoken in Turkey (Erzurum) in July 2002. The plant was identified by one of the authors (M.A.) and deposited at the Department of Pharmacognosy, Faculty of Pharmacy, Gazi University, Turkey (no. 02A011). The freshly picked flowers and leaves of the plant were air-dried at room temperature for 3 weeks under shade. Once dried, the plant was ground and stored at −20°C before being subjected to the extraction procedures.
The extracts used in the present study, obtained from the ethanol and water extraction of the flowers and leaves of HPP, were named as follows: flower ethanol extract (FEE), leaf ethanol extract (LEE), flower water extract (FWE), and leaf water extract (LWE). Sub-extracts were then prepared by successive solvent extractions of FEE by n-hexane (FEEHE), chloroform (FEECE), ethyl acetate (FEEEAE), and n-butanol (FEEBE); the remaining aqueous part was then called FEER.
Preparation of the plant extracts
The dried and powdered flowers and leaves of the title plant (100 g each) were extracted twice with 80% (v/v) EtOH and distilled water individually, for 30 min at 40ºC. Each extract (FEE, FWE, LEE, and LWE) was then filtered, combined, and evaporated to dryness in a rotary evaporator at a temperature below 40ºC under reduced pressure (yields: 19.87% FEE; 17.47% FEW; 14.77% LEE; 14.97% LWE). The residues were kept in small sterile vials under refrigerated conditions until used.
Solvent extraction of FEE
Dried and powdered flowers (500 g) of the plant were extracted twice with 80% EtOH for 24 h at 40ºC. The ethanol extracts were then filtered, combined, and evaporated to dryness in a rotary evaporator at 40ºC under reduced pressure to yield 99.38 g of crude extract. Then the residue was dissolved in 500 mL MeOH–H2O mixture (90% v/v) and extracted six times with n-C6H14 (FEEHE; 13 g). After removal of MeOH from the remaining layer, the volume was brought to 500 mL with sterile distilled water; this was extracted five times with CHCl3 and the combined extracts gave FEECE (5.56 g) on evaporation. The remaining aqueous layer was extracted five times with EtOAc to yield FEEEAE (25.39 g). Finally, FEEBE (8.50 g) was obtained by extracting the remaining aqueous layer five times with n-BuOH saturated with distilled water; the remaining aqueous layer was kept and called FEER (42.51 g). FEECE and FEEEAE were then subjected to chromatographic separations to evaluate further fractions.
Fractionation of FEECE
FEECE (250 mg) was further fractionated by silica gel column chromatography using n-C6H14, n-C6H14–CH2Cl2 (1:1 v/v), CH2Cl2–CHCl3 (9:1 v/v), and CHCl3–MeOH (8:2 v/v) as solvent systems. Resulting fractions were combined under six major fractions according to their TLC profiles on silica gel plates (Kieselgel 60 silica gel plates; Merck, Germany) with CHCl3–MeOH–H2O (61:32:7, by volume) as solvent system: Fr.1–2 (24 mg), Fr. 3 (20.6 mg), Fr. 4 (3.6 mg), Fr. 5–11 (26.5 mg), Fr. 12 (100.9 mg), and Fr. 13–30 (43.3 mg).
Fractionation of FEEEAE
FEEEAE (10 g) was subjected to silica gel column chromatography, eluting with CHCl3–MeOH (95:5 v/v, 90:10 v/v, 80:20 v/v, 70:30 v/v, 60:40 v/v) and CHCl3–MeOH–H2O (12:5.5:1, by volume) as solvent systems. Resulting fractions were combined under six major fractions: Fr. 3–6 (120 mg), Fr. 18–28 (850 mg), Fr. 32–54 (3.0 g), Fr. 55–63 (560 mg), Fr. 64–72 (1.05 g), and Fr. 89–96 (420 mg), depending on their TLC profiles on Kieselgel 60 silica gel plates (Merck) with EtOAc–MeOH–glCH3COOH–H2O (100:11:11:27, by volume) as solvent system.
Determination of antibacterial activity
Determination of minimum inhibitory concentration of the plant extracts
The minimum inhibitory concentrations (MIC) of the plant extracts were tested by the serial dilution method using sterile tryptic soy broth (TSB). DMSO (10 μL) solutions of the plant extracts were placed in sterile test tubes into which 980 μL of medium and 10 μL of E. coli O157:H7 culture were added. The final concentration of the extracts in each medium ranged from 4000 to 62.5 μg/mL (from 2.5 to 0.5%), and the resulting DMSO concentration was 1% (v/v). Then all the tubes were incubated at 37ºC for 18–24 h. The lowest concentration of a plant extract that retained its inhibitory effect resulting in no growth (absence of turbidity) of E. coli O157:H7 was recorded as the MIC value of the extract (CitationBarbour et al., 2004; CitationMora et al., 2005). Two control experiments were run to study the impact of the TSB medium itself and TSB with 1% DMSO on E. coli O157:H7. The experiment was set with two different culture concentrations, one suspension with E. coli O157:H7 having an approximate count of 109 CFU/mL and the other with count of about 105 CFU/mL.
Antibacterial activity test
The antibacterial activity test involved inoculation of E. coli O157:H7 in TSB, addition of the plant extracts dissolved in EtOH, followed by incubation and periodic sampling to determine survival. E. coli O157:H7 suspensions with approximate counts of 109 and 105 CFU/mL were inoculated into 7 mL of TSB containing 0.5, 1.0, 1.5, 2.0, and 2.5% of each plant extract, sub-extract, and fraction, and incubated at 37ºC for 7 days. Two control experiments [control (K) and alcohol control (AK)] were also run to study the impact of EtOH, which was used as a solvent for dissolving the samples. During the analysis, triplicate samples of 1 mL from each tube were taken on the first, second, fourth, fifth, and seventh days. These samples were diluted in sterile 0.85% (w/v) NaCl solution and the number of colonies was determined by plate count procedure in nutrient agar plates. Plates were incubated at 37ºC for 24 h, and then enumerated for viable organisms. The entire experiment was carried out twice with triplicate parallel counts.
HPLC analysis of FEE
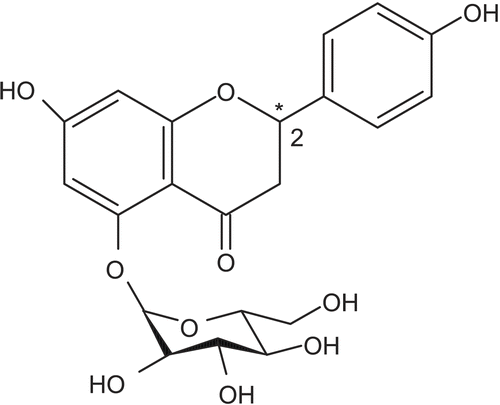
A flavanone agylcone, naringenin (5,7,49-trihydroxyflavanone), and its glucosides helichrysin A (naringenin-5-β-d-glucoside, 2R-enantiomer) and helichrysin B (naringenin-5-β-d-glucoside, 2R,S-enantiomers) (), are the main components of Helichrysum species. In order to standardize FEE, naringenin and total helichrysins A and B concentrations in FEE were determined by HPLC using an RP-18 Lichrosphere 5 μm (250 mm×4.6 mm) Supelco (serial no. 98040778) column, a Hewlett-Packard model 1050 pump, a Rheodyne model 7125 injector equipped with a 20 μL loop, a Hewlett-Packard model 1050 UV detector, and an HP-3996A integrator. H2O–CH3CN–MeOH (60:20:20, by volume) with a flow rate of 0.8 mL/min and UV wavelength of 280 nm was used as mobile phase.
Preparation of the standard solutions
Naringenin, helichrysin A, and helichrysin B (1 mg each) were dissolved in MeOH (10 mL) and 1 mL of this solution was diluted in MeOH (10 mL). Then 1.0, 1.5, 2.0, and 2.5 mL of the latter solution were diluted independently and completed up to 10 mL with MeOH.
Preparation of FEE
The flower part (1 g) of HPP was extracted with 50 mL EtOH (80%) under magnetic stirring at 40°C for 24 h. The ethanol phase was then filtered under vacuum. The residue was re-extracted with 50 mL of EtOH (80%) for 24 h and the ethanol phase was filtered. The combined extracts were evaporated under vacuum. After dissolving the concentrated extract in MeOH, it was brought up to 10 mL (stock solution) and 1 mL of stock solution was diluted with 10 mL of MeOH. This solution was subjected to HPLC for the quantification of naringenin and total helichrysins A and B.
HPLC analysis
Appropriate solutions prepared from standards and test solutions were filtered (Alltech Nylon 66 membrane, 0.45 Angstroms, (Deerfield, IL) and degassed. Prior to the application of the test samples, they were filtered through a filtration kit (Hamilton Bonaduz, Switzerland) and injected in triplicate (20 μL volume for each injection). A graph was obtained according to the ‘Linear Regression’ method in the Agilent LC Diagnostic Rev. A01.02 computer software (Istanbul, Turkey).
Statistical analysis
The computer program SPSS version 12.0 (SPSS Inc., Chicago, IL, USA) was implemented to process the data gathered from the results of the study. One-way analysis of variance (ANOVA) and Scheffe’s test was applied for comparison of the mean values. A p value of <0.01 was regarded as significant.
Results
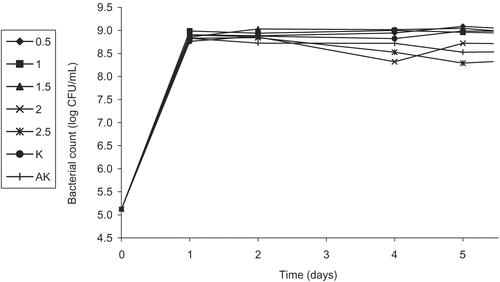
Because CitationJuneja et al. (2000) determined a loss in the antibacterial activity of green tea catechins due to high inoculation levels of the pathogen E. coli O157:H7, we carried out the antibacterial activity tests in two ways depending on the concentration of the E. coli O157:H7 culture solution: as an overnight suspension involving an approximate count of 109 CFU/mL and a diluted culture with a count of about 105 CFU/mL.
Overnight culture test
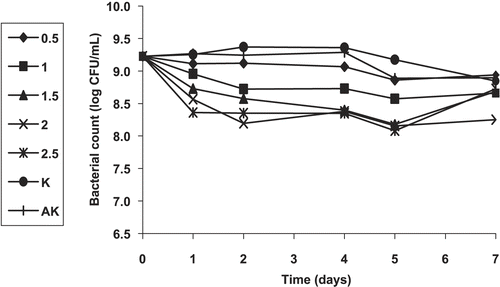
In this part of the study, we first investigated the effect of plant extracts at different concentrations on the survival of an overnight culture of E. coli O157:H7 at 37ºC for a week. All extracts (except for FEE) did not show any significant activity in this screening test, even at a concentration of 2.5%. Although the antibacterial activity of FEE at 0.5% concentration against E. coli O157:H7 was lower than that of the other concentrations of the extract, all concentrations of FEE had a bacteriostatic or bactericidal effect against E. coli O157:H7 (). A 2.5% concentration of this extract had the greatest inhibitory effect on the growth of the pathogen especially on the fifth day, but the same concentration at first seemed to have a stimulatory effect on the pathogen on the seventh day. Next, we tested the effect of sub-extracts of FEE at different concentrations on the survival of E. coli O157:H7 at 37ºC for a week. Of the five sub-extracts, FEEHE, FEEEAE, FEEBE, and FEER were observed to be inactive on E. coli O157:H7 at all concentrations. However, FEECE showed partial inhibition of the growth of the pathogen at a concentration of 2.5%, especially on the fifth and the seventh days ().
Diluted culture test
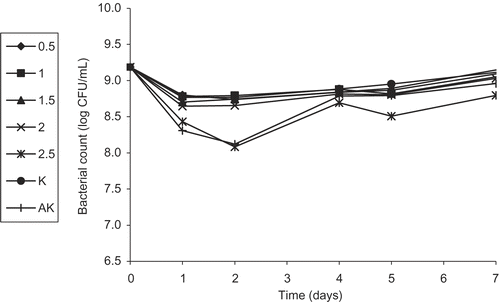
In this part of the study, we first investigated the effect of plant extracts at different concentrations on the survival of a diluted culture of E. coli O157:H7 at 37ºC for a week. All concentrations (except for 0.5%) of FEE showed a bacteriostatic or bactericidal effect on E. coli O157:H7 starting from the first day. However, the antibacterial activity of the sample with 0.5% concentration started after the fourth day of the analysis (). Concentrations of 1.5, 2.0, and 2.5% of FWE did not show any activity until the fourth day but started showing inhibitory effects on the fifth and the seventh days (). In the same way, LWE of 2.0 and 2.5% concentrations did not show any inhibitory effect until the second day, but started mediating antibacterial activity on the fourth, fifth, and seventh days, while at 1.5% concentration, a partial inhibition was shown on the seventh day (). On the other hand, LEE did not show any activity even at a concentration of 2.5%. Since FEE was found to be the most effective extract of the plant, a study was carried out with sub-extracts.
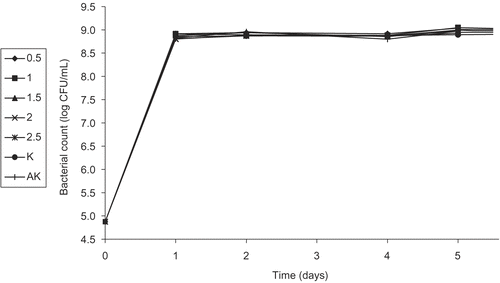
Figure 4. Activity of different concentrations (%) of the flower ethanol extract (FEE) of Helichyrsum plicatum subsp. plicatum against diluted culture of Escherichia coli O157:H7 (K, control; AK, alcohol control).
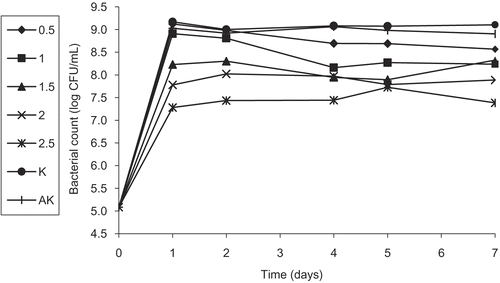
Figure 5. Activity of different concentrations (%) of the flower water extract (FWE) of Helichyrsum plicatum subsp. plicatum against diluted culture of Escherichia coli O157:H7 (K, control; AK, alcohol control).
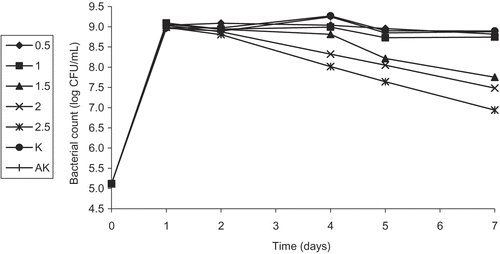
Figure 6. Activity of different concentrations (%) of the leaf water extract (LWE) of Helichyrsum plicatum subsp. plicatum against diluted culture of Escherichia coli O157:H7 (K, control; AK, alcohol control).
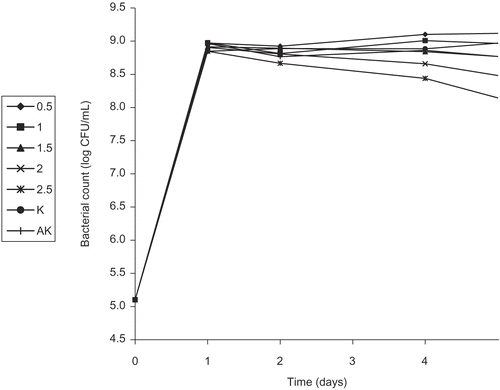
We tested the effect of sub-extracts of FEE at different concentrations on the survival of E. coli O157:H7 at 37ºC for a week. Of the five sub-extracts, FEECE, FEEBE, and FEER were observed to be inactive on E. coli O157:H7 at all concentrations. However, at 2.0 and 2.5% concentrations of FEEEAE, partial inhibition of the growth of the pathogen was observed only for the fifth and seventh days (). FEEHE started to express its bacteriostatic or bactericidal effect on the seventh day at a concentration of 2.5% ().
HPLC analysis
A series of the standard mixture solutions of naringenin and total helichrysins A and B were tested to determine the linearity between the standard mixture concentration and the peak areas. The results of the regression analysis on calibration curves and retention times are presented in . The HPLC fingerprint of FEE is shown in .
Table 1. Validation data from calibration curves of three flavonoids in the flower ethanol extract (FEE) of Helichyrsum plicatum subsp. plicatum.
Figure 9. HPLC fingerprint of the flower ethanol extract (FEE) of Helichyrsum plicatum subsp. plicatum.
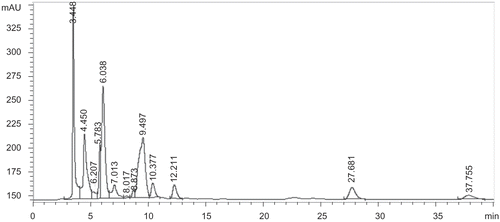
As shown in , helichrysins A and B are two enantiomers of naringenin-5-β-d-glucoside. In order to determine the individual ratio of these enantiomers, chiral HPLC columns should be used. In the present study, the analysis was performed using an RP-18 column. The total content of helichrysins A and B was estimated based on the helichrysin A standard, and found to be 6.78±0.6% in the ethanol extract. Naringenin was estimated as 42.6±1.3 mg/100 g.
Discussion
The results from the present study regarding the overnight culture of E. coli O157:H7 have shown that the flower ethanol extract of HPP might be useful in the continuing challenge to control the growth of E. coli O157:H7, rather than its sub-extract FEECE. The greater antibacterial activity of FEE at a concentration of 2.5% (particularly on the fifth day), compared to the sub-extract FEECE at the same concentration for the same day analysis, indicated that not only one component of FEE might be effective against E. coli O157:H7. The results of our study concerning the diluted culture of E. coli O157:H7 have shown that the bacterostatic or bactericidal activity of the flower ethanol extract of HPP was most significant against E. coli O157:H7. The greater antibacterial activity of the FEE than its sub-extracts, in particular the chloroform and ethyl acetate extracts, might be due to synergistic effects of the individual sub-extracts against E. coli O157:H7. Our results also support a modest loss and a delay in antibacterial activation time of HPP due to high inoculation levels (109 CFU/mL) of the pathogen E. coli O157:H7, when compared to the inoculation level of 105 CFU/mL.
Due to the well-known antimicrobial activity of traditional medicines used worldwide, a number of studies have been conducted to evaluate the antimicrobial potency of several Helichrysum species. In these studies, several extracts and isolated compounds were reported to possess antimicrobial activity. Chloroform and ethyl acetate extracts from H. compactum flowers were shown to possess antimicrobial activity against B. subtilis, Pseudomonas aeruginosa, S. aureus, E. coli, and Candida albicans (all MIC values were 40 μg/mL) (CitationSüzgeç et al., 2005). CitationNostro et al. (2000) reported moderate activity against E. coli for the diethyl ether extract of H. italicum, while the petroleum ether, dichloromethane, dichloromethane–methanol (9:1), and methanol extracts were devoid of such activity. In contrast, all of the extracts were active against Propionibacterium acnes. Effects of the acetone and methanol extracts and the essential oils from four South African species, H. dasyanthum (Willd.) Sweet, H. excisum (Thunb.) Less., H. felinum Less., and H. petiolare (L) DC., on B. cereus and S. aureus were investigated and the acetone extract from H. dasyanthum exhibited promising antimicrobial activity (CitationLourens et al., 2004).
Several antimicrobial constituents were also isolated from Helichrysum species. CitationAfolayan and Meyer (1997) isolated a flavone derivative, 3,5,7-trihydroxyflavone, with potent antimicrobial activity from H. aureonitens Sch. Bip. Phloroglucinol and acetophenone derivatives with antibacterial activity have been isolated from the aerial parts of H. italicum, H. stoechas, and H. decumbens by CitationTomas-Barberan et al. (1990). Phloroglucinol derivatives demonstrated an eight-fold increase in activity against Gram-positive bacteria compared to the acetophenone derivative, but the latter was also active against Gram-negative bacteria (E. coli). Another phologlucinol derivative, helihumulone, with potent antimicrobial activity was also isolated from the acetone extract of H. cymosum subsp. cymosum (L.) D. Don (Citationvan Vuuren et al., 2006). CitationDrewes et al. (2006) isolated a diterpene, ent-beyer-15-en-19-ol, with potent activity against B. cereus and S. epidermidis from the hexane extract of H. tenax. However, essential oils from Helichrysum species may have some antimicrobial activity depending on the chemotypic variation (Citationvan Vuuren et al., 2006).
As summarized above, Helichrysum species possess antimicrobial activity mainly against Gram-positive bacteria and a Gram-negative bacterium, E. coli. However, CitationSag˘dıç et al. (2002) reported that the methanol extract from H. compactum Boiss. was ineffective against E. coli O157:H7. In fact, the methanol extract was found to stimulate the growth of this organism in their preliminary activity screening study on the effects of several Turkish spice extracts against this food pathogen. This might be due to the low flavonoid content of H. compactum as reported by CitationSüzgeç et al. (2005).
In conclusion, among the ethanol and water extracts from the flowers and leaves of HPP, only FEE showed an efficacious inhibitory effect against E. coli O157:H7 in the present study. However, further purification and testing should be performed on the active flower ethanol extract to identify the major active ingredient(s) responsible for its antimicrobial activity.
Acknowledgement
This study was financially supported by the Research Fund of Gazi University (project no. 02/2003-16).
Declaration of interest: The authors report no conflicts of interest.
References
- Afolayan AJ, Meyer JJM (1997): The antimicrobial activity of 3,5,7-trihydroxyflavone from the shoots of Helichrysum aureonitens. J Ethnopharmacol 57: 177–181.
- Barbour EK, Al Sharif M, Sagherian VK, Habre AN, Talhouk RS, Talhouk SN (2004): Screening of selected indigenous plants of Lebanon for antimicrobial activity. J Ethnopharmacol 93: 1–7.
- Bianchini A, Tomi P, Costa J, Bernardini AF (2001): Composition of Helichrysum italicum (Roth.) G. Don fil. subsp. italicum essential oils from Corsica (France). Flavour Frag J 16: 30–34.
- Drewes SE, van Vuuren SF (2008): Antimicrobial acylphloroglucinols and dibenzyloxy flavonoids from flowers of Helichrysum gymnocomum. Phytochemistry 69: 1745–1749.
- Drewes SE, Mudau KE, van Vuuren SF, Viljoen AM (2006): Antimicrobial monomeric and dimeric diterpenes from the leaves of Helichrysum tenax var. tenax. Phytochemistry 67: 716–722.
- Eloff JN (1988): Which extractant should be used for the screening and isolation of antimicrobial components from plants? J Ethnopharmacol 60: 1–8.
- Erdogrul OT, Cakıroglu E, Karaman S (2001): Antibacterial activities of Helichrysum plicatum subsp. plicatum extracts. The Sciences 1: 176–178.
- Fujita T, Sezik E, Tabata E, Yesilada E, Honda G, Takeda Y, Tanaka T, Takaishi Y (1995): Traditional medicine in Turkey VII. Folk medicine in middle and west Black Sea Regions. Econ Bot 49: 406–422.
- Garcia D, Gomez N, Raso J, Pagan R (2005): Bacterial resistance after pulsed electric fields depending on the treatment medium pH. Innovative Food Sci Emerg Technol 6: 388–395.
- Juneja LR, Okubo T, Hung P (2000): Catechins. In: Naidu AS, editor. Natural food antimicrobial systems. Boca Raton, FL: CRC Press. pp 381–398.
- Kim HO, Park SW, Park HD (2004): Inactivation of Escherichia coli O157:H7 by cinnamic aldehyde purified from Cinnamomum cassia shoot. Food Microbiol 21: 105–110.
- Lourens ACU, Reddy D, Baser KHC, Viljoen AM, van Vuuren SF (2004): In vitro biological activity and essential oil composition of four indigenous South African Helichrysum species. J Ethnopharmacol 95: 253–258.
- Li Q, Logue CM (2005): The growth and survival of Escherichia coli O157:H7 on minced bison and pieces of bison meat stored at 5 and 10ºC. Food Microbiol 22: 415–421.
- Mathekga AD, Meyer JJ, Horn MM, Drewes SE (2000): An acylated phloroglucinol with antimicrobial properties from Helichrysum caespititium. Phytochemistry 53: 93–96.
- Meyer JJM, Afolayan AJ, Taylor MB, Erasmus D (1997): Antiviral activity of galangin isolated from the aerial parts of Helichrysum aureonitens. J Ethnopharmacol 56: 165–169.
- Mora A, Blanco JE, Blanco M, Alonso MP, Dhabi G, Echeita A, González A, Bernárdez MI, Blanco J (2005): Antimicrobial resistance of Shiga toxin (veratoxin)-producing Escherichia coli O157:H7 and non-O157 strains isolated from humans, cattle, sheep and food in Spain. Res Microbiol 156: 793–806.
- Moreira MR, Ponce AG, del Vale CE, Roura SI (2005): Inhibitory parameters of essential oils to reduce a foodborne pathogen. LWT-Food Sci Technol 38: 565–570.
- Nostro A, Germano MP, D’Angelo V, Marino A, Cannatelli MA (2000): Extraction methods and bioautography for evaluation of medicinal plant antimicrobial activity. Lett Appl Microbiol 30: 379–384.
- Nostro A, Bisignano G, Cannatelli MA, Crisafi G, Germanò MP, Alonzo V (2001): Effect of Helichrysum italicum extract on growth and enzymatic activity of Staphylococcus aureus. Int J Antimicrob Ag 17: 517–520.
- Rios JL, Recio MC, Villar A (1991): Isolation and identification of the antibacterial compounds from Helichrysum stoechas. J Ethnopharmacol 33: 51–55.
- Sag˘dıç O, Kuscu A, Ozcan AM, Ozcelik S (2002): Effects of Turkish spice extracts at various concentrations on the growth of Escherichia coli O157:H7. Food Microbiol 19: 473–480.
- Schinella GR, Tournier HA, Prieto JM, Mordujovich de Buschiazzo P, Rios JL (2002): Antioxidant activity of anti-inflammatory plant extracts. Life Sci 70: 1023–1033.
- Sezik E, Tabata M, Yesilada E, Honda G, Goto K, Ikeshiro Y (1991): Traditional medicine in Turkey I. Folk medicine in Northeast Anatolia. J Ethnopharmacol 35: 191–196.
- Sezik E, Yesilada E, Honda G, Takaishi Y, Takeda Y, Tanaka T (2001): Traditional medicine in Turkey X. Folk medicine in Central Anatolia. J Ethnopharmacol 75: 95–115.
- Süzgeç S, Mericli AH, Houghton PJ, Cubukcu B (2005): Flavonoids of Helichrysum compactum and their antioxidant and antibacterial activity. Fitoterapia 76: 269–272.
- Tepe B, Somken M, Askin Akpulat A, Somken A (2005): In vitro antioxidant activities of the methanol extracts of four Helichrysum species from Turkey. Food Chem 90: 685–689.
- Tomas-Barberan F, Iniesta-Sanmartin E, Tomas-Lorente F, Rumbero A (1990): Antimicrobial phenolic compounds from three Spanish Helichrysum species. Phytochemistry 29: 1093–1095.
- van Vuuren SF, Viljoen AM, van Zyl RL, van Herden FR, Bas¸er KHC (2006): The antimicrobial, antimalarial and toxicity profiles of helihumulone, leaf essential oil and extracts of Helichrysum cymosum (L.) D. Don subsp. cymosum. S Afr J Bot 72: 287–290.